Abstract
Lipid-based drug delivery systems have been widely employed over the past few decades for efficient drug delivery. These systems offer several benefits, including protection of drugs from the biological environment, thereby improving their therapeutic efficacy. Various types of lipid-based drug delivery systems have been developed, such as solid lipid nanoparticles, liposomes, emulsions, emulsomes, pharmacosomes, lipospheres, Nano emulsions, niosomes, and transferosomes. Among these, emulsomes have garnered significant attention due to their unique properties, which overcome many limitations associated with other systems. This review emphasizes lipid-based drug delivery systems, with a particular focus on emulsomes. It explores the success of emulsomes as drug carriers, detailing their advantages, structure, and composition, which include a lipid core, antioxidants, phosphatidylcholine, negatively charged particles, surfactants, and cholesterol. Additionally, it outlines various preparation methods for emulsomes, such as lipid film hydration, reverse-phase evaporation, high-pressure extrusion technique, sonication, cast film method, ethanol injection method and detergent removal method, along with their diverse applications.
Keywords
Lipid-based drug delivery systems, emulsomes, drug carriers, therapeutic efficacy, composition, preparation methods, drug delivery applications.
Introduction
In the vesicular drug delivery system (VDDS), the active pharmaceutical ingredient is enclosed within a vesicular structure. The vesicles used in these systems include liposomes, niosomes, archeosomes, transferosomes, sphinosomes, pharmacosomes, ufasomes, and emulsomes. This approach enhances the drug's systemic circulation while minimizing toxicity. The introduction of innovative vesicular technologies has revolutionized diagnostics and treatments across various biomedical fields. These systems are commonly employed in gene delivery, brain tumour targeting, oral formulations, and addressing challenges related to pharmaceutical stability and permeability [1]
Emulsomes
Emulsomes are an innovative lipid-based vesicular system consisting of a solid lipid core enveloped by a phospholipid bilayer. The solid core is composed of materials that remain in a solid state at room temperature, stabilized by a surrounding layer of phospholipids and cholesterol. These vesicular drug delivery systems encapsulate active drug molecules within their structure. Vesicular drug delivery systems, including emulsomes, offer significant advantages such as prolonged drug presence in systemic circulation, enhanced therapeutic efficacy, controlled drug release, and minimal toxic effects [2,3]. Although derived from liposomes, emulsomes differ in several aspects, particularly due to their ability to encapsulate lipophilic drugs in both the solid core and the phospholipid bilayer. This characteristic makes emulsomes highly effective for delivering poorly water-soluble drugs that conventional drug delivery systems struggle to manage or might otherwise cause undesirable side effects [4,5,6].
A wide range of drugs can be encapsulated within emulsomes, including antifungal agents, neuroprotective drugs, AZT derivatives, ?-blockers, antiepileptics, antibiotics, antineoplastic agents, and anti-inflammatory drugs. As an emerging drug delivery system, emulsomes show great promise in treating life-threatening diseases such as hepatitis, HIV, fungal infections, leishmaniasis, and other viral and fungal infections. Their solid fat core, often composed of triglycerides, enhances the entrapment of lipophilic drugs, making emulsomes an efficient solution for addressing the challenges associated with poorly water-soluble drugs [7,8].
Structure Of Emulsomes

Figure:1
Emulsomes are lipid-based nanoparticles commonly used as drug delivery systems. Their structure can be described as a combination of liposomes and solid lipid nanoparticles, as they consist of an oil phase enclosed by a phospholipid bilayer. The phospholipid bilayer in emulsomes is formed by a head-to-tail arrangement, where the hydrophilic heads of the phospholipids face outward, and the hydrophobic tails face inward. This bilayer provides stability to the emulsomes and helps protect the encapsulated oil phase from degradation the structure and composition of emulsomes is shown in Figure 1 and Figure 2[9].
Composition Of Emulsomes
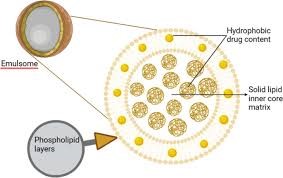
Figure:2
Advantages Of Emulsomes
- A cost-effective alternative to conventional lipoidal formulations.
- Enhanced drug concentration in damaged or targeted tissues.
- Protection of the drug from the harsh gastrointestinal environment.
