Development and Assessment of Sustained Release Matrix Tablet of Telmisartan the UV-Visible spectra analysis of Telmisartan in methanol and water solvents discovered a consistent absorption peak at approximately 234 nm, indicating its characteristic absorbance wavelength. The FT-IR spectrum highlighted functional groups such as hydroxyl, carbonyl, and aromatic rings present in Telmisartan, essential for identifying molecular characteristics. Formulation F8 emerged as the most promising option for sustained-release matrix tablets of Telmisartan, demonstrating superior flow properties, compressibility, and tablet characteristics. Compatibility studies with various excipients indicated stable conditions at lower temperatures, but interaction challenges arose at elevated temperatures and humidity levels. Differential Scanning Calorimetry (DSC) analysis provided insights into the thermal behavior of Telmisartan and excipients, aiding in formulation optimization. Furthermore, formulation F8 exhibited prolonged floating time and sustained drug release in dissolution profiles, suggesting its suitability for once-daily dosing regimens. Stability studies confirmed F8's robustness and reliability over a one month period, indicating its potential for clinical use. Further investigations may be required to assess long term stability under different environmental conditions.
Telmisartan Sustained Release Matrix Tablet, DSC, FT-IR etc.
Piroxicam is a nonsteroidal anti-inflammatory drug (NSAID) that is primarily used to relieve symptoms of inflammation and pain associated with conditions like arthritis. It belongs to the oxicam class of NSAIDs and works by inhibiting the production of prostaglandins, which are substances in the body that play a role in inflammation and pain.
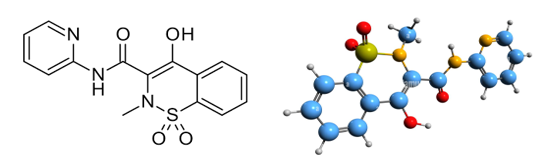
Figure 01: Structure and IUPAC name of Piroxicam: 4-Hydroxy-2-methyl-N-(2-pyridinyl)-2H-1, 2-benzothiazine-3-carboxamide 1, 1-dioxide.
Class and Mechanism of Action: Piroxicam belongs to the oxicam class of NSAIDs. Like other NSAIDs, it works by inhibiting the enzyme cyclooxygenase (COX), which is involved in the production of prostaglandins. By reducing prostaglandin levels, piroxicam helps alleviate inflammation, pain, and fever.
Indications: Piroxicam is primarily used to treat symptoms of various inflammatory conditions, such as osteoarthritis, rheumatoid arthritis, ankylosing spondylitis, and acute gout. It is also sometimes used to relieve menstrual cramps and other types of pain.
Administration: Piroxicam is typically administered orally in the form of tablets or capsules. It is usually taken once daily, although the dosage may vary depending on the condition being treated and the individual's response to the medication. Piroxicam may also be available in topical formulations for local application.
Side Effects: Common side effects of piroxicam include gastrointestinal discomfort, such as stomach pain, indigestion, nausea, and diarrhea. It may also cause dizziness, headache, rash, and fluid retention. Serious side effects, although rare, can include gastrointestinal bleeding, kidney problems, and allergic reactions.
Precautions: Piroxicam should be used with caution in individuals with a history of gastrointestinal ulcers or bleeding, kidney problems, or heart disease. It should be avoided in patients with a known allergy to NSAIDs. Long-term use of piroxicam or other NSAIDs may increase the risk of cardiovascular events such as heart attack or stroke.
Interactions: Piroxicam may interact with other medications, including blood thinners, other NSAIDs, corticosteroids, and certain medications used to treat high blood pressure or heart conditions. It is important to inform your healthcare provider about all medications you are taking before starting piroxicam.
Monitoring: Regular monitoring of kidney function, liver function, and blood pressure may be necessary during treatment with piroxicam, especially in individuals with preexisting medical conditions.
Pregnancy and Lactation: Piroxicam is not recommended during the third trimester of pregnancy due to the potential risk of complications to the fetus. It should also be avoided during breastfeeding, as it may pass into breast milk and cause harm to the nursing infant.
Overall, piroxicam is an effective medication for relieving inflammation and pain associated with various conditions, but it should be used cautiously and under the supervision of a healthcare professional to minimize the risk of side effects and complications.
