Abstract
Rabies is a zoonotic, fatal and progressive neurological infection caused by rabies virus of the genus Lyssavirus and family Rhabdoviridae. It affects all warm-blooded animals and the disease is prevalent throughout the world and endemic in many countries except in Islands like Australia and Antarctica. Over 60,000 peoples die every year due to rabies, while approximately 15 million people receive rabies post-exposure prophylaxis (PEP) annually. Bite of rabid animals and saliva of infected host are mainly responsible for transmission and wildlife like raccoons, skunks, bats and foxes are main reservoirs for rabies. The incubation period is highly variable from 2 weeks to 6 years (avg. 2-3 months). Though severe neurologic signs and fatal outcome, neuropathological lesions are relatively mild. Rabies virus exploits various mechanisms to evade the host immune responses. Being a major zoonosis, precise and rapid diagnosis is important for early treatment and effective prevention and control measures. Traditional rapid Seller's staining and histopathological methods are still in use for diagnosis of rabies. Direct immunofluorescent test (dFAT) is gold standard test and most commonly recommended for diagnosis of rabies in fresh brain tissues of dogs by both OIE and WHO. Mouse inoculation test (MIT) and polymerase chain reaction (PCR) are superior and used for routine diagnosis. Vaccination with live attenuated o3r inactivated viruses, DNA and recombinant vaccines can be done in endemic areas. This review describes in detail about epidemiology, transmission, pathogenesis, advances in diagnosis, vaccination and therapeutic approaches along with appropriate prevention and control strategies
Keywords
DNA, epidemiology, polymerase chain reaction, post-exposure prophylaxis, Mouse inoculation test
Introduction
Rabies is a deadly zoonotic viral disease that affects the central nervous system (CNS) of mammals, including humans. The disease is almost fetal both in humans and animals as there is no cure once the signs and symptoms appear. Though all animals are susceptible to rabies some serve as hosts and vectors which include Dogs, cats, mongoose, foxes, ferrets, and bats in India, the most common vectors are the dogs (96%) and cats (2%), besides other mammal like mongoose, foxes etc(1). He central nervous system of animals, ultimately causing disease in the brain and death. The World Organization for Animal Health (WHO) reported that children under 15 years of age account for 40% of all dog bites cases and 35-50% of all rabies deaths. This could be attributes to their very curious and inquisitive nature as well as their small stature which might result to bite wounds in highly innervated areas of the body which makes it easier for the rabies virus to travel to the central nervous system (2).
History of Rabies
Rabies has a long and interesting history that is lot in antiquity. According to Athezndorus, it was first observed in mankind in the days of the Ascipiadae, the god of medicine Aesculapius, Acteon, the famous hunter of myth who was torn to pieces by his hounds when he surprised Diana and her attendants at the bath Was thought to have to have been destroyed by rabid dogs .In the Iliad, Homer is thought to refer to rabies when he mention that Sirius, the dog star of the dog star of the Orion, exerts a malignant influence upon the health of mankind . The dog, Sirius, was associated with mad dogs all through the eastern Mediterranean and Egypt, and later Rome. Homer further uses the term “raging dog in the epithets that are thrown at hector by Teucer. The Greeks had a special god in their mythology to counteract the effects of rabies, Aristaeus son of Apollo. He is represented as the header of rabies (3).