- Improved solubility and bioavailability of poorly water-soluble drugs.
- A cytotoxicity profile that ensures both safety and effectiveness.
- Prevention of the development of multi-drug resistance.
- Alteration of the drug’s pharmacokinetic profile.
- Increased therapeutic efficacy with reduced toxicity. Prolonged drug effectiveness through a slow-release profile.
- Potential for surface modifications to enable cellular targeting.
- High loading capacity for poorly water-soluble drugs.
- Outstanding stability.
- Cost-effective production with ease of large-scale manufacturing.
Disadvantages Of Emulsomes
- Limited drug loading capacity.
- Causes adverse effects during parenteral administration.
- Minimal use of surfactants in parenteral administration due to their detergent-like effects.
- High oil content negatively impacts formulation stability [10].
Components Of Emulsomes
Lipid Core
At 25°C, the lipid core of emulsomes typically exists in a solid or crystalline phase, or a combination of solid and liquid crystal phases. In the pharmaceutical industry, various lipids and lipid-like excipients are readily available. These compounds are collectively referred to as lipids and can include either a single lipid or a mixture of lipids. They are generally fatty acids, their derivatives, or substances that are biosynthetically or functionally related to these compounds. Lipids are hydrophobic and insoluble in water, and they vary based on factors like fatty acid composition, melting point, and hydrophilic-lipophilic balance (HLB). Excipients used for instant release and bioavailability enhancement tend to have a high HLB and are in a semi-solid form, while those intended for sustained release have a low HLB and a high melting point. Due to the limited shelf life of o/w emulsions, solid triglycerides at 25°C are considered an ideal core material. Emulsomes, typically composed of unbranched fatty acids with chain lengths between C-10 and C-18, are made using triglycerides [11].
ANTIOXIDANT
The lipid core of the emulsome particles in this invention may incorporate one or more antioxidants. Preferred antioxidants include alpha-tocopherol or its derivatives, which belong to the vitamin E family, as well as butylated hydroxytoluene (BHT). Antioxidants help prevent the formation of oxidative breakdown products, such as peroxides, in unsaturated lipids. To meet the antioxidant requirements, the lipid core can also be created using saturated fatty acids [12].
Negatively Charged Particle
To enhance the zeta potential and stabilize the particles, negatively charged phospholipids such as phosphatidic acid, phosphatidylinositol, phosphatidylserine, or negatively charged lipid compounds like oleic acid can be incorporated into emulsomes. The addition of these negatively charged lipid molecules leads to the formation of oppositely charged phospholipid bilayers.
This results in an increase in the loading capacity of the aqueous compartment within the bilayers. The enhanced loading capacity is driven by the electrical repulsion between the bilayers in the aqueous spaces. Furthermore, a positive charge improves particle dispersion, reducing the likelihood of coalescence, flocculation, or fusion [9,10].
Surfactants
A phospholipid molecule forms part of the layer that surrounds the lipid core. This phospholipid layer plays a stabilizing or surface-active role, reducing surface tension. It is believed that a monolayer of surface-active phospholipids forms around the lipid core, with their polar head groups positioned at the interface. Additional phospholipids can create one or more roughly concentric bilayers around the lipid core, with the number of bilayers varying. These bilayer envelopes can contain one or more aqueous compartments, which may house water-soluble medications. The use of multiple concentric bilayer structures in emulsome design allows the particles to carry a significant load of both lipid-soluble and water-soluble drugs. Drug entrapment efficiency within the vesicles is also influenced by the transition temperature of the surfactants. Drugs are most effectively entrapped when the surfactants have the lowest phase transition temperature, and efficiency decreases as the phase transition temperature rises [13].
Phosphotidylcholine
Lecithin is rich in phosphatidylcholine, which does not dissolve readily in water. Depending on temperature and hydration levels, the phospholipids in this solution can organize into lamellar structures, micelles, or bilayer sheets. This results in the formation of a surfactant typically classified as amphipathic. Lecithin can be easily obtained from accessible sources such as egg yolks or soybeans, where it is referred to as egg lecithin and soya lecithin, respectively. These compounds are essential components of biological membranes. Incorporating lecithin has been shown to increase drug entrapment efficiency to 96.1% while simultaneously reducing vesicle size due to increased hydrophobicity [14,15].