Piroxicam: benefits and problems:
Piroxicam, like other NSAIDs, offers several benefits in managing inflammation and pain associated with various conditions. However, it also presents certain potential problems and risks. Let's explore both aspects:
Benefits:
Anti-inflammatory Effects: Piroxicam effectively reduces inflammation by inhibiting the production of prostaglandins, which are key mediators of the inflammatory response. This makes it useful in managing conditions like arthritis, bursitis, and tendonitis.
Pain Relief: By reducing inflammation, piroxicam helps alleviate pain associated with inflammatory conditions such as osteoarthritis, rheumatoid arthritis, and gout. It can also be effective in reducing pain from other sources, such as menstrual cramps or minor injuries.
Fever Reduction: Piroxicam possesses antipyretic properties, meaning it can help reduce fever by inhibiting prostaglandin synthesis in the hypothalamus, the part of the brain that regulates body temperature.
Convenience: Piroxicam is typically administered once daily, which can be more convenient for patients compared to medications that require more frequent dosing.1-5
Problems and Risks:
Gastrointestinal Side Effects: NSAIDs, including piroxicam, are associated with an increased risk of gastrointestinal adverse effects such as stomach ulcers, bleeding, and perforation. These side effects can be severe and even life-threatening, particularly in older adults and those with a history of gastrointestinal issues.
Cardiovascular Risks: Piroxicam, like other NSAIDs, may increase the risk of cardiovascular events such as heart attack and stroke, especially when used long-term or in high doses. This risk is more pronounced in individuals with preexisting heart disease or risk factors for cardiovascular disease.
Kidney Toxicity: Piroxicam can cause kidney damage, particularly in individuals with preexisting kidney disease, dehydration, or other risk factors. Regular monitoring of kidney function is recommended during treatment with piroxicam.
Allergic Reactions: Some individuals may experience allergic reactions to piroxicam, ranging from mild skin rash to severe anaphylaxis. Patients with a known allergy to NSAIDs should avoid piroxicam.
Interactions with Other Medications: Piroxicam can interact with certain medications, including blood thinners, corticosteroids, and some antihypertensive drugs, potentially increasing the risk of adverse effects or reducing the efficacy of these medications.
Pregnancy and Lactation: Piroxicam is not recommended during the third trimester of pregnancy due to potential harm to the fetus, and it should be avoided during breastfeeding as it can pass into breast milk.
Central Nervous System Effects: Piroxicam can rarely cause central nervous system side effects such as dizziness, headache, and confusion, particularly at higher doses or in susceptible individuals.
In summary, while piroxicam can provide effective relief from inflammation and pain, it is important to use it judiciously, under the guidance of a healthcare professional, and to weigh its benefits against potential risks, especially in individuals with underlying health conditions. Regular monitoring for side effects and adherence to dosage recommendations can help minimize the likelihood of adverse outcomes.
Orodispersible tablets (ODTs), also known as fast-dissolving tablets or melt-in-mouth tablets: represent an innovative dosage form designed to enhance patient convenience and compliance. These tablets have gained increasing popularity in the pharmaceutical industry due to their unique characteristics and potential benefits for patients, especially those with swallowing difficulties or who prefer not to take tablets with water.
- ODTs are solid dosage forms that disintegrate rapidly in the mouth upon contact with saliva, without the need for water or chewing. This rapid disintegration is facilitated by various formulation techniques and excipients that promote quick dissolution or dispersion of the tablet particles.
- As a result, the active pharmaceutical ingredient (API) is released and absorbed through the oral mucosa, leading to faster onset of action compared to conventional tablets.
- One of the key advantages of ODTs is their convenience and ease of administration. Patients who have difficulty swallowing large tablets or capsules, such as pediatric, geriatric, or dysphagic individuals, can benefit significantly from ODTs.
- Elderly patients, in particular, may have compromised swallowing function or reduced saliva production, making traditional dosage forms challenging to consume. ODTs provide a viable alternative that improves patient compliance and medication adherence.
- Furthermore, ODTs offer enhanced portability and versatility, making them ideal for on-the-go use and situations where access to water is limited or inconvenient. Patients can carry ODTs easily in their pockets or purses and administer them discreetly whenever needed, without the need to find
- Water for swallowing. This feature is particularly advantageous for travelers, athletes, and individuals with busy lifestyles who require quick and convenient medication options.
