Abstract
Ebola virus disease (EVD) is a severe and lethal disease caused by the Ebola virus (EBOV). It typically starts from a single case of probable zoonotic transmission and can spread through direct contact or infected bodily fluids. Diagnosis involves case definition and laboratory tests. Recent advances in medical countermeasure research have led to the approval of an EBOV-targeted vaccine and a randomized clinical trial of investigational therapeutics for EVD. The 2013-16 outbreak in the Western African and Democratic Republic of the Congo has improved understanding of EVD and viral persistence in survivors, leading to new strategies for prevention and clinical management. Ebola virus disease (EVD) is a severe and frequently lethal disease caused by Ebola virus (EBOV). EVD outbreaks typically start from a single case of probable zoonotic transmission, followed by human-to-human transmission via direct contact or contact with infected bodily fluids or contaminated fomites. EsVD has a high case-fatality rate; it is characterized by fever, gastrointestinal signs and multiple organ dysfunction syndrome. Diagnosis requires a combination of case definition and laboratory tests, typically real-time reverse transcription PCR to detect viral RNA or rapid diagnostic tests based on immunoassays to detect EBOV antigens. Recent advances in medical countermeasure research resulted in the recent approval of an EBOV-targeted vaccine by European and US regulatory agencies. The results of a randomized clinical trial of investigational therapeutics for EVD demonstrated survival benefits from two monoclonal antibody products targeting the EBOV membrane glycoprotein. New observations emerging from the unprecedented 2013-2016 Western African EVD outbreak (the largest in history) and the ongoing EVD outbreak in the Democratic Republic of the Congo have substantially improved the understanding of EVD and viral persistence in survivors of EVD, resulting in new strategies toward prevention of infection and optimization of clinical management, acute illness outcomes and attendance to the clinical care needs of patients.
Keywords
Ebola Virus, zoonotic transmission, infected bodily fluids, clinical management, laboratory tests, clinical trails
Introduction
To date, 12 distinct filoviruses have been described. The seven filoviruses that have been found in humans belong either to the genus Ebolavirus;
- Bundibugyo virus [BDBV]
- Ebola virus [EBOV]
- Reston virus [RESTV]
- Sudan virus [SUDAN]
- Tai Forest virus [TAFV]
- Marburgvirus [MARV]
- Ravn virus [RAVV]
The WHO International Classification of Diseases (ICD-11) of 2018 recognizes two major subcategories of filovirus disease (FVD): Ebola disease caused by BDBV, EBOV, SUDV or TAFV, and Marburg disease caused by MARV or RAVV.
Ebola virus disease (EVD) is defined as a disease only caused by EBOV. This subcategorization of FVD is largely based on the increasing evidence of molecular differences between ebolaviruses and Marburg viruses, differences that may influence virus– host reservoir tropism, pathogenesis and disease phenotype in accidental primate hosts
FILOVIRUS TAXONOMY AND EBOLA VIRUS TRANSMISSION:
[A]Taxonomy of the genus Ebolavirus. Thus far, five ebolaviruses have been associated with human infections, and four of them have been identified as pathogens.
[B]The natural reservoir host of Ebola virus has yet to be identified. Multiple data indicate a direct or indirect role of bats in EBOV ecology, but to date, EBOV has not been isolated from, nor has a near-complete EBOV genome been detected in any wild animal. However, it is tempting to speculate that Ebola virus disease is a zoonosis (that is, an infectious disease caused by an agent transmitted between animals and humans).
[C]Scanning electron microscopic (SEM) image of EBOV particles (green) budding from grivet cells.
[D]Transmission electron microscopic (TEM) image of EBOV particles (green) budding from grivet cells.
[E]The kingdom name has been approved by the International Committee on Taxonomy of Viruses (ICTV).
Since the discovery of filoviruses in 1967 FVD outbreaks (excluding at least five laboratories acquired infections) have been recorded in or exported from Africa. The epidemiological definition of outbreak is one or more cases above the known endemic prevalence. For example, the single case of TAFV infection recorded in a setting in which FVD had never been reported before (Côte d’Ivoire) is still considered an outbreak. All FVD outbreaks, with the exception of that caused by TAFV, were characterized by extremely high case–fatality rates (CFRs, also known as lethality). Until 2013, the most extensive outbreak, caused by SUDV, involved 425 cases and 224 deaths (CFR 52.7%). The overall limited numbers of FVD cases (1967–2013: 2,886 cases including 1,982 deaths), the typical remote and rural locations of outbreaks and the often-delayed announcement of new outbreaks to the international community have prevented the systematic study of clinical FVD in humans.
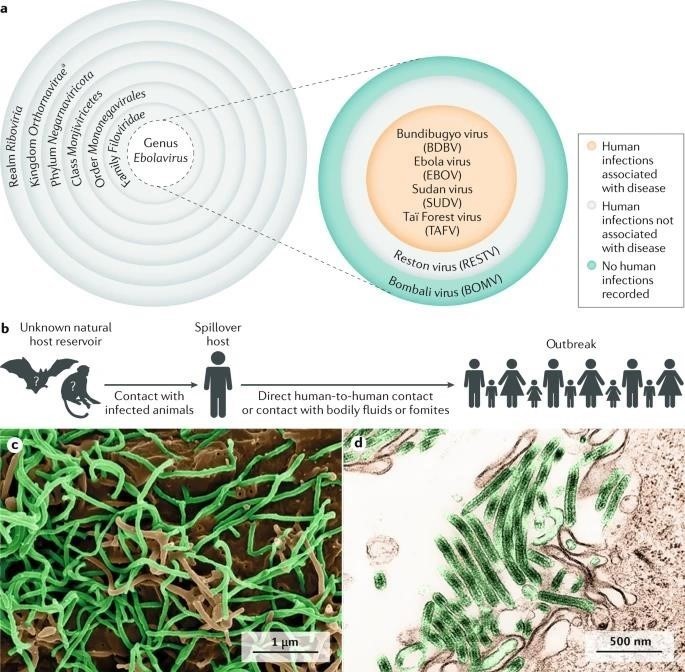
FIGURE :1
Until 2013, most EVD outbreaks originated from Middle Africa: the Democratic Republic of the Congo, Gabon and the Republic of the Congo. From late 2013 to early 2016, EBOV caused the largest outbreak to date, which spread from Guinea to other countries in Western Africa, leading to 28,652 human infections and 11,325 deaths. The location and scale of the 2013– 2016 outbreak was entirely unexpected. Consequently, local, national and international organizations were caught unprepared for an outbreak caused by what, until then, was considered an exotic pathogen of largely negligible consequence for global public health. After the WHO declared the outbreak a Public Health Emergency of International Concern, the global and local responses to the outbreak intensified. Ultimately, the outbreak was contained, but it devastated individuals, families, communities, health-care systems and economies. In most affected countries, the response included the establishment of Ebola (virus disease) Treatment Units (ETUs) in which medical professionals and biomedical scientists managed large cohorts of patients with suspected or confirmed EVD in controlled settings. In addition to the Western African outbreak, an ongoing outbreak in the ITURI, Nord-Kivu and Sud-Kivu Provinces of the Democratic Republic of the Congo are the second largest outbreak in terms of the number of cases and deaths, with 3,418 infections and 2240 deaths (as of 28 January 2020).