CHOLESTROL
Emulsomes function as vesicles, with cholesterol serving as a key component. The incorporation of cholesterol significantly impacts the stability of the vesicles. It has been reported to enhance the buffering capacity and regulate the fluidity of the overall formulation. Cholesterol is added to all formulations as a stabilizing agent, as it can induce the formation of a liquid crystal phase by altering the fundamental packing structure of the vesicle. Additionally, it stabilizes the outer phospholipid layers, leading to improved drug entrapment efficiency and reduced drug leakage [16] Cholesterol plays a vital role in enhancing the drug entrapment efficiency of emulsomes. Studies suggest that as cholesterol concentration increases, drug entrapment efficiency also improves. However, excessively high cholesterol levels can negatively impact drug entrapment. When cholesterol exceeds a certain threshold, it disrupts the normal bilayer structure, leading to a reduction in drug entrapment efficiency [17].
Preparation Of Emulsomes [5,11,18]:
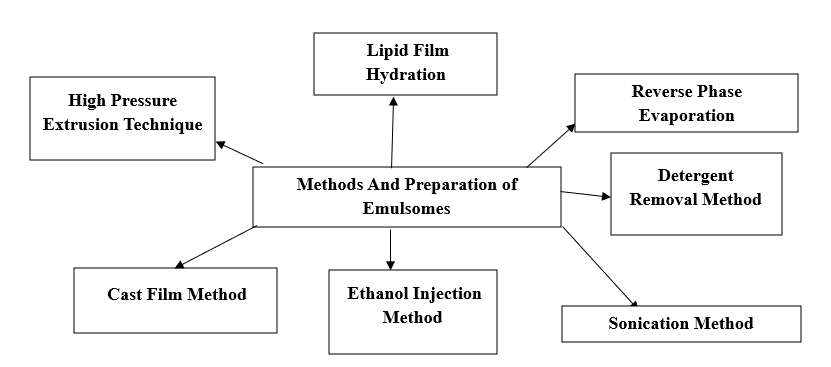
1. Lipid film hydration
- Surfactants or lipids are dispersed in an aqueous environment after being cast as thin film layers from their organic solution.
- The film is formed using a flask rotary evaporator at low pressure or by manual shaking.
- The lipids expand and peel off the flask walls at a temperature slightly above the surfactant phase transition temperature.
- Hydration occurs for a specified duration (hydration time) with mild, continuous shaking.
- Alternative methods to facilitate lipid swelling and dispersion include:
Manual handshaking: Provides mechanical energy for lipid swelling, resulting in the formation of multi-lamellar vesicles (MLVs).
Non-shaking method: The lipid film is exposed to water-saturated nitrogen steam for 15 minutes, followed by swelling in an aqueous solution, which produces large unilamellar vesicles (LUVs) lipid film hydration method is shown in Figure3.
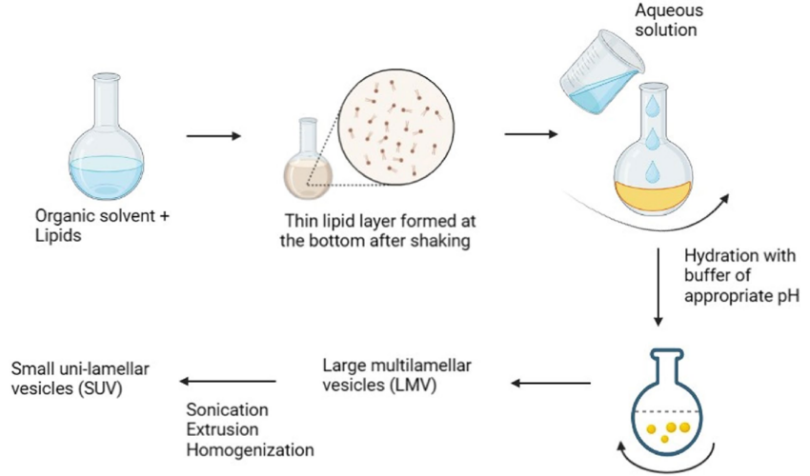
Figure: 3
2. Reverse-Phase Evaporation (REV) Technique:
- Procedure Overview:
- A two-step process is used.
- Involves creating a phospholipid water-in-oil emulsion using a buffer and an organic phase.
- Emulsion Preparation:
- Phospholipids and water are emulsified mechanically or acoustically.