- The formulation of ODTs involves careful selection of excipients and processing techniques to achieve the desired characteristics, such as: rapid disintegration, good mechanical strength, and acceptable taste and mouth feel.
- Commonly used excipients in ODT formulations include superdisintegrants (e.g., Crospovidone, Croscarmellose sodium), which promote rapid tablet disintegration, as well as sugar-based bulking agents, sweeteners, and flavoring agents to improve palatability.
- In addition to improving patient convenience and acceptability, ODTs offer several other potential benefits. For instance, the rapid onset of action associated with ODTs can be advantageous for medications intended for the treatment of acute conditions, such as pain relief or migraine attacks. By bypassing the gastrointestinal tract and delivering the API directly to the systemic circulation via the oral mucosa, ODTs can achieve faster therapeutic effects compared to traditional oral dosage forms.
- Moreover, ODTs may enhance drug bioavailability and therapeutic efficacy in certain cases. The absorption of drugs through the oral mucosa can bypass first-pass metabolism in the liver, leading to higher systemic drug concentrations and improved bioavailability.
Ideal properties of ODTs:
-
- Should dissolve or disintegrate in the mouth within seconds, eliminating the need for water.
- Accommodate high drug capacity.
- Require acceptable taste masking and other excipients.
- Provide a pleasant mouth feel.
- Possess sufficient strength to endure formulation and post-manufacturing handling.
- Suitable for situations like motion sickness or sudden allergic reactions, requiring rapid action.
- Enhance bioavailability, particularly for insoluble or hydrophobic drugs, through rapid disintegration and dissolution.
Advantages of ODTs:
- Rapid intervention in drug therapy.
- Convenient for administration, enhancing compliance for disabled, bedridden individuals, travelers, and busy individuals without access to water.
- Pleasant mouth feel reduces the perception of medication as bitter, especially in pediatric patients.
- Protects the drug from degradation by pH and gastrointestinal enzymes.
- Enhances compliance by eliminating the discomfort associated with injections.
- Ensures accurate dosing compared to liquids.
Disadvantages of ODTs:
- ODTs have a hydrophilic nature and should be stored in dry conditions.
- They exhibit fragile characteristics similar to effervescent granules.
- Special packaging is necessary to stabilize and ensure the safety of ODTs.
EXPERIMENTAL:
Chemical and reagent: Piroxicam IP was obtained as a gift sample from Pure Pharma Limited, Indore. Carmellose Sodium (Ac-Di-Sol) was sourced from National Chemicals, Mumbai, India. Xylitol, Mannitol, and magnesium stearate were all received as gift samples from Pure Pharma Limited, Indore. The procured samples were tested to confirm their identity and this included UV-visible wavelength scan, and recording of FT-IR spectra. FT-IR spectra were recorded. The sample was prepared as a KBr pellet for recording the spectra. The UV-Visible spectra of Piroxicam were recorded using methanol as solvent was recorded using water as solvent on Shimadzu 1900 series Instrument. 6-10
Preformulation Studies for Piroxicam Orodispersible Tablets: 11-20
Evaluation Parameters:
1. Appearance: Small, flat, round/oval, smooth surface, uniform color.
2. Size and Shape: Small, circular/oval, designed for rapid oral disintegration.
3. Weight Variation: Weigh 20 tablets individually, calculate average weight.
4. Thickness: Measure using Vernier calipers.
5. Hardness: Measure with Monsanto hardness tester.
6. Friability: Roche friabilator, weight loss should not exceed 1%.
7. Drug Content: UV spectrophotometer analysis at 331 nm.
8. Wetting Time: Measured with tissue paper method.
9. Disintegration Time: Aim for rapid disintegration in the mouth.
10. Dissolution Test: Conducted in suitable dissolution medium.
11. Solubility: Assess solubility of Piroxicam in various solvents.
Formulation Development of Piroxicam Orodispersible Tablets:
Preparation Method:
-
- Sieving: Ensure uniform particle size distribution.
- Mixing: Homogeneous distribution of drug and excipients.
- Compression: Use Cadmach compression machine with 10/32 round biconvex punches.