TABLE -1
EBOLA VIRUS DISEASE OUTBREAKS STATISTICS:
|
Country[year]
|
Case-fatality rate [%]
|
Number of cases
|
|
COD [then Zaire] [1976]
|
88.1
|
318
|
|
COD [then Zaire] [1977]
|
100.0
|
1
|
|
Gabon [1994-1995]
|
61.5
|
52
|
|
COD [then Zaire] [1995]
|
77.3
|
317
|
|
Russia [1996]
|
100.0
|
1
|
|
Gabon [1996]
|
67.7
|
31
|
|
Gabon, also exported to
South Africa [1996-1997]
|
74.2
|
62
|
EBOLA VIRUS DISEASE OUTBREAKS:
Owing to observations from the 2013?2016 Western African outbreak and, to a limited degree, from subsequent EVD outbreaks in the Democratic Republic of the Congo clinicians can now better describe predictable phases in the progression of EVD in humans. Typically, EVD begins with a nonspecific febrile illness followed by severe gastrointestinal symptoms and signs. In highly viraemic patients who often also have dysregulated immune responses, EVD progresses to a complex multiple organ dysfunction syndrome that can be fatal. A subset of patients, usually with lower viraemia, have less-severe disease progression and organ dysfunction. Ultimately, these patients develop robust immune responses leading to clearance of viraemia and a resolution phase. However, recovery can be complicated by long lasting clinical sequelae and/or virus persistence in immune-privileged sites that can lead to disease flares and even sexual transmission. In this Primer, we outline the current improved understanding of EVD based on the most recently published human clinical data.
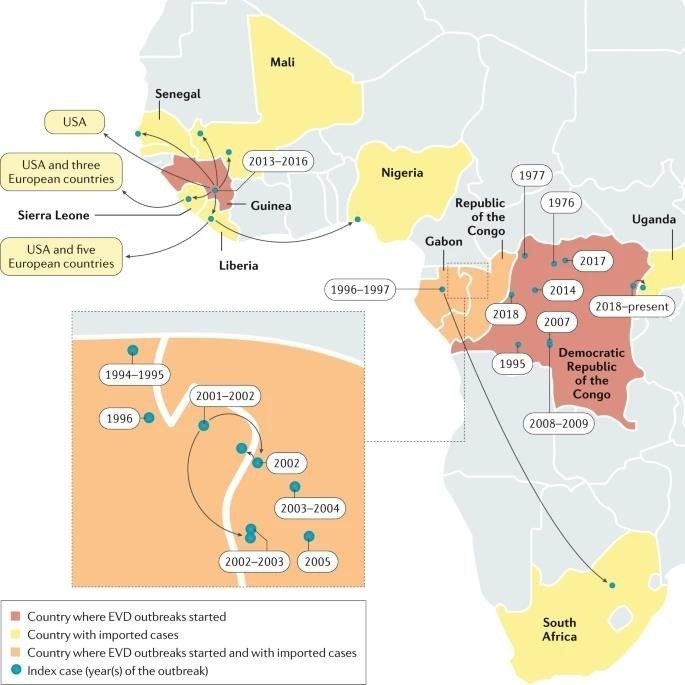
FIGURE:2
EPIDEMIOLOGY:
Classic epidemiology
Since the discovery of EBOV in 1976 in the Democratic Republic of the Congo (then Zaire) at least 17 EVD outbreaks have originated in Gabon, Guinea, the Republic of the Congo or Zaire/Democratic Republic of the Congo. EBOV infections in humans, including 14,742 deaths (average CFR 43.8%).
OUTBREAKS AND TRANSMISSION:
Most outbreaks can be traced back to a single spillover introduction of EBOV into the human population from an unknown reservoir by unknown means. Subsequently, the virus is transmitted by direct, typically non-aerosol, human-to-human contact or contact with infected tissues, bodily fluids or contaminated fomites. EBOV may have been transmitted from its natural reservoir host(s) to humans to cause disease only about 20–30 times, although it is probable that limited EVD outbreaks may have been overlooked or not reported. The potential for infection of an index case and subsequent spread locally and globally has been estimated by considering reservoir species distribution, along with governance, communications, isolation, infrastructure, health care and international connectivity. These predictions are crucial to identify regions that require increased surveillance and investments. Tracking EBOV within the human population after a zoonotic transmission event can be challenging, especially as the single natural reservoir has not been identified. A strong risk factor linked to human-to human EBOV propagation is contact with infected bodily fluids. Indeed, infectious EBOV has been recovered from breast milk, saliva, urine, semen, cerebrospinal fluid, and aqueous humour, in addition to blood and blood derivatives, and detected in amniotic fluid, tears, skin swabs and stool by reverse transcription (RT)-PCR. Although EBOV RNA has been detected in illness related bodily fluids (such as diarrhoea and vomitus), infectivity is unclear.
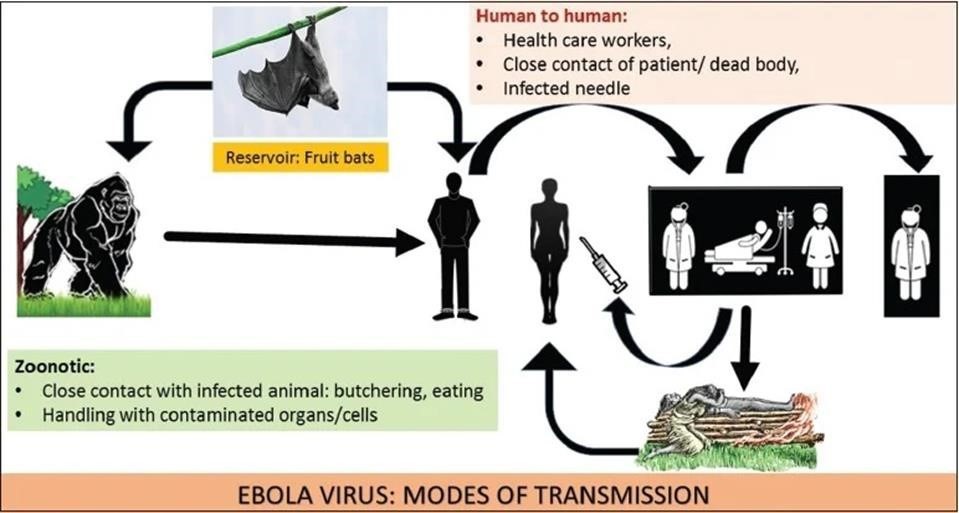
Figure: 3
Taking care of an individual with EVD at home or in a health-care facility or following traditional funeral practices, which involve contact with the deceased’s body, substantially increases the risk of acquiring infection. Although rare, sexual transmission of EBOV was proven or strongly suspected during the Western African EVD outbreak. Fortunately, the risk of widespread outbreaks in middle-income and high-income countries remains relatively low, partially owing to our ability to keep the reproductive number (R0, the average number of individuals to whom an infected person will transmit the pathological agent over the course of the infectious period)
REPLICATION OF EBOLA VIRUS:
Entry is mediated by attachment of virus to host receptors like DC-SIGN and DC - SIGNR through GP glycoprotein. The virion enters the cell by macropinocytosis or clarthrin - mediated endocytosis. GP interacts with host NPC, in late macropinosome and promotes fusion of virus membrane with the vesicle membrane. The ribonucleocapsid is then released into the cytoplasm. Sequential transcription, viral MRNAs are capped and polyadenylated by polymerase stuttering in the cytoplasm. VP30 is an important transcription activation factor for viral genome transcription, Membrane-associated proteins are the matrix protein (VP40), VP24, and the GP (peplomer glycoprotein). The nucleocapsid is encapsulated by an outer viral envelope originating from the host cell membrane with characteristic 10 nm long viral glycoprotein (GP) spikes that Membrane-associated proteins are the matrix protein (VP40), VP24, and the GP (peplomer glycoprotein).