- Organic solvents such as chloroform, isopropyl ether, or freon are used to dissolve phospholipids.
- Organic Phase Removal:
- Organic solvent is removed under low pressure (vacuum).
- Initial removal leads to a gel-like matrix formed by phospholipid-coated water droplets.
- Further solvent removal under reduced pressure converts the gel-like matrix into a smooth paste.
- Formation of Large Unilamellar Vesicles (LUVs):
- In the smooth paste, LUVs are dissolved.
- Drug entrapment efficiencies of 60–65% are achievable.
- Versatility:
- Both small and large molecules can be encapsulated using this technique.
- Challenges:
- Drugs and biologically active molecules are exposed to:
- Organic solvents.
- Mechanical agitation.
- Significant exposure can lead to:
- Conformational changes in biomolecules (e.g., enzymes, RNA).
- Protein denaturation or DNA strand breakage.
- Additional Considerations:
- To support proper emulsification, combining two organic solvents may be required.
Adjusting the solvent mixture density closer to that of the aqueous phase is necessary for optimal emulsification conditions reverse-Phase Evaporation (REV) Technique is shown in figure4.
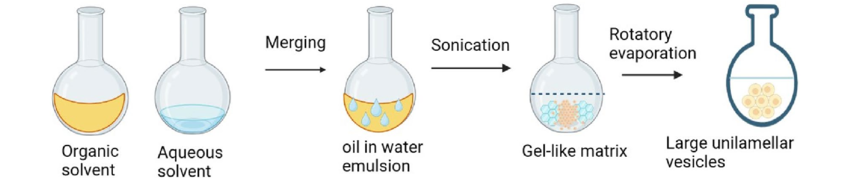
Figure: 4
3. High-pressure extrusion technique
- This method is used to produce unilamellar vesicles by forcing multilamellar vesicles through a small orifice under high pressure.
- A filter membrane, typically made of polycarbonate with a pore size of 0.8 to 1.0 µm, is utilized in the process.
- After 5–10 passes through the membrane, unilamellar vesicles in the size range of 60–180 nm is obtained.
- Some researchers have used a microfluidizer to produce nanosized vesicles.
- A microfluidizer is an instrument that forces the feed material through a narrow orifice under high pressure.
- This process removes multiple layers of the phospholipid bilayer to create nanosized unilamellar vesicles.
4. Sonication method
- Solid lipids, cholesterol, and phosphatidylcholine were dissolved in chloroform with 3–4 drops of methanol in a round-bottom flask in varying molar ratios.
- A specific quantity of the drug was added to this solution.
- The organic solvent was evaporated under reduced pressure using a rotary evaporator until a thin lipid layer formed on the walls of the flask.
- The dry lipid film was hydrated with 10 mL of phosphate-buffered saline (pH 7.4).
- The resulting suspension was homogenized through ultrasonication for 15 minutes at 40% frequency to produce nano-sized emulsomes.
5. Cast film method
- Emulsomes are prepared by mixing phospholipids with triglycerides (solid lipids).
- The mixture is suspended in an aqueous solution at a temperature below the solid-to-liquid transition temperature.
- The resulting emulsion consists of liquid particles with a diameter ranging from 10–250 nm.
- The particle size is preferably determined based on weight percentage rather than particle number.
- The solvent is removed under reduced pressure using a rotary evaporator or by a stream of inert gases.
- The resulting dry lipid film is hydrated by shaking it with an aqueous solution.
- The drug to be encapsulated can be added either to the aqueous solution or the organic solution, depending on the drug's properties.
6. Ethanol injection method
- A technique for creating small unilamellar vesicles (SUVs) has been described.
- An ethanol-surfactant solution is rapidly injected into excess saline or another aqueous environment using a small needle.
- Vesicles form spontaneously as the ethanol evaporates.
- Small liposomes (sub-100 nm) with a tight size distribution can be produced in a single step by infusing ethanolic lipid solution into water, without the need for extrusion or sonication.
- The ethanol injection method is an efficient way to create emulsomes with a small average radius.
- In this method, the lipid or lipid mixture is dissolved in an alcohol-based solvent.
- An aliquot of 200, 500, or 600 µL is quickly injected using a 1 mL syringe into the dispersant solution, containing:
- 9.8 mL water/saline diluted to 1:50,
- 9.5 mL water/saline diluted to 1:20, or
- 9.8 mL water/saline diluted to 1:17.