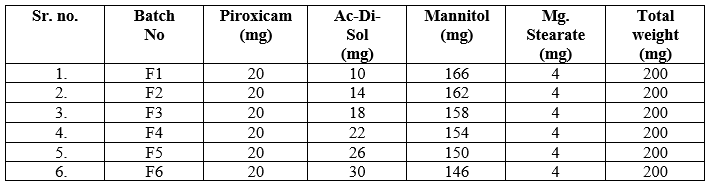
Table 01: Super disintegrants Selection:
Optimization of Piroxicam Orodispersible Tablets:
Procedure for Subliming Agents:
-
- Formulated with camphor, menthol, and Thymol at various concentrations.
- Subjected to sublimation at 40°C for 1 hour.
- Assessed for friability, disintegration time, and hardness.
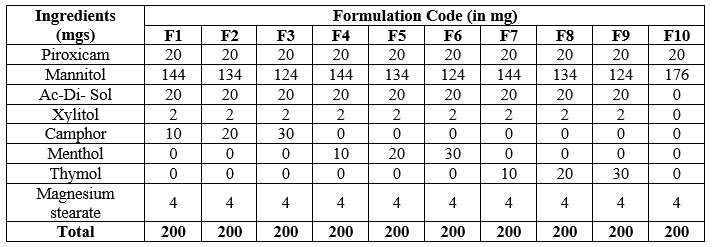
Table 02: Piroxicam Orodispersible Tablets (F1 to F10) Formulations:
Physical Characterization and Compatibility Study:
- Physical Characterization: Analyzed for appearance, weight variation, thickness, hardness, friability, drug content, wetting time, disintegration time, dissolution test, and solubility.
- Compatibility Study: Drug and excipients mixed in equal proportions and stored at various conditions (40°C, 25°C/RH 60%, 40°C/RH 75%) for one month, analyzed by UV-visible spectrophotometer and FT-IR.
Stability Studies:
Stability Studies: Conducted for 3 month at different temperatures (40°C, 25°C/RH 60%, 40°C/RH 75%). Samples were analyzed weekly for Piroxicam content using UV-visible spectrophotometer.
RESULTS AND DISCUSSION:
Confirmation of Identity of Piroxicam other excipients:
Piroxicam IP was obtained as a gift sample from Pure Pharma Limited, Indore. Sodium Starch Glycolate was sourced from National Chemicals, Mumbai. Microcrystalline Cellulose, Colloidal Silicon Dioxide, Mannitol, and Sodium Saccharine were also received as gift samples from Pure Pharma Limited, Indore. The samples' identities were confirmed via UV-visible wavelength scan and FT-IR spectra. FT-IR spectra were recorded, and the UV-visible spectra of Piroxicam were recorded using methanol and water as solvents on a Shimadzu instrument.

Figure 02: Piroxicam ?-max maximum absorbance at 354 nm
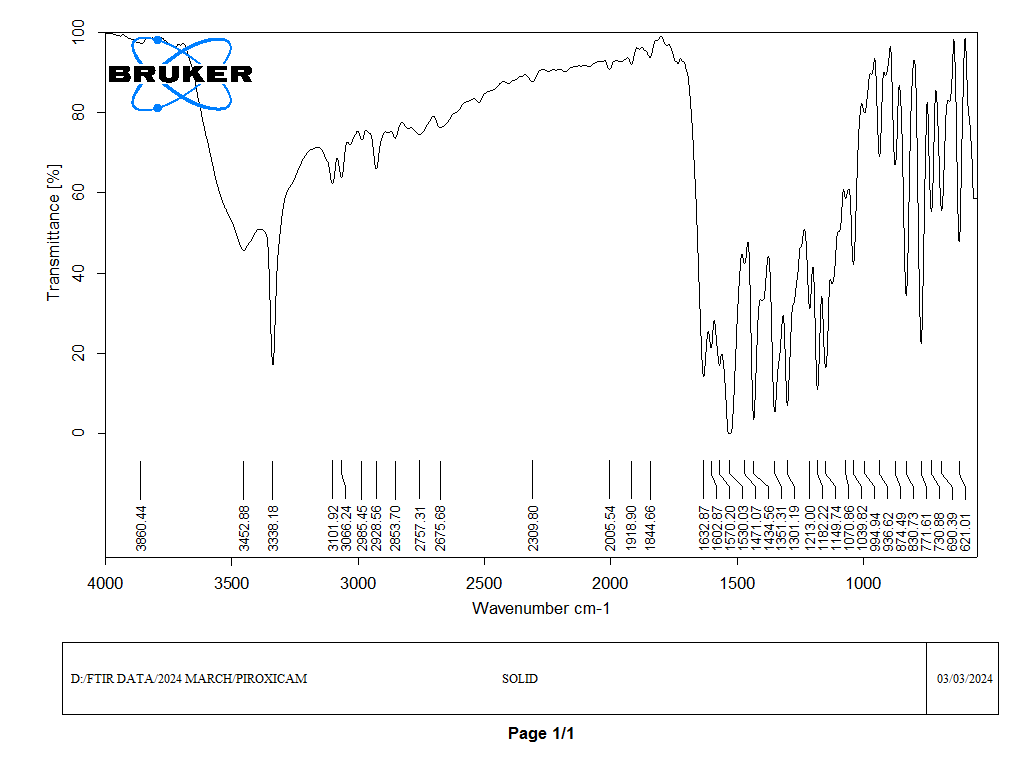
Figure no.03: FT-IR Interpretation of pure Piroxicam.