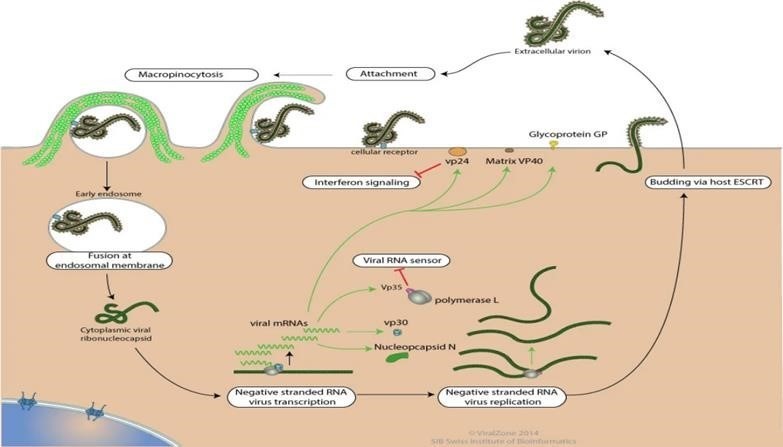
FIGURE :5
RISK FACTORS AND OUTCOMES:
Demographical risk factors for EBOV infection and subsequent development of EVD, such as age, sex and ethnicity, are not well-defined. By current understanding, sex differences in susceptibility have not been identified, but women as care-givers may be at higher risk of being exposed to EBOV, and the incidence of EVD increases almost linearly with age to a peak at 35–44 years. Although children typically constitute a disproportionately small number of EVD cases, they have shorter incubation periods, and a more rapid disease course. Children have a higher risk of death than older populations, with children of <5>
Infected pregnant women are at high risk of miscarriage or stillbirths, and newborn babies of infected mothers rarely survive. Indeed, EBOV can be transmitted transplacentally and also lead to foetal death related to placental insufficiency. Transmission of EBOV from infected pregnant women to their embryos or foetuses or from infected mothers to their children occurs frequently and is associated with elevated in utero and neonatal lethality. The risk of fatal loss in survivors of EVD who become pregnant after recovery remains unclear; some data suggest an increased risk over baseline, especially early after recovery, although healthy pregnancy outcomes are possible. EBOV RNA has been detected at high concentrations in amniotic fluid, placenta, foetal tissue and breast milk.
MECHANISMS / PATHOPHYSIOLOGY:
ANIMAL MODES:
Exposure of immunocompetent laboratory mice, Syrian hamsters [mesocricetus auratus) and domesticated guinea pigs (Cavia porcellus) to EBOV does not yield severe (or any) disease, and EBOV must be adapted via serial passages in rodents before lethal infection is achieve. Even when adapted viruses are used, these rodent models do not fully mimic human disease. Because non-human primates (NHPs) are evolutionarily much more closely related to humans than rodents, NHP models of EVD are often considered to be more useful for the study of human EBOV infection and EVD. Indeed, much of the information on viral pathogenesis has been derived from studies with wild-type EBOV predominantly in crab eating macaques (Macaca fascicularis) and rhesus monkeys (M. mulatta). On the basis of experimental animal data, two factors that may influence development and severity of human EVD may be the EBOV exposure route and dose. Direct contact with infected biological materials or contaminated non-biological materials via cuts or scratches or via contact with mucosal membranes (oral or, theoretically, nasopharyngeal or conjunctival mucosa) is considered the most frequent mode of human-to-human EBOV transmission. However, these transmission pathways are difficult to simulate in experimental settings. Thus, animal models of EVD have been established using injection and aerosol methods of EBOV exposure to model accidental needlestick injury and respiratory routes of exposure, respectively, despite the lack of evidence that these exposure routes have any relevant roles during natural EVD outbreaks.
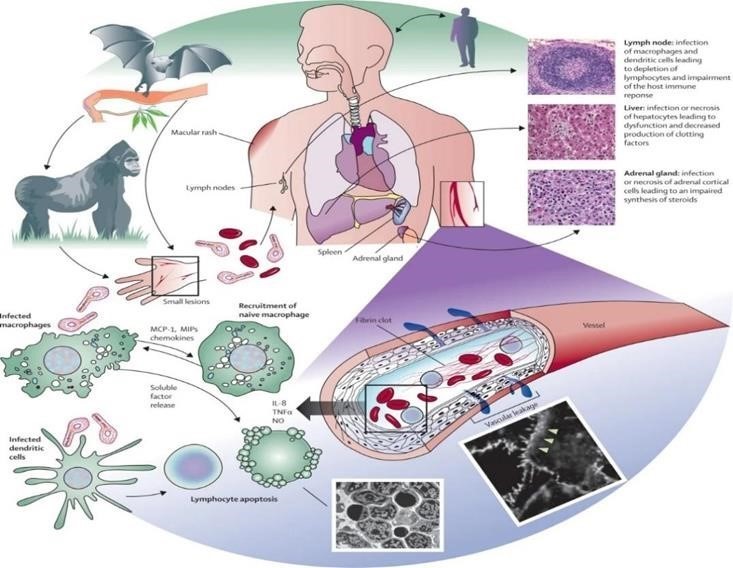
FIGURE:6
HOST – PATHOGEN DETERMINANTS OF OUTCOME:
In humans, outcome could only be correlated with very limited clinical data, providing only low-resolution associations. In proxy animal disease models, the homogeneity of highly stringent uniformly lethal models prevents the identification of any host-specific or Filovirus specific variability, much less mechanistic. EBOV tissue and cell tropism are primarily determined by the EBOV glycoprotein GP, GP attachment factors on the host cell surface and the intracellular binding of GP to the NPC intracellular cholesterol transporter receptor. Most human cells can become infected, but mononuclear phagocytes (for example, Kupffer cells in the liver, macrophages and microglia) and dendritic cells are primary EBOV targets. As the primary target cells become infected, they probably facilitate further virus dissemination and migrate to the regional lymph nodes and to the liver and spleen. In vitro, infected macrophages are activated by binding to EBOV GP to secrete pro-inflammatory cytokines, in particular interleukins IL-1?, IL-6 and IL-8, and tumour necrosis factor (TNF). These secretions probably result in the recruitment of additional EBOV-susceptible macrophages to the site of infection and, ultimately, the breakdown of endothelial barriers. In NHP models, this breakdown frequently causes third spacing leading to oedema and hypovolaemic shock. Although described, this manifestation is less well characterized in human patients. In vitro, dendritic cells react to EBOV infection with partial suppression of major histocompatibility complex class II responses, expression of tissue factor and TNF ligand superfamily member 10 (TNFSF10), increased production of chemokines (for instance, C-C motif chemokine 2 (CCL2), CCL3, CCL4 and IL-8) and suppressed secretion of pro-inflammatory cytokines. Together with possible abortive infection, the aberrant cytokine responses and TNFSF10 expression are probably key to the extensive lymphocyte death. Such lymphocyte depletion possibly contributes to the susceptibility of patients with EVD to acquiring secondary infections), hypotension, disseminated intravascular coagulation, and ultimately multiple or dysfunction syndrome that is typical of EVD.
EBOV GENOME AND LIFE CYCLE:
[A] Ebola virus (EBOV) has a linear, non-segmented, negative-sense, single-stranded RNA genome (~19 kb) expressing seven structural proteins and several non-structural proteins from seven genes: NP encodes nucleoprotein NP, VP35 polymerase cofactor VP35, VP40 matrix protein VP40, GP glycoprotein GP, and secreted glycoproteins (not shown), VP30 transcriptional activator VP30, VP24 RNA complex-associated protein VP24, and L large protein L.
[b]the binding of EBOV particles to the attachment factors on the host cell surface is mediated by the homotrimeric structural glycoprotein GP which is formed of three heterodimers consisting of subunits GP and GP that are connected by a disulfide bond.
- Binding to the host cell membrane triggers viral particle endocytosis
- In the late endosome, GP is sequentially cleaved by cathepsin B (CATB) and cathepsin L (CATL)
- To expose the receptor-binding site of the GP subunit. A low pH induces GP interaction with the EBOV receptor NPC1, with subsequent GP2-mediated fusion of the particle envelope with the endosomal membrane and thereby expulsion of the ribonucleoprotein complex (predominantly RNA + NP) into the cytosol
- There, the filovirus genome is replicated
- And the filovirus genes are transcribed into mRNAs
- Viral proteins are translated in the cytosol or, in the case of GP into the endoplasmic reticulum (ER)
- Mature progeny ribonucleoprotein complexes and viral proteins are transported to the plasma membrane, where particle budding occurs
- NPC1, NPC intracellular cholesterol transporter; ORF, open reading frame.