- The solution is vigorously stirred by hand for 20–30 seconds.
- The ethanol solution is then rapidly pumped into a 5% glucose solution.
- The resulting vesicles have an average diameter of 60 nm and are expected to remain stable for at least one week.
7. Detergent removal technique
In this method, lipids are combined with a detergent to form a micelle mixture. The detergent is then removed through various techniques, resulting in the formation of micelles. These micelles attract phospholipid molecules from the bulk solution, causing additional lipid molecules to aggregate and form a bilayer. Detergent removal can be achieved using methods such as dialysis, column chromatography, or adsorption. Detergents with a high critical micelle concentration (CMC), such as sodium cholate, sodium deoxycholate, and octyl glycoside, are commonly used in this process.
Stability Ascepts of Emulsomes
In nanoparticle-mediated drug delivery systems (DDSs), stability refers to the nanocarrier's ability to maintain its biophysical properties such as size, zeta potential, and drug retention over time. Emulsomes, with their high absolute zeta potential values, are expected to exhibit significant physical stability, minimizing the risk of coalescence. Compared to other lipid-based formulations like liposomes, emulsomes are more stable in suspensions, a property that holds great potential for clinical applications [19,20]. Emulsomes are formed by combining two key components: a phospholipid layer surrounding the lipidic core, which provides vesicular steric stability. This allows for the development of pharmaceutically stable emulsomal formulations without the need for additional solubilizers or surfactants. Furthermore, PEGylation of the emulsome surface enhances steric stabilization and prolongs circulation time in the body. The stability of the nanocarrier is significantly influenced by the physicochemical properties of the lipids used and the storage temperature [21].
These characteristics make emulsomes particularly suitable for developing sustained-release formulations for oral administration. The zeta potential measurement of electrostatically stabilized vesicles is a critical parameter for evaluating storage stability, as it helps understand dispersion and aggregation behaviour. High-energy input during sonication reduces particle size and zeta potential, resulting in stable, dense emulsomes [22].
Characterization Of Emulsomes
Characterizing the prepared emulsomes is essential for ensuring their effectiveness and consistency in application. Monitoring both physical and chemical properties is necessary to guarantee that the emulsomes preparation is reproducible and meets its intended purpose. Key properties of emulsomes include average size, size distribution, shape, polydispersity index, surface charge, and encapsulation efficiency.
Transmission electron microscopy (TEM) confirms that emulsomes are spherical in shape, similar in size and morphology to empty emulsomes. These biocompatible vesicular structures consist of a solid lipid core surrounded by multiple layers of phospholipids. The solid core enables emulsomes to encapsulate more lipophilic therapeutic molecules and provides a longer half-life compared to emulsion formulations with a liquid core. Due to their lipid composition, emulsomes are biocompatible and offer promising potential for delivering poorly water-soluble drugs like curcumin and silybin. Recent studies have demonstrated that a dehydration-rehydration technique, followed by temperature-controlled extrusion, can be used to combine phospholipids and triglycerides, resulting in stable, dispersed emulsomes [13].
Application Of Emulsomes [4,5,7,12,13,27,28,29,30,31]
1. Anti-fungal therapy
Amphotericin B (AmB) is a polyene macrolide antifungal antibiotic with limited oral bioavailability. Its use is associated with adverse effects such as fever, chills, nausea, vomiting, headache, renal failure, anemia, hypokalaemia, and hypomagnesemia. Lipid-based formulations of AmB offer significant advantages over conventional formulations, particularly in reducing renal toxicity.
2. Anti-inflammatory action
Lornoxicam is a novel non-steroidal anti-inflammatory drug (NSAID) belonging to the oxicam family, with a plasma half-life of approximately 3 hours. It is commonly used for relieving musculoskeletal and joint pain and is administered through the skin using soya lecithin-based emulsomal nanoparticles. Lornoxicam is effective in treating conditions such as rheumatoid arthritis, osteoarthritis, and ankylosing spondylitis.
3. Drug targeting
One of the key advantages of emulsomes is the ability to modify or tailor their phospholipid bilayer, making them ideal for surface coating with specific ligands. These ligands possess free ends that specifically bind to surface overexpressed receptors, enabling targeted delivery. By coating emulsomes with ligands such as O-Palmitoyl Amylopectin or O-Palmitoyl Mannan, they can be directed for tissue-specific targeting.