The UV-visible spectra of Piroxicam were recorded using methanol and water as solvents on a Shimadzu 1900 series instrument. Piroxicam typically shows maximum absorbance at around 354 nm, which is used for its quantitative analysis in pharmaceutical formulations.
FT-IR (Fourier Transform Infrared Spectroscopy) is used to analyze the functional groups in Piroxicam, a nonsteroidal anti-inflammatory drug (NSAID) with analgesic, anti-inflammatory, and antipyretic properties. Interpreting the FT-IR spectrum involves matching peaks with known vibrational frequencies to identify specific functional groups in the molecule shown figure 02 and 03.
Preformulation Studies Piroxicam Orodispersible Tablets: 18-21
Evaluation of tablet: Evaluation parameter:
Appearance of Tablet: Color: Uniform white. Odor: No unusual odors. Surface Texture: Smooth, free from cracks or roughness.
Particles: No visible particles or foreign matter.
Size and Shape of Tablet: Size: Uniform within specified range. Shape: Consistent, as per design (round or oval).
Tablet Uniformity of Weight: Uniformity: Consistent appearance with no significant deviations in color, shape, texture, or size.
Thickness and Diameter: Ensures consistent dosing, handling, packaging, and administration.
Hardness (Crushing Strength): Measured in kilograms or Newton’s. Ensures tablets withstand mechanical stress and remain intact until use.
Friability Test: Low friability ensures tablets remain intact during handling and transportation, minimizing dose variability.
Wetting Time: Indicates how quickly the tablet absorbs saliva and starts to disintegrate, ensuring fast onset of action and better patient compliance.
Disintegration Time: Measures how quickly the tablet breaks down in the mouth, ideally within a few seconds for rapid drug release and absorption.

Figure 05: Wetting time of Piroxicam Orodispersible tablet formulation F09.

Table no.03: Evaluation and results of Piroxicam Orodispersible Tablets (F1 to F10) formulations:
Each data represents Mean ±SD (n=3
Dissolution test:
In-Vitro dissolution studies: In vitro dissolution studies for Piroxicam Orodispersible Tablets was carried out using USP paddle method at 50 rpm in 900 ml of phosphate buffer pH 6.8 as dissolution media, maintained at 37±0.5°C. 5 ml aliquot of the solution was withdrawn from the dissolution apparatus after suitable time intervals, and the samples were replaced with fresh dissolution medium. Absorbance of these solutions was measured at 354 nm using a shimadzu UV- Shimadzu 1900 UV/VIS spectrophotometer.

Figure 06: Calibration curve of Piroxicam Orodispersible Tablets.
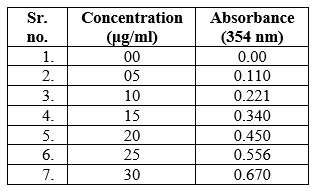
Table no.04: Calibration curve of Piroxicam Orodispersible Tablets.