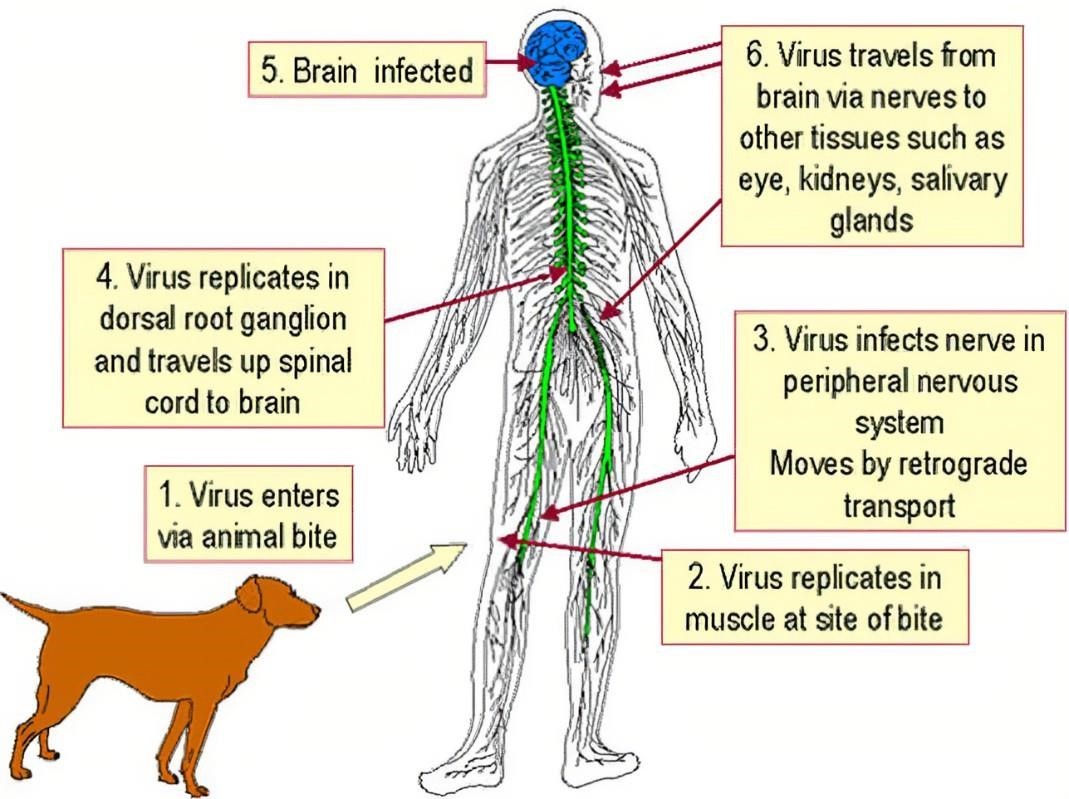
Figure 1. Transmission of Rabies Virus
Structure of Rabies Virus
This is approximately 180nm long and 75nm wide. the rabies genome encodes five proteins: nucleoprotein (N), phosphoprotein (P), matrix protein (M), glycoprotein (G), and polymerase (L). All rhabdovirus have two major structural components: a helical ribonucleoprotein core RNP, genomic RNAis tightly encased by the nucleoprotein. The other viral proteins, the phosphoproteins and the large protein (L-protein or polymerase) are associated with the RNP. They have terminal spikes which are tightly arranged on the surface of virus. The M protein is associated both with the envelope and the RNP and may be the central protein of rhabdovirus assembly Rabies is the RNA virus. The genome encoded 5 proteins namely M, N, G, L and P protein. The arrangements of these proteins and the RNA determine the structure of rabies(4).

Figure 2. Structure of Rabies Virus
Various Proteins Carried by Rabies Virus
|
Symbol
|
Name
|
Function
|
|
N
|
Nucleoprotein
|
It mainly coats the RNA.
|
|
P
|
Phosphoprotein
|
L cofactor and various regulatory functions. Has many isoforms from multiple initiation
|
|
M
|
Matrix
|
It mainly Keeps nucleoprotein condensed. Important for assembly; has roles in regulation
|
|
G
|
Glycoprotein
|
It Uses muscular nAChR, NACM, and p75NTR as receptors. It is responsible for the virus pathogenicity and development of immunity to rabies.
|
|
L
|
Large structural protein
|
RNA replicase of the Mononegavirales type (5) .
|
Clinical Manifestations of Rabies Virus
Rabies is a viral infection that affects the central nervous system and can be fatal if not treated before symptoms appear. The clinical manifestations of rabies typically develop in several stages:
- Period of Incubation: It mainly often 1-3 months, but sometimes ranging from days to years. The virus is moving towards the brain through the nerve system even if there are no symptoms. The exposure, which typically occurs by an animal bite, may go unnoticed by the individual.
- Prodromal phase: The early symptoms of prodromal phase are occurring last two to ten days. The various symptoms include:
Fever
Headache
Malaise (general feeling of being unwell).
Pain, tingling, or itching at the site of the bite (often a key indicator).
Anxiety, agitation, and discomfort.
- Progressive neurological symptoms (acute neurologic phase): Having trouble swallowing, which can lead to throat spasms. It is the cause of hydrophobia, or a fear of water. The dread of air or aerophobia, is brought on by painful spasms in the throat that are caused by a draft. Paralysis or weakness of muscles, particularly in the limbs. The other symptoms such as convulsions, confusion, agitation, delirium, or hallucinations. Partial paralysis with the potential to become complete paralysis.
- Coma and death (if untreated): As the virus progresses, the patient may enter a coma. Respiratory failure due to paralysis of the respiratory muscles is the leading cause of death, often within 1-2 weeks after the onset of severe symptoms.