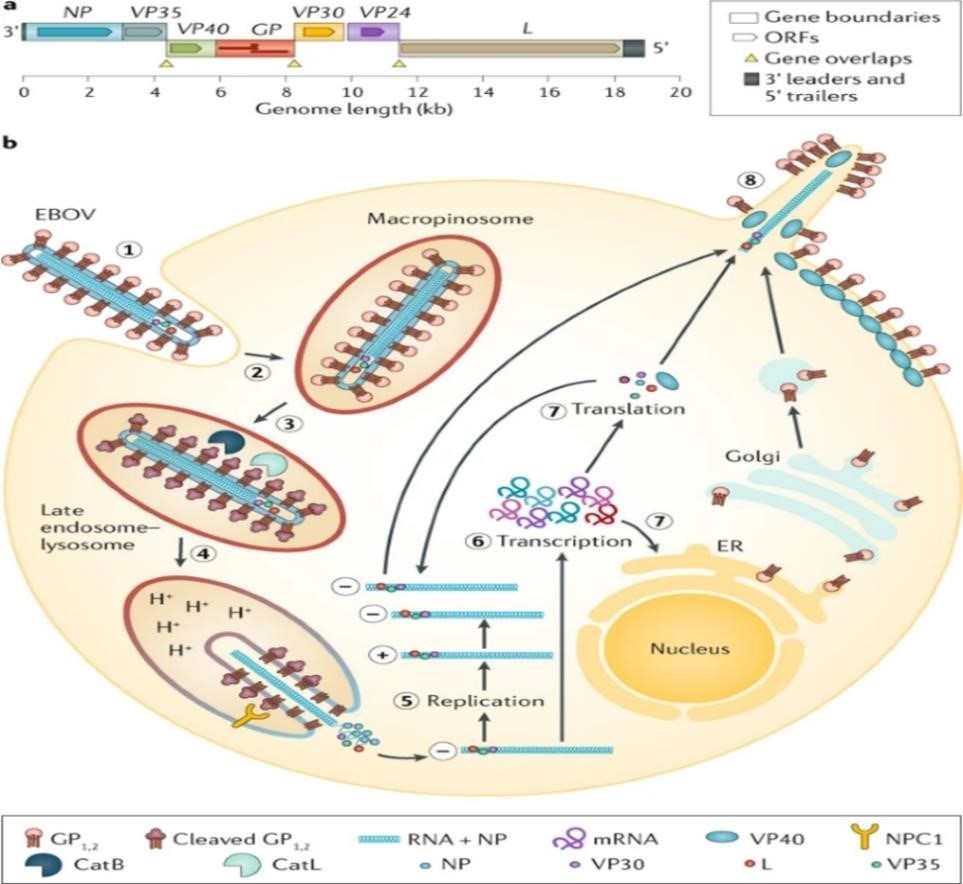
FIGURE:7
EVD PATHOGENESIS:
Ebola virus particles enter the body through dermal injuries (microscopic or macroscopic wounds) or via direct contact via mucosal membranes. Primary targets of infection are macrophages and dendritic cells. Infected macrophages and dendritic cells migrate to regional lymph nodes while producing progeny virions. Through suppression of intrinsic, innate and adaptive immune responses, systemic distribution of progeny virions and infection of secondary target cells occur in almost all organs. Key organ-specific interactions occur in the gastrointestinal tract, liver and spleen, with corresponding markers of organ injury or dysfunction that correlate with human disease outcome.
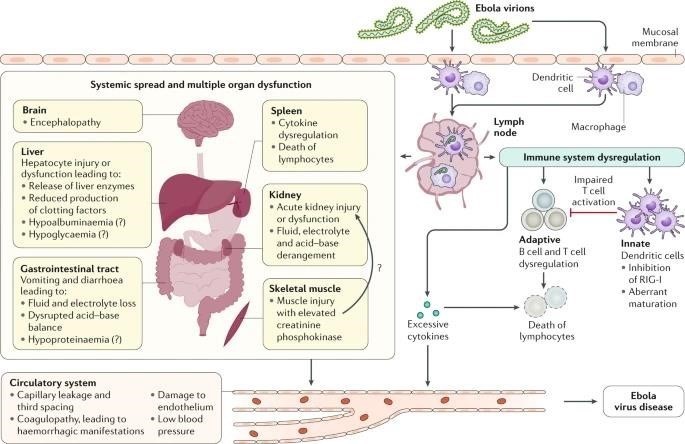
FIGURE:8
IMMUNE RESPONSES:
EBOV inhibits induction of intrinsic (cell-based antiviral defence mechanisms via proteins that are constitutively expressed and target specific viruses) and innate (cell-based antiviral defence mechanisms via proteins that are induced by infection and rely on pattern recognition receptors) host immune responses. This inhibition permits efficient virus replication in host cells, thereby accelerating viral spread. To this end, the virus invests a substantial amount of its genome coding capacity. Perhaps the best studied inhibitor is the EBOV polymerase cofactor VP35, which is also a type I interferon (IFN) antagonist.
EBOV VP35 suppresses production of type I IFN (by impairing IRF-3 phosphorylation) through its ability to bind double-stranded RNA and through direct interactions with the host proteins TBK-1, IKKE and PACT. In addition, VP35 suppresses micro-RNA silencing (an important post-translational regulatory pathway) in the host cell, and GP antagonizes a cellular antiviral restriction factor, BST. A second EBOV-encoded protein, RNA complex associated protein VP24, also inhibits the antiviral response by preventing the nuclear accumulation of phosphorylated signal transducer and activator of transcription 1?/? (STAT1), which is induced by type I IFN and acts as a transcription factor to increase expression of antiviral proteins. Finally, EBOV VP40 is incorporated into exosomes that seem to have the potential to disrupt or kill host immune cells.
EBOV-specific cellular and humoral immune responses develop but are often outpaced in the host–pathogen EVD ‘arms race’ in which timing seems crucial. This host–pathogen competition might also be applied to vaccine-mediated mechanisms of protection. A vaccinated individual is assumed to be protected once outside the window for a vaccine induced mounting of a humoral response. USA and Western African cohorts suggest that robust adaptive immune activation, which includes antigen-specific T cell and B cell responses, occurs during acute illness. Efforts are ongoing to define the characteristics of effective and ineffective B cell and T cell responses during acute infection and over time. These limited results suggest a ‘race’ between EBOV proliferation and the ability of the human host to mount an effective and regulated anti-EBOV immune response.
CLINICAL COURSE OF ACUTE EVD OVER TIME:
- Renal dysfunction is common and not well-characterized in patients with EVD; it is probably a multifactorial combination of hypovolaemia (related to gastrointestinal fluid losses, decreased fluid input, fever, hypoalbuminaemia and sepsis pathophysiology), intrinsic renal injury or cytokine-mediated nephrotoxicity.
- Whereas respiratory symptoms and signs may reflect respiratory compensation for a primary metabolic acidosis, primary causes of hypoxaemic respiratory failure include acute lung injury (related to systemic inflammatory response syndrome and/or sepsis or Ebola virus (EBOV)-related Cytokinaemia), pulmonary oedema (in the setting of capillary leak or direct infection) and viral pneumonia. Respiratory muscle fatigue may also contribute to ventilatory respiratory failure.
- Haemorrhagic manifestations include oozing from venipuncture sites, haemoptysis (coughing up blood), haematemesis (vomiting blood), melaena (dark stools as a result of bleeding) and vaginal bleeding.