Once administered in the body, emulsomes become coated with serum factors known as opsonin’s, which are recognized by the reticuloendothelial system and subsequently taken up. This process, called opsonization, facilitates the clearance of opsonin-coated particles. This unique property makes emulsomes particularly useful for treating tumours and infections in the liver and spleen.
4. Auto-Immunity
Emulsomes can serve as adjuvants in mucosal vaccines. When combined with anti-CD3 monoclonal antibodies (mAb), emulsomes reduce antibody production against type II collagen and alleviate joint disease severity by lowering inflammatory cytokines in the joints. Anti-CD3 therapy, administered nasally or orally, enhances the Th2 response and activates LAP+ (latency-associated peptide) regulatory T cells, contributing to arthritis suppression.
Vaccines composed of the following components can benefit from emulsomes:
- Protein or peptide antigens.
- Hydrophobic compounds, if necessary, to enhance the formulation.
- An immune-potentiating membranous carrier, such as emulsomes, which helps preserve the antigenic integrity of protein or peptide epitopes and enhances the vaccine’s immunogenicity.
This approach has been demonstrated to be a safe and effective mucosal and non-invasive therapy for rheumatoid arthritis.
5. Cancer treatment
Emulsome formulations encapsulating the anti-cancer drugs methotrexate and curcumin have demonstrated effectiveness in cancer treatment, highlighting the potential of emulsomes as a powerful carrier for antineoplastic drugs.
6. Dermal therapy
Dithranol, also known as anthralin, has long been used to treat psoriasis, a non-contagious autoimmune skin disorder. However, its use has declined due to side effects such as skin irritation, erythema, peeling, and discoloration. Encapsulating dithranol in the lipidic core of emulsomes significantly enhances its skin permeability, increasing drug retention in skin tissues and reducing adverse effects. The formulation by design (FbD) approach has been applied to develop various compritol-based emulsomes. Formulations containing 63–75% compritol and 25–37% phospholipids (PL) demonstrated the highest entrapment efficiency. However, increasing the PL content reduced skin permeation due to the formation of multilamellar barriers. In a mouse-tail model antipsoriatic study, emulsome formulations showed superior pharmacodynamic performance compared to commercial products, with no signs of erythema or wrinkles on the mice's skin. These findings indicate that emulsomes can enhance dithranol's therapeutic efficacy while minimizing its side effect.
7. AIDS Treatment
Zidovudine is approved drug for AIDS treatment. Due to high lipophilicity, drug shows some serious side effects with altered pharmacokinetics. By incorporating zidovudine in emulsomes, problem of low bioavailability and other side effects have been overcome.
8. Ophthalmic delivery
Sparfloxacin, classified as a Biopharmaceutical Classification System (BCS) Class II drug, exhibits low solubility in aqueous media, with its therapeutic effect limited by its dissolution rate. It is a third-generation fluoroquinolone derivative commonly prescribed for external eye infections such as conjunctivitis and bacterial keratitis. Sparfloxacin demonstrates in vitro antibacterial activity against both gram-negative and gram-positive bacteria and is available as a 0.3% (w/v) ophthalmic solution. The standard dosage is 1–2 drops every 4 hours, or hourly for severe infections. To address the limitations of traditional sparfloxacin formulations, such as short residence time, drug drainage, and frequent administration, sparfloxacin emulsomes can be incorporated into an in-situ gelling system. This innovative delivery system provides controlled drug release on the ocular surface. The lipid bilayer of the emulsomes, combined with their slow diffusion within the hydrogel, ensures prolonged drug release and improved trans-corneal penetration. This thermosensitive in-situ emulsomal gel enhances patient compliance and has shown promising antibacterial activity in both in vivo and in vitro studies, demonstrating its potential as an effective ocular drug delivery system. As a result, emulsomal in-situ gels offer a viable alternative to conventional eye drops for sparfloxacin administration.
9. Hepatoprotective activity
Silybin (SIL) is a natural compound derived from milk thistle plants, commonly used to treat hepatitis, cirrhosis, and protect the liver from toxic substances. It also prevents hepatic lipid peroxidation and ischemia. However, its therapeutic potential is limited due to its low aqueous solubility (0.43 mg/mL in water), low oral bioavailability, and poor intestinal absorption.