The Global Burden of Rabies (Analysing Data Across All Countries)
In India
- Rabies Cases: India reports a significant number of rabies cases, with thousands of deaths annually, mostly in rural areas where vaccination and access to medical care are limited.
- Efforts: The government has launched initiatives to vaccinate dogs and provide rabies education, but challenges remain due to the large stray dog population (6) .
In China
- Rabies Cases: China has seen a decline in rabies cases over the past decade due to aggressive vaccination campaigns and public awareness efforts.
- Efforts: The country has implemented strict control measures, including mass vaccination of dogs and improved surveillance (7).
In United States
|
Country
|
Estimated Annual
Cases
|
Rabies Control Measures
|
|
India
|
18,000-20,000+
|
Vaccination campaigns, public awareness, stray dog control
|
|
China
|
~500-1,000
|
Aggressive dog vaccination, public awareness, surveillance
|
|
Philippines
|
~200-300
|
Aggressive dog vaccination, public awareness, surveillance
|
|
United States
|
<5>
|
High pet vaccination coverage, wildlife control
|
|
Brazil
|
~30-50
|
Urban rabies control programs, rural area challenge
|
|
Tanzania
|
~1,500-2,000
|
Limited access to vaccines, focus on dog vaccination
|
|
Ethiopia
|
~2,000-3,000
|
Low access to vaccines, educational efforts in progress
|
|
Thailand
|
~100-200
|
Dog vaccination, public education
|
|
South Africa
|
~150-300
|
Dog and wildlife vaccination, public awareness
|
|
Russia
|
~20-50
|
Rabies control through vaccination and animal control
|
|
Mexico
|
~10-20
|
Extensive vaccination programs, rural areas under focus
|
|
Bangladesh
|
~2,000-3,000
|
Challenges in rural areas, focus on vaccination
|
|
Vietnam
|
~100-200
|
Increasing vaccination efforts, focus on public awareness
|
|
Nigeria
|
~2,000-3,000
|
Limited vaccine access, ongoing public health efforts
|
|
Kenya
|
~1,000-2,000
|
Stray dog control, public health campaigns
|
|
France
|
~0-1
|
High control, rare cases usually from imports
|
Current Treatment Approaches for Rabies
The central nervous system is impacted by the viral disease rabies, which is nearly invariably lethal once symptoms start to show. However, if treatment is started as soon as possible before symptoms appear, it may be successful. The primary methods used now to treat rabies are as follows:
- Prophylactic Post-Exposure (PEP): If a person has been bitten or exposed to the rabies virus, this is the best course of action.
Rabies Immunoglobulin (RIG): The Individuals who are exposed to rabies are given a dose of RIG by producing antibodies that aid in neutralizing the virus while the recipient's immune system reacts to the vaccination, this offers passive immunity.
Rabies Vaccine: Over the course of several weeks, the individual receives a series of rabies shots in addition to RIG. The vaccine stimulates the production of antibodies by the individual's immune system against the virus (9).
- Inducing a Coma (The "Milwaukee Protocol"): In rare cases, when symptoms have already developed (which is very difficult to treat and often fatal), some protocols have been attempted. One of these is the "Milwaukee Protocol," which involves:
- Inducing a coma in the patient to try to give their body a chance to fight the virus.
- The treatment typically includes antiviral medications, sedatives, and support for organ function, although success has been very limited, and the long-term outcomes are not favorable.
- Supportive Care: In cases where the rabies virus has progressed to the symptomatic stage, treatment becomes primarily supportive. This involves: Managing pain, agitation, seizures, and other symptoms that may arise. Providing hydration, respiratory support, and maintaining organ function.
- Experimental Treatments: Some ongoing studies are exploring new ways to treat rabies, such as:
- Antiviral medications: Researchers are investigating drugs that might inhibit the rabies virus replication, though no proven antiviral treatments have been established yet.
- Gene therapy and monoclonal antibodies: These approaches aim to either boost the immune system's ability to respond to the virus or directly target the virus itself.
Current Rabies Vaccines
- Modified live vaccine (MLV): The live rabies vaccine must be non-pathogenic to animals, have the capacity to multiply large viral titers in cells, produce protective immunity upon delivery, and be genetically and thermally stable. The majority of researchers altered the virus by serially passing it through different cells in order to guarantee the safety of potential vaccinations. Attenuated live vaccines have been developed as a result of this technology to manage the infectious disease. In 1974, Canada introduced the Evelyn-Rokitnicki-Abelseth (ERA) attenuated live vaccination strain. The ERA strain was investigated by the Korean Veterinary Authority in the late 1970s as a potential replacement for the fluory low egg passage vaccine, which had side effects due to tissue debris in the vaccination (10). The ERA strain propagated in primary porcine kidney cells was cloned three times using an end-dilution method. The experimental vaccine containing the cloned ERA strain was prepared and examined using biological methods. Domestic animals inoculated with the vaccine via an intramuscular route for safety, including dogs, sheep, goats, and cats, did not show any clinical signs. The Flury strain, a chicken embryo-origin MLV vaccine, has been produced and given to animals in some Asian countries. The management of MLV vs. inactivated vaccines is harder as the former is more sensitive to changes in temperature. Consequently, the impact of MLV rabies vaccines is expected to decline in several countries, including Korea.