Each data represents Mean ±SD (n=3)
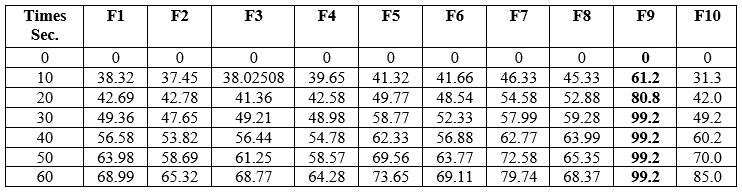
Table no.05: Dissolution study of Piroxicam Orodispersible Tablets formulation.
Each data represents Mean ±SD (n=3)

Figure 07: Dissolution study of Piroxicam Orodispersible Tablets formulation
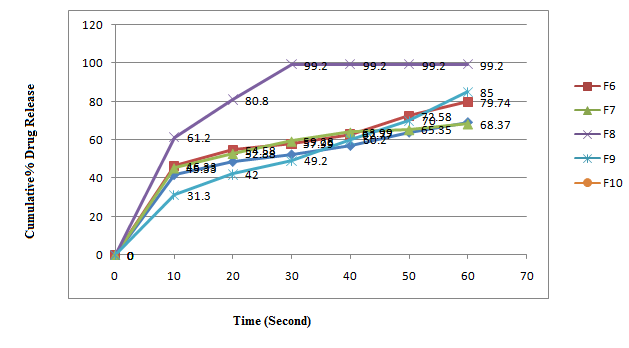
Figure 08: Dissolution study of Piroxicam Orodispersible Tablets formulation
Formulation Development of Piroxicam Orodispersible Tablets:
Formulation F6 stands out as the most suitable option for the development of Piroxicam Orodispersible Tablets due to its optimized superdisintegrants concentration, proper diluents content, consistent drug dosage, uniform tablet weight, effective lubrication, and proper manufacturing techniques.

Table no. 06: Procedure for selecting superdisintegrants:
Optimization Piroxicam Orodispersible Tablets:
To plan experiments efficiently, statistical techniques such as factorial design, response surface methodology, or Taguchi methods are utilized. Factors are systematically varied within predetermined ranges, and experiments are conducted at selected combinations of factor levels.
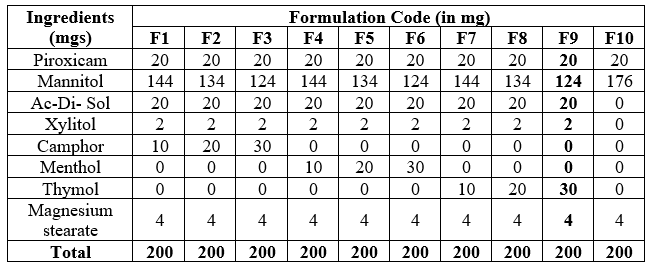
Table 07: Piroxicam Orodispersible Tablets (F1 to F10) formulations:
Based on the Design of Experiments (DOE), formulation F9 appears to be the most promising among the provided formulations for several reasons:
1. Optimal Superdisintegrants (Ac-Di-Sol) Concentration: F9 contains 20 mg of Ac-Di-Sol, which is consistent with the amounts used in other formulations. This ensures adequate disintegration properties without compromising tablet integrity.
2. Adjusted Mannitol Content: F9 contains 124 mg of Mannitol, which is lower than formulations F1 to F7 but higher than F8 and F10. This balance likely provides sufficient bulk and ensures proper tablet formation while facilitating rapid disintegration.
3. Absence of Additional Flavoring Agents: F9 does not contain additional flavoring agents like Camphor, Menthol, or Thymol. While these agents may enhance palatability, their absence in F9 simplifies the formulation and may reduce potential side effects or interactions.
4. Uniform Total Tablet Weight: All formulations, including F9, maintain a total tablet weight of 200 mg, ensuring consistency in dosage delivery and manufacturing processes.
5. Proper Lubrication: Magnesium stearate (4 mg) is included in F9, ensuring proper lubrication during tablet compression and preventing sticking, which is crucial for maintaining tablet integrity.
6. Optimization: The methodology likely considered various factors such as disintegration time, dissolution profile, and tablet hardness to determine the best formulation, and F9 emerged as the optimal choice based on these criteria.
Overall, formulation F9 stands out as the most suitable option for the intended purpose based on the analysis, balancing disintegration properties, tablet composition, and simplicity of formulation.