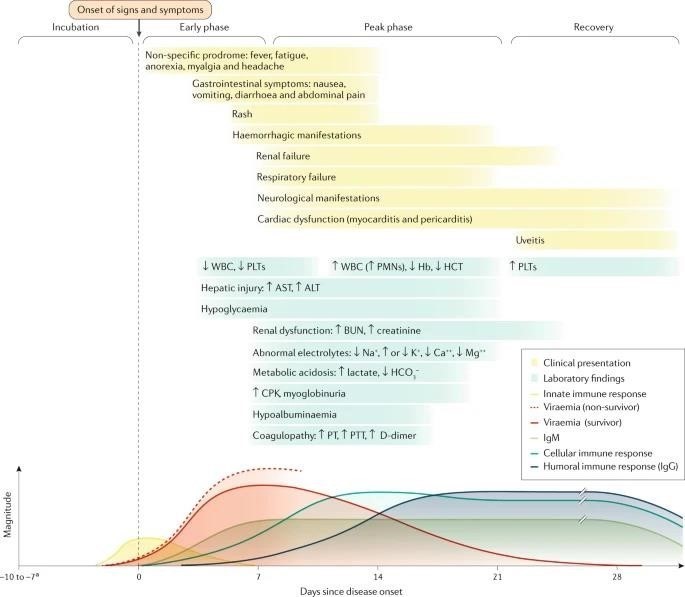
FIGURE:9
- Neurological manifestations include meningoencephalitis and cerebrovascular accidents (such as strokes). ALT, alanine aminotransferase; AST, aspartate aminotransferase; bun, blood urea nitrogen; CPK, creatine phosphokinase; Hb, haemoglobin, haematocrit; PLT, platelet; PMN, polymorphonuclear leukocyte; PT, prothrombin time; PTT, partial thromboplastin time; WBC, white blood cell count.
- Incubation periods of 2–21 days have been reported. Bottom panel adapted with permission from [The American Association of Immunologists].
As the EBOV load increases, typically the severity of EVD clinical manifestations increases as well. The onset of detectable viraemia and manifestations of clinical signs and symptoms in most patients occurs 6–10 days after exposure. Later in the first week of illness following disease onset, patients may have persistent fever and increased gastrointestinal fluid losses and hypotension from dehydration and, to a minor extent, vascular leakage.
During the terminal phase (days 7–12 following disease onset), tissue hypoperfusion and vascular leakage, often in conjunction with dysregulated inflammation, lead to multiple organ dysfunction syndrome and/or damage, including acute kidney injury. Kidney injury is evidenced by oliguria or anuria and abnormalities in electrolytes including potassium and sodium. A subset of patients develops central nervous system manifestations and encephalopathy. Although several underlying causes could be involved, EBOV RNA has been detected in the cerebrospinal fluid of patients with EVD, suggesting that meningoencephalitis may be directly mediated by the virus.
The mean initial viral load at this ETU decreased over the course of the outbreak, as did the CFR, although such decreases occurred in the setting of numerous potential explanatory factors.
Other risk factors that have been sporadically linked with a fatal outcome include;
- Age ?45 years,
- fever >38?°C
- Weakness
- Dizziness
- Diarrheal
- Conjunctivitis
- Difficulty breathing or swallowing
- Confusion or disorientation
- Coma
- Haemorrhagic signs
DIAGNOSIS OF ACUTE EVD
Several molecular testing methods are available. RT - PCRS evaluate for the presence of the nucleoprotein or envelope glycoprotein. These tests require an inactivation phase and must be performed in bio safety level 4 isolation. Some reagents need to be kept at -20°C. Additionally, the test requires a dedicated area and careful attention, as some byproducts of testing can contaminate the test. Testing generally requires 3 to 4 hours to perform. Although RT - PCRS are the most sensitive tests, the limitations of the safety requirements and equipment needed make this problematic in resource-limited settings. They are instead often found in reference laboratories. Automated nucleic acid tests are easier and quicker to perform. A comparative study comparing the RT - PCR method and an automated nucleic acid test, the filmarray test, showed 85% agreement between the 2 tests.
Several rapid lateral flow assays with quicker turnaround times than PCR testing is also available. A meta-analysis that compared rapid tests to RT - PCR showed a pooled sensitivity of 85% and a pooled specificity of 95%. Some rapid tests have a sensitivity as low as 77%.
Although more sensitive and specific tests are needed, these remain potent tools in rural areas with limited resources and an epidemic setting.
Serologic tests can be helpful. However, the earliest that Ebola IGM can be detected is 6 days after the onset of the illness. Due to the nature of Ebola virus disease, patients may have died before IGM would be detectable.
Radiography
Chest x-rays should be done to evaluate for pulmonary oedema secondary to capillary leak syndrome.
TABLE: 2
EBOV DETECTION TESTS:
Rapid viral antigen detection tests
|
Test
[manufacturer]
|
Test type
|
Target
|
Samples
|
Sensitivity
|
Specificity
|
Virus detected
|
|
Dual Path
Platform (DPP) Ebola antigen system
(chembio)
|
Immunochromatogr
aphic lateral flow assay
|
VP4 0
|
Venous whole blood
(EDTA)
, venous plasma (EDTA) and capillary y fingerstick ck whole blood
|
Qualitativ
e; less
sensitive
than PCR;
requires confirmatory testing
|
From limited data, does not crossreact with another ebolavirus
|
EBOV
|
|
Oral Quick Ebola rapid antigen test
(OraSure Technologie
s)
|
Immunochromatogr
aphic lateral flow assay
|
VP4
0
|
Oral fluid and whole blood
|
97.1% (From oral fluid from deceased individual
s); LLOD: 53?ng per ml for whole blood samples and 106mg per ml for oral fluid
|
98–100% from venous whole blood samples; 99.1– 100% from oral fluid from deceased individuals
|
BDBV, EBOV
and
SUDV;
does not differenti ate
between ebolavirus
|
|
SD ne
Ebola Zaire Ag test (SD
Biosensor)
|
Immunoprecipitation n lateral flow assay
|
GP, NP
and
VP4
0
|
Plasma, serum and whole blood
|
84.9% for whole blood and plasma
|
99.7% for whole blood and plasma
|
EBOV
|
TABLE :3
|
Test manufacturer
|
Test type
|
Target
|
Samples
|
Sensitivity
|
Specificit y
|
Virus detected
|
|
Filmarray NGDS
BT-E
(BIOFIRE)
|
Fluoresce nt nested multiplex
RT-PCR
|
EBOV
nucleic acids
|
Whole blood,
plasma and serum
|
LLOD:
1,000?pfu per ml or4.36?×?103geno me equivalent Sd per ml for live virus
|
EBOV; no cross reactivity with
another ebolavirus or Marburg virus
|
EBOV
|
|
Ebola virus NP real-time RTPCR
(Thermofisher
(CDC))
|
Qualitative realtime
RTPCR
with fluorescent reporter dye detected at each
PCR
cycle
|
EBO
V NP
RNA
|
Whole blood, serum, plasma and urine
|
99.80%;
LLOD: 600–700 TCID50
copies per ml
|
100% for
EBOV
|
EBOV
|
|
EBOV VP40 real-
time RTPCR
(CDC)
|
Real-time RT-PCR with fluorescent dye- labelled probes to detect
PCR
amplicons
|
EBOV
VP40
RNA
|
Whole blood, serum, plasma and urine
|
LLOD:400-600 TCID50 per ml from whole blood; 250–600 TCID50 per ml, depending on body fluid sample and extraction method used
|
100% for
EBOV
|
EBOV
|
|
Gene XPERT Ebola (Cepheid)
|
Real-time RT-PCR with fluorescent signal from probes for quality control
|
EBOV
NP and
GP nucleic acids
|
Whole blood and oral fluids
|
100%; LLOD: 232.4 genomic
copies per ml
|
99.5% from whole
blood;
100% from
oral fluid
|
EBOV
|
PREVENTION:
Public health organizations work to contain outbreaks of Ebola by monitoring for new cases and taking precautions to keep healthcare workers safe while caring for people with Ebola. Steps you can take include:
- Use protective equipment (such as a mask, goggles, apron and gloves) when caring for someone with Ebola. Avoid touching any of their body fluids and wash your hands after contact, even if you wear gloves.
- Use condoms or don’t have sex until tests confirm that ebolavirus isn’t present in your semen (or your sex partners, if they had Ebola). Even if you feel better, the virus can live in semen for a long time. There isn’t evidence that it’s contagious in vaginal fluids for as long.
- Avoid contact with anything that may have touched infected body fluids. Don’t touch semen unless tests confirm that it no longer carries the virus.
- Avoid touching the body of someone who died from Ebola, or use protective equipment if you have to. This includes funeral customs.