- Inactivated rabies vaccine: Inactivated rabies vaccines require that high RABV titers be produced in tissues or cells. RABV can be grown in brain tissue, and nerve tissue vaccines (NTVs) consisting of inactivated rabies vaccine produced from RABV-infected brain tissue of sheep, goats, and mice were developed about 100 years ago and have been used in some Asian and African countries. The antigen for the first vaccine was obtained from RABV-infected rabbit brain and spinal cord tissue and inactivated with 0.8% phenol or 0.1% merthiolate at 37°C for 3 and 5 days. The second antigen was prepared from calf brain and spinal cord tissue infected with wild RABV. Worldwide, the following RABV strains have been used for inactivated rabies vaccine such as CVS 11, Pittman-Moore–NIL2, RC-HL derived from the Nishigahara strain, and Pasteur virus (11). These inactivated rabies vaccine strains are grown in culture systems with baby hamster kidney, hamster lung, guinea pig brain, chick embryo. The potency and safety of the inactivated rabies vaccines via intramuscular route are quite good.
- Oral Modified live virus vaccine: It is a type of vaccine that can protect dogs from disease by introducing a weakened from of the virus into the dogs body. Modified live rabies vaccine for oral immunization started with the SAD Berne strain, which was developed from the ERA strain in 1969. Oral rabies vaccine has been produced for free-ranging animals and wildlife species that serve as vectors. However, the SAD Berne strain had a degree of residual pathogenicity in wild animals and induced a partial immune response in young foxes (12). Raccoon dogs and dogs ingesting ten doses of SAG2 bait remained healthy, and all vaccinated animals had high rabies neutralizing antibody levels for 180 days after inoculation. The SAG2 vaccine is the only oral vaccine registered with the European Medicine Agency. In Europe, other commercial MLVs for oral immunization are Lysvulpen, SAD B19, and SAD P5/88 (13).
- Oral rabies vaccines derived from Plants: Oral rabies vaccines derived from plants. Plants have provided new systems for the large-scale production of recombinant proteins at low cost, simplifying the production process. A variety of genetically engineered vaccines using tobacco mosaic virus and tomato bushy stunt virus have been developed for expressing foreign antigens in plants (14). Rabies antigen expressed in plant tissue was immunogenic and protective in mice immunized intramuscularly and orally. One of the more advanced approaches for expressing foreign antigens in plants is to construct transgenic plants. To produce a plant-derived rabies antigen, the native signal peptide within the rabies glycoprotein gene was replaced with that of the pathogenesis-related protein of Nicotiana tabacum. Codon optimization of the rabies glycoprotein gene is necessary for providing plant-preferred codons. Plant-derived antigens induced strong mucosal and humoral immune responses after administration via either an oral or an intramuscular route in mice (15). These antigens have several advantages, including post-translational modifications, stability for storage, and ease of delivery. The rabies glycoprotein has been expressed in several plants, including tobacco, tomato, spinach, carrot, and maize. These antigens obtained from transgenic plants conferred protection to mice against challenge (16,17). Although plant-derived antigens have many advantages, some problems should be solved before oral rabies vaccines originating from plants are given to domestic animals. The most important are the need to improve the expression in raw transgenic plants and to reduce the substantial variability in the level of protein expression among different lines and subsequent generations. Additionally, it is necessary to shorten the time needed to obtain antigens from different plants.