Incorporating SIL into emulsomes significantly enhances its bioavailability. SIL is encapsulated within the solid lipid core of the emulsome, providing a sustained-release profile both in vitro and in vivo, unlike a standard SIL solution. Emulsomes are recommended for SIL delivery in the treatment of liver diseases due to their enhanced stability and reduced risk of coalescence, attributed to the high absolute zeta potential.
10. Increase bioavailability of lipophilic drugs
Emulsomes have been shown to be effective drug delivery carriers for lipophilic drugs that have poor aqueous solubility in biological fluids, which often limits their absorption and bioavailability. The core of emulsomes consists of solid lipids that encapsulate lipophilic drugs, enabling their sustained release. Additionally, emulsomes offer the advantage of higher drug content, leading to increased entrapment efficiency and reduced dosing frequency.
CONCLUSION
In conclusion, emulsomes represent a significant advancement in nanotechnology for targeted drug delivery and therapeutics. Their unique structure, combining the benefits of both liposomes and solid lipid nanoparticles, allows for the efficient encapsulation and controlled release of lipophilic drugs, improving bioavailability and therapeutic efficacy. The ability to tailor the lipid composition and surface properties of emulsomes enables the targeting of specific tissues, enhancing their potential in treating a variety of diseases, including cancer, infections, and autoimmune disorders. Additionally, their biocompatibility, stability, and versatility in formulation make emulsomes a promising candidate for overcoming the challenges faced by conventional drug delivery systems. With ongoing research and development, emulsomes hold the potential to revolutionize the delivery of poorly water-soluble drugs, providing more effective and safer therapeutic options for patients.
REFERENCES
- Mahapatra DK, Bharti SK, editors. Medicinal chemistry with pharmaceutical product development. CRC Press; 2019 Feb 4.
- Amselem S, Yogev A, Zawoznik E, Friedman D. Emulsomes, a novel drug delivery technology. InProceedings of the International Symposium on Controlled Release of Bioactive Materials 1994; 21(2):1368-69.
- Vyas SP, Subhedar R, Jain S. Development and characterization of emulsomes for sustained and targeted delivery of an antiviral agent to liver. J Pharm Pharmacol, 2006; 58(3):321-326.
- Gupta S Vyas SP. Development and characterization of Amphotericin B bearing emulsomes for passive and active macrophage targeting. J Drug Target, 2007; 15(3):206-217.
- Gill V, Nanda A. Emulsomes: A Lipid Bases Drug Delivery System. World J. Pharm. Res. 2021 Aug 10; 10(12):113-29.
- Paliwal R, Paliwal SR, Mishra N, Mehta A, Vyas SP. Engineered chylomicron mimicking carrier emulsomes for lymph targeted oral delivery of methotrexate. Int J Pharm., 2009; 380(1-2):181-188.
- Pal A, Gupta S, Jaiswal A, Dube A, Vyas SP. Development and evaluation of tripalmitin emulsomes for treatment of experimental visceral leishmaniasis. J Liposome Res, 2012; 22(1): 62-71.
- Amselem, Shimon, Zawoznik E, Yogev A, Friedman, D (2018) Emulsomes, a new type of lipid assembly. In Handbook of Nonmedical Applications of Liposomes: 3rded.CRC Press; 2018; 209–223.
- Amalnath S, Ganesh NS, Chandy V (2021) A well explained review on: emulsomes as vesicular drugdelivery system. World J Pharm Sci. 2021;10(10):10-32
- Gill B, Singh J, Sharma V, Kumar SH. Emulsomes: An emerging vesicular drug delivery system. Asian J Pharm. 2014;6(2):87-94.
- Varshosaz J, Raghami F, Rostami M, Jahanian A. PEGylated trimethylchitosan emulsomes conjugated to octreotide for targeted delivery of sorafenib to hepatocellular carcinoma cells of HepG2. J Liposome Res. 2019;29(4):383-98.
- Jos S, Krishnakumar K, Dinesh Kumar B. Emulsomes drug delivery: A review. Int J Res Pharm Sci. 2019;4(4):13-7.
- Singh S, Khurana K, Chauhan SB, Singh I. Emulsomes: new lipidic carriers for drug delivery with special mention to brain drug transport. Future Journal of Pharmaceutical Sciences. 2023 Sep;9(1):78.