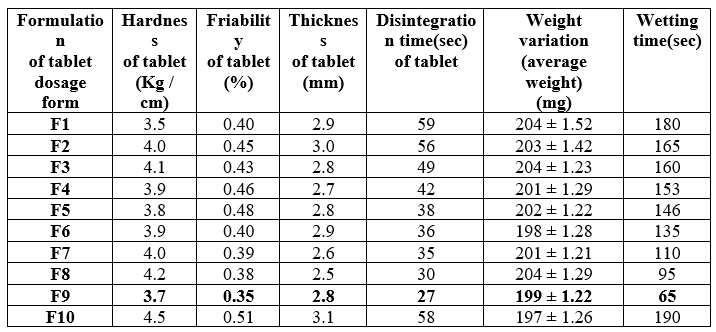
Table 08: Optimization of Piroxicam Orodispersible Tablets (F1 to F10) formulations results:
Based on the provided table, formulation F9 appears to be the most suitable formulation for several reasons:
1. Hardness of Tablets: F9 has a hardness of 3.7 Kg/cm, which falls within the acceptable range for tablet hardness, indicating sufficient mechanical strength. 2. Friability: F9 has the lowest friability of 0.35%, indicating minimal tablet breakage or crumbling during handling or transportation. 3. Thickness of Tablet: The thickness of F9 tablets is 2.8 mm, which is within the desired range for ease of swallowing and packaging. 4. Disintegration Time: F9 exhibits the shortest disintegration time of 27 seconds, indicating rapid breakdown of the tablet into smaller particles when exposed to saliva, facilitating quick dissolution and absorption. 5. Weight Variation: F9 has a weight variation of 199 ±1.22 mg, demonstrating consistency in tablet weight, which is crucial for accurate dosing and uniformity of drug delivery. 6. Wetting Time: F9 shows a wetting time of 65 seconds, indicating rapid dispersion and absorption of saliva by the tablet, which is essential for Orodispersible tablets to dissolve quickly in the mouth without the need for water.
Release kinetics of In-vitro Drug release: The kinetics of In-vitro drug release was determined by applying the drug release data to various kinetic models such as zero order, first order, Higuchi, Peppas-Korsmeyer and Hixson Crowell. The result obtained were represented in table 09, and shown in figure 09.
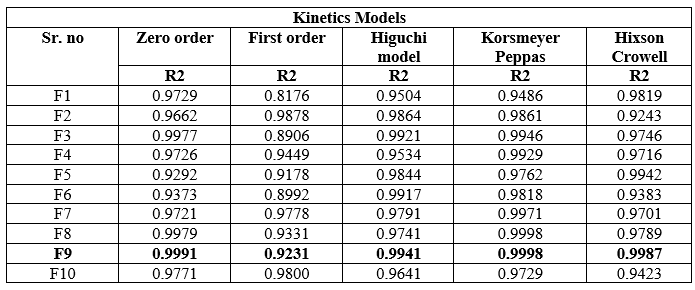
Table 09: Different Release kinetics of In-vitro Drug release
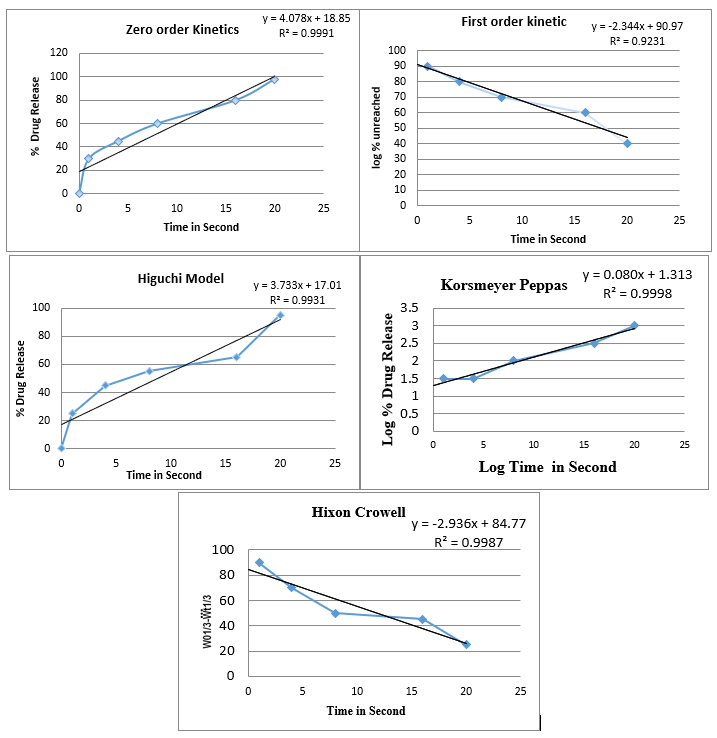
Figure 09: Graph of different Release kinetics of In-vitro Drug release
Physical Characterization Piroxicam Orodispersible Tablets:
The Piroxicam Orodispersible Tablets formulations were analyzed using UV-spectrophotometric methods to quantify both free and entrapped Piroxicam.