- Avoid contact with body fluids and tissues of animals (dead or alive) that could have Ebola.
- Don’t eat bush meat (the meat of wild animals).
- If you’ve recently returned from travel to a place where there’s an Ebola outbreak, monitor yourself for symptoms for 21 days. Get medical care right away if you develop symptoms.
- Isolate yourself from others if you could have Ebola for the same period or for at least 1 year.
CANDIDATE VACCINES:
Amid increasing concerns about unmitigated transmission during the 2013–2016 Western African EVD outbreak in mid-2014, a statement from a stakeholder meeting held by the WHO urged acceleration of the development and evaluation of EVD candidate vaccines. As the EBOV glycoprotein GP is the major viral immunogen, all candidate vaccines in advanced development are designed to stimulate a host immune response against this protein, among others. In the Western African outbreak, several candidate vaccines were evaluated in clinical trials. Owing to the success of the Ebola CA SUFFIT! phase III ring vaccination trial in Guinea, the RVSV?G-ZEBOV-GP, a live-attenuated recombinant vesicular virus candidate vaccine currently approved by the US Food and Drug Administration and the European Commission and is actively administered to help contain the currently ongoing EVD epidemic that started in Nord-Kivu Province of the Democratic Republic of the Congo in 2018. Using a ring vaccination strategy, whereby contacts of infected individuals (primary ring) and contacts of those contacts (secondary ring) are vaccinated, this candidate vaccine has been administered to 276,520 people in the eastern Democratic Republic of the Congo as of 26 January 2020.
TABLE :4
EVD candidate vaccines in phase I–III clinical trials
|
Candidate vaccine (s)
|
Vaccine design
|
Study design
|
Outcome
es
|
Results
|
Notes
|
Trails
|
|
RVSV?GZEB
O
V-GP (Also known as BPSC-1001 and V920)
|
Replication n competent
t RVSIV
expressing g EBOV
GP in place of
VSIV G
|
Phase I trial evaluating safety and immunogenicity of
RVSV?GZEBO
V-GP escalating doses
|
Primary: adverse effects up to 6 months. Secondar y:
humoral immunity up to 6 months
|
No pre-existing immunity; anti-
EBOV
matrix antibodies detected in
28% o
participant
s, with levels that
peaked at day 56 after vaccination n
|
Adverse effects: arthralgia, oligoarthritis is myalgia, headache and injection site pain
|
NCT0228
3
099
|
|
rAd26
ZEBO
V-GP and
MVA-
BN-
Filo
|
Replication n defective human adenovirus s (Ad) 26 vectors expressing g
EBOV
GP;
|
Phase I trial evaluating safety and immunogenicity of MVA-BN
Filo and rAd26 ZEBOV-GP
as heterologous
|
Primary: adverse effects up to 78 days.
Secondar y
: immune response s up to 1 year
|
Anti-
EBOV GP
antibodies detected in
97% o
23% o
participant
s receiving rAd26
ZEBOV-
GP o
|
Adverse effects:
with rAd26
ZEBOV-
GP: fever, injection site reactions headache, myalgia, nausea, fatigue and chills; with
MVABN-
Filo: injection site reactions
|
|
Preliminary analyses on data evaluating the first 93,965 vaccinated individuals revealed a lower estimated attack rate among individuals who were vaccinated (0.017%) than in unvaccinated individuals (0.656%). The WHO reported an estimated vaccine efficacy of 97.5% (95% CI 95.8–98.5%). However, determination of true vaccine efficacy is impossible in the absence of a placebo-controlled group. Notably, a model of the EBOV infection risk during the 2018 EVD outbreak in Équateur Province in the Democratic Republic of the Congo found that the introduction of ring vaccination with RVSV?G-ZEBOV-GP vaccine resulted in a decrease of 70.4% of the geographical area of risk and 70.1% of the level of EBOV infection risk. However, if ring vaccination is delayed by as little as 1 week, the size of this effect is considerably diminished.
The same candidate vaccine is also used in the ongoing outbreak in the Democratic Republic of the Congo as emergency post-EBOV exposure prophylaxis in, for instance, health-care workers.
TREATMENT /MANAGEMENT
Infection Prevention and Control
The importance of employing immediate, effective Infection Prevention and Control (IPC) for patients with Ebola virus disease cannot be understated. The reproduction number of EBOV is estimated to be between 1.51 and 2.53, meaning for every patient infected with EBOV, between 1 and 3 additional people are expected to be infected. Effective IPC is paramount to controlling the spread of EVD. In endemic areas in West Africa, patients are often directed to Ebola Treatment Units (ETUS) that have tight protocols for testing and isolation. However, patients may first seek treatment at any healthcare facility or not at all. This is sometimes due to the belief that the ETUS were the source of the infection. In areas with inadequate contact tracing, people infected with an Ebola virus may present to facilities that do not have adequate IPC measures. This can amplify spread, as happened in the West African EVD epidemic between late 2013 and 2016. In this epidemic, more than 800 healthcare workers died of EVD; most of these occurred where adequate IPC precautions were not employed. The ability to screen patients for symptoms and signs consistent with EVD, practical training on IPC, facilities to isolate patients with EVD and those under investigation, and swift reporting of patients suspected of having Ebola to public health agencies are crucial to an effective IPC effort to control the disease. The first case seen in the United States was in a community hospital in Dallas, Texas, in 2014. Two nurses were infected while caring for the patient. Following this, extra engineering controls were added for the medical intensive care unit to contain the virus, including zippered fabric walls to denote where PPE was required and the implementation of unidirectional entry and exit from the unit to decrease contamination of PPE. Strict PPE was also implemented for laboratory personnel testing materials from patients with EVD or those under investigation.
Ebola virus can be found in many bodily fluids, including;
- BLOOD
- FAECES
- VOMIT
- BREAST MILK
- URINE
- SEMEN
- SALIVA
- TEARS
- MUCUS
- SWEAT

FIGURE :10
Avoidance of funeral or burial rituals that require handling the body of person who has died from Ebola. Avoidance of contact with bats and nonhuman primates or blood, fluids, and raw meat prepared from these animals.
Healthcare workers who may be exposed to people with Ebola should wear appropriate personal protective equipment (PPE) and practice proper infection control and sterilization measures.
Early testing and isolation of the patient plus barrier protection for caregivers (mask, gown, goggles, and gloves) is very important to prevent other people from getting infected. Spoken instructions during doffing, double gloving, glove disinfection, and following CDC guidance may improve effectiveness and compliance. The centres for Disease Control and Prevention recommend:
- single-use, impermeable gowns or coveralls
- either a powered, air-purifying respirator (PAPR) or an N95 respirator
- two pairs of single-use gloves with extended cuffs
- single-use boot covers
- a single-use apron
SUPPORTIVE CARE FOR ACUTE EVD:
Aggressive supportive care includes appropriate intravenous fluid replacement with crystalloid fluids and perhaps vasopressors, to prevent patients with EVD from developing HYPOVOLAEMIC shock from profound intravascular volume depletion and/or septic shock that may include vascular leak syndromes.
In the early stages of disease when the patient is ambulatory and able to eat and drink without nausea and excessive vomiting, oral rehydration solutions can be administered to replace gastrointestinal and insensible fluid losses (insensible water loss is attributed to evaporation from the skin and respiratory tract). Establishing early on intravenous access is crucial for administration of balanced crystalloid solutions (for example, Ringer’s lactate) as the patient’s condition worsens and nausea, vomiting, asthenia (weakness), and malaise and lassitude (lethargy) make adequate oral fluid intake impossible.
As illness progresses to more severe stages (peak phase), increased gastrointestinal fluid losses (secretory phase) predominate, and patients with EVD may produce large amounts of emesis and stool. Stool volumes of 5–10 per day have been reported, leading to heavy fluid and electrolyte losses. Managing patients’ bodily fluids is also an important infection control consideration in the health-care environment and can be accomplished with physical and pharmacological controls.