- Ghode SP, Ghode P. Applications perspectives of emulsomes drug delivery system. Int J Med Phar Sci| 2020 Jan;10(01):2-4.
- Jain S, Jain V, Mahajan SC. Lipid based vesicular drug delivery systems. Advances in Pharmaceutics. 2014;14(1):574-673.
- Kumar R, Nirmala KHLS (2013) Emulsomes: an emerging vesicular drug delivery system. J Drug Deliv Ther 3(6):133–142.
- Afreen U, Shailaja AK. Pharmacosomes and Emulsomes: An Emerging Novel Vesicular Drug Delivery System. Glob J Anes Pain Med. 2020;3(4):287-97.
- Briuglia ML, Rotella C, McFarlane A (2015) Influence of cholesterol on liposome stability and on in vitro drug release. Drug Deliv Trans Res 5(4):231–242.
- Nasr M, Mansour S, Mortada ND, Elshamy AA Vesicular aceclofenac systems: a comparative study between liposomes and niosomes. J Micro encapsule. 2008;25(7):499–512.
- Yilmaz EN, Bay S, Ozturk G, Ucisik MH. Neuroprotective effects of curcumin-loaded emulsomes in a laser axotomy-induced CNS injury model. Int J Nanomedicine. 2020 Nov; 20(1):9211-29.
- Zhou X, Chen Z (2015) Preparation and performance evaluation of emulsomes as a drug delivery system for silybin. Arch Pharm Res 38(12):2193 2200.
- Dubey S, Vyas PS (2021) Emulsomes for lipophilic anticancer drug delivery: development, optimization and in vitro drug release kinetic study. IJAP 13(2):114-121
- Zakaria MY, Zaki I, Alhomrani M, Alamri AS, Abdulaziz O, Abourehab MA (2022) Boosting the anti MERS-CoV activity and oral bioavailability of resveratrol via PEG-stabilized emulsomal nano-carrier: factorial design, in-vitro and in-vivo assessments. Drug Deliv 29(1):3155–3167.
- Ghosh A, Kaur CD, Gupta A, Saraf S (2017) Surface engineered lamivudine loaded emulsome for targeting drug delivery to lymphatic system for effective treatment of hiv. Int J Appl Pharm Biol Res 2(1):25–37.
- Mehanna MM, Mneimneh AT (2021) Formulation and applications of lipid-based nano vehicles: spotlight on self-emulsifying systems. Adv Pharm Bull 11(1):56–67.
- H Ucisik M, B Sleytr U, Schuster B. Emulsomes meet S-layer proteins: an emerging targeted drug delivery system. Curr Pharm Biotechnol. 2015;16(4):392-405.
- Gill V, Kumar MS, Khurana B, Arora D, Mahadevan N. Development of Amphotericin B loaded modified emulsomes for visceral leishmaniasis: in vitro. IJRAPR, 2011;1(2):14-20.
- Ucisik MH, Kupcu S, Schuster B, Sleytr UV. Characterization of CurcuEmulsomes: nano formulation for enhances solubility and delivery of curcumin. J Nanobiotechnology, 2013; 11(1):37.
- Raza K, Katare OP, Setia A, Bhatia A, Singh B. Improved therapeutic performance of dithranol against psoriasis employing systematically optimized nanoemulsomes. J Microencapsulation, 2013; 30(3): 225-236.
- Wu HY, Maron R, Tukpah AM, Weiner HL. Mucosal anti-CD3 monoclonal antibody attenuates collagen-induced arthritis that is associated with induction of LAP+ regulatory T cells and is enhanced by administration of an emulsomes based Th2-skewing adjuvant. Journal of Immunology, 2010; 185(6): 3401-3417.
- Lowell JH, Kaminski RW, Vancott TC, Slike B, Kersey K, Zowoznik E, Loomis-price L, Smith G, Redfield RR, Amselem S, Birx DL. Proteosomes, emulsomes and cholera toxin B improved nasal immunogenicity of human immunodeficiency virus gp160 in mice: induction of serum, intestinal, vaginal and lung IgA and IgG. Journal of infectious disease, 1997; 175(2): 292-301.


 Santhosh Kumar C.*
Santhosh Kumar C.*





 10.5281/zenodo.14881136
10.5281/zenodo.14881136