CONCLUSION
Rabies is still a major public health issue, especially in areas with limited access to post-exposure therapies and preventative measures. Rabies is avoidable with prompt and proper therapies, even though it is nearly always fatal once clinical symptoms manifest. The disease is now better managed and prevented thanks to developments in monoclonal antibody treatments, vaccine development, and diagnostics. However, disparities still exist, especially in low-income areas with limited access to care and immunization. Dog bites are a major cause in Asia and Africa, where the majority of cases occur. Dogs are the primary source of rabies, accounting for about 99 percent of human fatalities from the disease. Despite the fact that animal rabies vaccinations are safe, immunogenic, and effective when given as prescribed, vaccinations lacking the appropriate immunogenic antigens must to be recognized and thrown out. Millions of people have been spared from this deadly illness because to animal vaccination, and in 2011, the World Organization for Animal Health (OIE) proposed the idea of regional dog vaccine banks. Typically, data would display the age demographics, impacted areas, and number of instances. Research may indicate that laboratory methods such as the direct fluorescent antibody (DFA) test, RTPCR, or virus isolation can be used to confirm rabies. The disease is now better managed and prevented thanks to developments in monoclonal antibody treatments, vaccine development, and diagnostics. However, disparities still exist, especially in low-income areas with limited access to care and immunization. Both before and after exposure, vaccination is still crucial for protection, and prompt administration greatly slows the progression of the disease.
REFERENCES
- Sudarshan MK, Madhusudana SN, Mahendra BJ, Rao NSN, Ashwath Narayana DH, Abdul Rahman S, et al. Assessing the burden of human rabies in India: results of a national multicentre epidemiological survey. International Journal of Infectious Diseases. 2007; 11:29-35.
- Dzikwi A, Ibrahim A, Umoh j. knowledge, attitude and practice about Rabies among children Receiving formal Nd Informal Education in Samaru, Zaria, Nigeria. Global Journal of health science.
- James H. Steele, Peter J. Fernandez, The Natural History of rabies 2nd edition Routledge.
- X.Zhang, Z. Zhu, C. Wang, Persistance of rabies antibody 5 year after post exposure prophylaxis with zero cell anti rabies vaccine and antibody response to a single booster dose clan. Vaccine 9,309(2011)
- Carter, John; Saunders, Venetia (2007). Virology: Principles and Applications. Wiley. p. 175. ISBN 978-0-470-02386-0.
- Scott TP, Coetzer A, Nel LH. Strategies for the elimination of dog-mediated human rabies by 2030. InRabies 2020 Jan 1 (pp. 671-688). Academic Press.
- Rupprecht CE, Willoughby R, Slate D. Current and future trends in the prevention, treatment and control of rabies. Expert review of anti-infective therapy. 2006 Dec 1;4(6):1021-38.
- Vigilato MA, Clavijo A, Knobl T, Silva HM, Cosivi O, Schneider MC, Leanes LF, Belotto AJ, Espinal MA. Progress towards eliminating canine rabies: policies and perspectives from Latin America and the Caribbean. Philosophical Transactions of the Royal Society B: Biological Sciences. 2013 Aug 5;368(1623):20120143.
- Poorolajal J, Babaee I, Yoosefi R, Farnoosh F. Animal bite and deficiencies in rabies postexposure prophylaxis in Tehran, Iran. Archives of Iranian medicine. 2015 Dec 1;18(12):0-
- Yang DK, Oh YI, Cho SD, et al. Molecular identification of the vaccine strain from the inactivated rabies vaccine. J Bacteriol Virol 2011; 41:47-54
- Roumiantzeff M. The present status of rabies vaccine development and clinical experience with rabies vaccine. Southeast Asian J Trop Med Public Health 1988; 19:549-61.
- Baer GM, Abelseth MK, Debbie JG. Oral vaccination of foxes against rabies. Am J Epidemiol 1971; 93:487-90.
- Cliquet F, Gurbuxani JP, Pradhan HK, et al. The safety and efficacy of the oral rabies vaccine SAG2 in Indian stray dogs. Vaccine 2007; 25:3409-18.
- Yusibov V, Hooper DC, Spitsin SV, et al. Expression in plants and immunogenicity of plant virus-based experimental rabies vaccine. Vaccine 2002; 20:3155-64.
- Loza-Rubio E, Rojas-Anaya E, Lopez J, Olivera-Flores MT, Gomez-Lim M, Tapia-Perez G. Induction of a protective immune response to rabies virus in sheep after oral immunization with transgenic maize, expressing the rabies virus glycoprotein. Vaccine 2012; 30:5551-6.
- McGarvey PB, Hammond J, Dienelt MM, et al. Expression of the rabies virus glycoprotein in transgenic tomatoes. Biotechnology (N Y) 1995; 13:1484-7.
- Rojas-Anaya E, Loza-Rubio E, Olivera-Flores MT, GomezLim M. Expression of rabies virus G protein in carrots (Daucus carota). Transgenic Res 2009; 18:911-9.


 Ankit Sharma*
Ankit Sharma*
 Sahil Bhatia
Sahil Bhatia


 10.5281/zenodo.14859869
10.5281/zenodo.14859869