Anti-emetic medications (for example, metoclopramide and ondansetron) have been used to control nausea and vomiting. Also, anti-diarrheal agents (for example, loperamide) have been used to reduce the frequency of diarrhoea. With potential adverse events such as intestinal ileus (that is, intestinal paralysis that can lead to obstruction), the risk–benefit ratio of using loperamide for inflammatory diarrhoea associated with EVD is uncertain.
Examples of physical controls used in the hospital include emesis bags, bedside commodes and FAECAL management systems (temporary containment devices composed of a rectal catheter and a collection bag) for non-ambulatory patients.
Although primary EVD-attributable respiratory disease is uncommon, patients who have respiratory signs (such as dyspnoea) and hypoxia may require conservative treatment with supplemental oxygen, particularly those with pulmonary oedema as an iatrogenic effect of aggressive fluid replacement.
Haemorrhagic complications can be treated with blood products when available, but clinicians should be aware of potential hypo-coagulable and hyper-coagulable states. Severe neurological manifestations, including meningitis, encephalitis, seizures and coma, have been reported in patients with acute EVD. Complex causes of encephalopathy include altered vital signs (hypoxemia and hypotension), metabolic dysfunction (hypoglycaemia and electrolyte derangement), organ dysfunction (uraemia and hepatic encephalopathy) and also central nervous system dysfunction related directly to EBOV meningoencephalitis or to indirect micro-vascular or macrovascular infarction. Benzodiazepines or other available sedating medications may be needed to keep patients from harming themselves, other patients or health-care providers. Fever and pain may be treated by acetaminophen.
CRITICAL CARE:
Patients with EVD who progress to critical illness, including multiple organ dysfunction syndrome, may require advanced life support modalities. However, given the resource constraints typical of most outbreak areas, the capacity to deliver critical care is limited by gaps at the personnel, equipment and facility levels. The knowledge of modern critical care of patients with EVD stems from the care of several patients who acquired EBOV infection in Western Africa but were managed in the USA and Europe and limited experience in an ETU in Sierra Leone that was equipped with intensive care unit (ICU) capabilities. Substantial preplanning was necessary to provide treatment for critically ill patients, to ensure the availability of physicians with experience in airway management and dialysis, for example, and the necessary equipment and appropriate PPE for potentially aerosol-generating procedures. Invasive procedures also place health-care workers at risk of transmission of blood-borne pathogens, including EBOV. In patients treated in the USA and Europe and those in the ICU-equipped ETU in Sierra Leone, intubation was accomplished via rapid sequence induction using neuromuscular blockade, followed by video laryngoscopy to provide direct visualization of the airway while reducing the likelihood of aerosol or bodily fluid exposure via coughing or vomiting. Validation of correct endotracheal tube placement was often difficult, as some CENTRES did not have the ability to auscultate the lungs or monitor end-tidal CO2 concentrations. In patients with EVD who developed acute kidney injury, continuous renal replacement therapy (CRRT) was performed. CRRT was chosen over intermittent HAEMODIALYSIS to decrease the frequency of exposure to blood and bodily fluids. Frequent laboratory monitoring, including electrolytes, was performed while patients remained on CRRT, and regional citrate anticoagulation. Effluent waste was found to be negative for EBOV by RT-PCR on three separate occasions at one centre, probably owing to the inability of EBOV particles to cross the dialyzer membrane. However, as the effluent waste was found to be positive for EBOV by RT-PCR in one of three samples at another centre, the CDC and some clinicians recommend that effluent waste be generally handled as potentially contaminated.
MONITORING:
The clinical parameters that can be monitored closely include:
- Blood chemistry (sodium, potassium chloride, ionized calcium, glucose and creatinine concentrations)
- markers of liver injury (AST and ALT)
- creatine phosphokinase
- C-reactive protein
- Haematological parameters (white blood cells, haematocrit and platelets count)
- Urine analyses (glucose, ketones, ascorbic acid, protein, blood, leukocytes, nitrite, PH, bilirubin and urobilinogen)
COMPLICATIONS:
In general, patients with EVD should either receive reliable testing for malaria or be treated empirically with artemether–lumefantrine or other artemisinin-based therapy. Regardless of testing results, all ill patients who meet criteria for severe malaria should receive intravenous artesunate empirically. Secondary infectious complications in patients with EVD, including sepsis induced by Gram-negative bacteria, have been observed. Patients with EVD may be at high risk of bacterial translocation of the commensal gut microbiota into the bloodstream, owing to substantial inflammation in the gastrointestinal tract.
Patients with EVD treated in Western Africa received empirical antibiotic therapy to prevent and treat bacterial infection and sepsis but also, particularly in children, to treat other potentially life-threatening infections that can mimic the clinical signs of EVD (for example, Salmonella Typhi bacteraemia). Initiation of broad-spectrum antibiotics is recommended in patients with EVD who are critically ill. Antimalarial treatments were administered to patients from the Western African outbreak with suspected EVD, since malaria co-infection was common in patients presenting to ETUs. Since patients with EVD may remain hospitalized for prolonged periods and may undergo invasive procedures, they should be monitored closely for the development of nosocomial infections such as central line-associated bloodstream infections, ventilator-associated pneumonia and urinary tract infections. As performing blood cultures for full identification of the causative bacterium and its antimicrobial susceptibility pattern can be logistically challenging in ETUs, broad-spectrum PCR-based methods may prove useful in some settings for rapid identification of secondary or nosocomial infections.
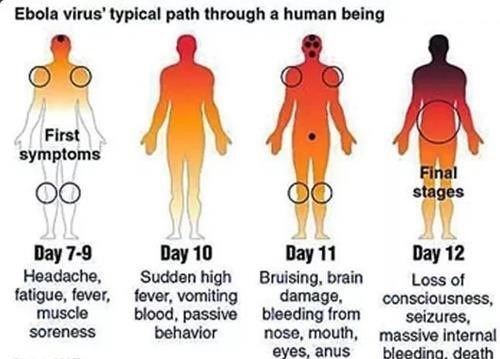
FIGURE:11
CLINICAL SEQUELAE IN SURVIVORS OF EVD:
Clinical sequelae in survivors of Ebola virus disease (EVD) that are supported by evidence that includes physical examination of the individuals. Studies reporting patient-reported symptoms are not included in this summary figure. EBOV, Ebola virus; PTSD, post-traumatic stress disorder.
In the PREVAIL III clinical trial, a prospective, controlled study assessing symptoms in survivors that had a >10% increase in prevalence compared with control close contacts, this symptom had an increased odds ratio (P?<?0.0001) compared with close contact controls.
In the PREVAIL III clinical trial, in which symptoms in survivors were compared with symptoms in control close contacts (regardless of any increase in their prevalence in survivors), this symptom had an increased odds ratio (P?<?0.01) compared with control close contacts.
- Most common abnormalities in neurological examinations are abnormal oculomotor examination, abnormal reflexes, tremor and abnormal sensory examinations.
- Data from uncontrolled cohorts, case series or case reports.
- Most common abnormalities include irregular heart rate, cardiac murmur, decreased breath sounds, rales (crackling lung sounds) and wheezes.
- Most common abnormalities include abdominal tenderness, mass or distension.
- Most common abnormalities include muscle tenderness and decreased range of motion
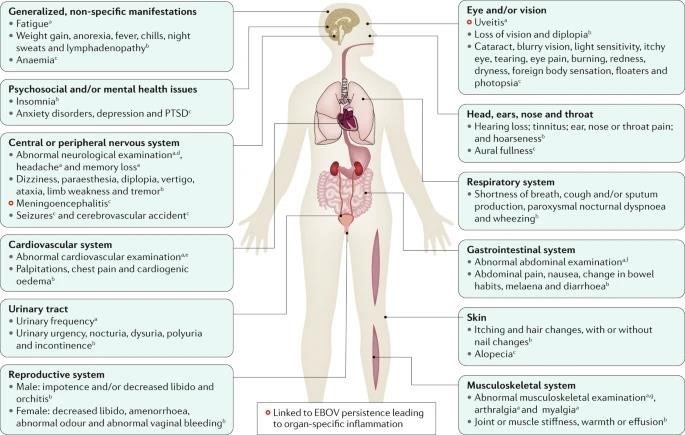
FIGURE:12
PHYSICAL SEQUELAE:
Up to 87% of survivors of EVD report arthralgias with symmetric polyarticular involvement affecting (in order of decreasing frequency) the knees, back, hips, fingers, wrists, neck, shoulders, ankles and elbows. The presence and severity of arthralgias has been reported to directly impede recovery of functional status. Although rarely actually characterized, physical findings are usually unremarkable without overt erythema or swelling. Imaging of a limited number of joints has thus far been unrevealing. In the only report of joint arthrocentesis (aspiration of synovial fluid from within a joint capsule), EBOV RNA could not be detected in the synovial fluid.
Ocular symptoms and signs, including
- retro-orbital pain
- blurry vision
- eye pain
- sensitivity to light
- conjunctival injection also seems to complicate EVD recovery in a substantial proportion of adult (14–60%) and paediatric [32%].
These symptoms and signs are most frequently due to uveitis, which has been reported most frequently within the first 12 weeks (but sometimes even after a year) following hospital or ETU discharge. However, the true incidence and prevalence of ocular complications are uncertain, as diagnosis requires advanced ocular equipment, including a slit lamp, and ophthalmological expertise, which are less commonly available in resource-limited settings. Careful characterization of the clinical phenotype and natural history of uveitis in survivors of EVD is ongoing, but emerging reports suggest involvement of all anatomical locations including anterior uveitis (affecting the anterior chamber, iris or ciliary body) in 46–62%, posterior uveitis (affecting the choroid or posterior retina) in 26%, and pan-uveitis in 21–25% of examined populations with uveitis. Patients may also develop structural ocular complications, most commonly cataracts, which require surgical intervention. In one study of 57 patients with uveitis after EVD, seven (12%) were also diagnosed with cataracts concurrently with uveitis, and at least three others developed cataract(s) following the onset of uveitis. These findings raise the concern for long-term visual disability if complications of uveitis are not diagnosed and treated early. Timely diagnosis enabling early appropriate cycloplegic and anti-inflammatory treatment (topical or systemic steroids depending on severity) for uveitis and recognition and management of complications are crucial to avoid long-term visual disability. Recurrent uveitis has been described.
- Neurological issues (headache, memory loss, mental status changes, seizures and insomnia), psychiatric conditions (anxiety and depressive disorders and posttraumatic stress disorder; see next section), dermatological disorders (alopecia and rashes), gastrointestinal issues (poorly defined abdominal pain syndromes), auditory issues (hearing loss and tinnitus), and generalized symptoms, including severe and persistent fatigue, have also been reported in a substantial number of survivors of EVD. Many of these conditions are associated with important functional limitations; more than one-third of survivors in a single study reported health problems lasting >1 year, and 29% indicated that their health problems limited their ability to walk or run.
- Additionally, gender-specific complications (for example, orchitis and amenorrhoea) and sexual dysfunction in both women and men have been reported
REFERENCES
- Kuhn, J. H. et al. ICTV virus taxonomy profile: Filoviridae. J. Gen. VIROL. 100, 911– 912 (2019).
- Kuhn, J. H. et al. new filovirus disease classification and nomenclature. Nat. Rev. MICROBIOL. 17, 261–263 (2019). Outlines the current official WHO International Classification of Diseases version 11 (ICD-11) subdivisions of filovirus disease (FVD), including Ebola virus disease (EVD).
- Siegert, R., Shu, H.-L., SLENCZKA, W., Peters, D. & Müller, G. On the ethology of an unknown human infection originating from monkeys [German]. DTSCH. Med. WOCHENSCHR. 92, 2341–2343 (1967).
- Kuhn, J. H., Amarasinghe, G. & Perry, D. L. in Fields Virology: Emerging Viruses 7th edn Ch. 12 (eds Sean P. J. Whelan, Peter M. Howley, & David M. Knipe) in the press (Wolters Kluwer, 2020).
- Formenty, P. et al. Human infection due to Ebola virus, subtype Côte d’Ivoire: clinical and biologic presentation. J. Infect. Dis. 179, S48–S53 (1999).
- Okware, S. I. et al. An outbreak of Ebola in Uganda. Trop. Med. Int. Health 7, 1068– 1075 (2002)
- Kuhn, J. H. Filoviruses. A compendium of 40 years of epidemiological, clinical, and laboratory studies. Arch.Virol. Suppl. 20, 13 360 [2008].
- Martines, R. B., Ng, D. L., Greer, P. W., Rollin, P. E. & Zaki, S. R. Tissue and cellular tropism, pathology and pathogenesis of Ebola and Marburg viruses. J. Pathol. 235, 153– 174 (2015). An updated and comprehensive summary of filovirus disease autopsy data.
- Siragam, V., Wong, G. & Qiu, X.-G. Animal models for filovirus infections. Zool. Res. 39, 15–24 (2018).
- Nakayama, E. & Saijo, M. Animal models for Ebola and Marburg virus infections. Front. Microbiol. 4, 267 (2013)
- World Health Organization. Ebola Outbreak2014–2016. http://www.who.int/csr/disease/ebola/en/ (2017).
- Chippaux, J.-P. Outbreaks of Ebola virus disease in Africa: the beginnings of a tragic saga. J. Venom. Anim. Toxins Incl. Trop. Dis. 20, 44 (2014).
- Check Hayden, E. Ebola failures prompt WHO rethink. Nature 521, 137 (2015
- Levett, J. Disastrous events and political failures. Prehosp. Disaster Med. 30, 227–228
- Ebola: a failure of international collective action. Lancet 384, 637 (2014).
- Ippolito, G., Di Caro, A. & Capobianchi, M. R. The chronology of the international response to Ebola in Western Africa: lights and shadows in a frame of conflicting position and figures. Infect. Dis. Rep. 7, 5957 (2015).
- Kiiza, P., Adhikari, N. K. J., Mullin, S., Teo, K. & Fowler, R. A. Principles and practices of establishing a hospital-based Ebola treatment unit. Crit. Care Clin. 35, 697–710 (2019). Informative discussion of the process and resources necessary to initiate an Ebola (virus) Treatment Unit [ETU]
- Janke, C. et al. Beyond Ebola treatment units: severe infection temporary treatment units as an essential element of Ebola case management during an outbreak. BMC Infect. Dis. 17, 124 (2017).
- Lamb, L. E., Cox, A. T., Fletcher, T. & McCourt, A. L. Formulating and improving care while mitigating risk in a military Ebola virus disease treatment unit. J. R. Army Med. Corps 163, 2–6 (2017).
- Leitenberg, M., Zilinskas, R. A. & Kuhn, J. H. The Soviet Biological Weapons Program — a History (Harvard Univ. Press, 2012).
- Radoshitzky, S. R., Bavari, S., Jahrling, P. B. & Kuhn, J. H. in Medical Aspects of Biological Warfare (Textbooks of Military Medicine) Ch. 23 (eds Bozue, J., Cote, C. K & Glass, P. J.) 569–614 (Borden Institute, US Army Medical Department Centre and School, Health Readiness Centre of Excellence, 2018).
- World Health Organization. Ebola Virus Disease Democratic Republic of Congo: External Situation Report 77/2020. https://reliefweb.int/sites/reliefweb.int/files/resources/SITR EPEVD DRC 20200128 eng.pdf (2020).
- Maganga, G. D. et al. Ebola virus disease in the Democratic Republic of Congo. N. Engl. J. Med. 371, 2083–2091 (2014).
- Ebola Outbreak Epidemiology Team. Outbreak of Ebola virus disease in the Democratic Republic of the Congo, April-May, 2018: an epidemiological study. Lancet 392, 213–221 (2018)


 Molaka Sphurthy Mitra*
Molaka Sphurthy Mitra*











 10.5281/zenodo.14354147
10.5281/zenodo.14354147