Result of determination of Free Piroxicam: Tablets were analyzed for free Piroxicam using a UV-spectrophotometric method at a wavelength of 354 nm against an appropriate blank. Tablets were dissolved in distilled water, and the solution was appropriately diluted to ensure the absorbance fell within the range of the standard curve. The absorbance of the diluted solution was measured at 354 nm using a UV-visible spectrophotometer.
Result of determination of Entrapped Piroxicam: Approximately 200 mg of dried Piroxicam Orodispersible Tablets were accurately weighed and dissolved in distilled water. The resulting solution was then appropriately diluted with distilled water to obtain absorbance readings within the range of the standard curve. The absorbance of the diluted solution was measured at 354 nm using a UV-visible spectrophotometer. By employing these methods, both free and entrapped Piroxicam in the Orodispersible Tablets were quantified, providing valuable insights into the formulation's efficacy and ensuring the desired drug release profile is achieved.
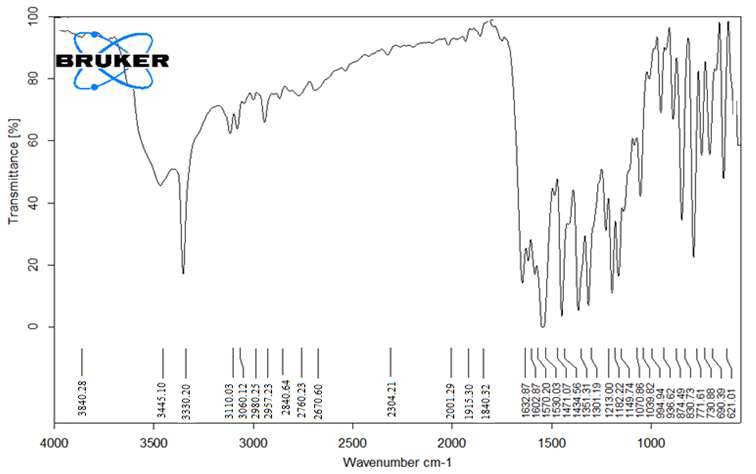
Figure 10: FT-IR-Spectra of Piroxicam from Orodispersible Tablets
Look for characteristic peaks associated with the functional groups present in Piroxicam For: O-H Stretch: Broad peak around 3200-3500 cm-1 due to the stretching vibration of the O-H bond in the carboxylic acid group. C=O Stretch: Strong, sharp peak around 1700-1720 cm-1 due to the stretching vibration of the carbonyl group (C=O) in the carboxylic acid. C-H Stretch: Typically observed in the range of 2900-3000 cm-1, representing the stretching vibration of C-H bonds, mainly from the aromatic rings and methyl groups. These peaks provide diagnostic information about the functional groups present in Piroxicam, aiding in its identification and characterization using FT-IR spectroscopy
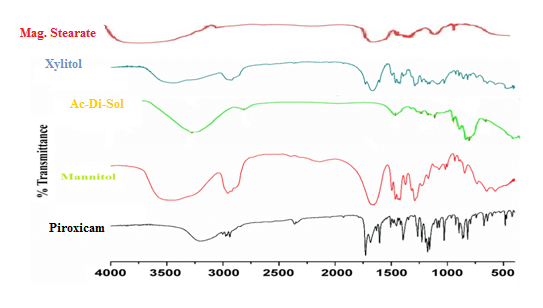
Figure 11: FT-IR-Spectra for Piroxicam physical mixture.
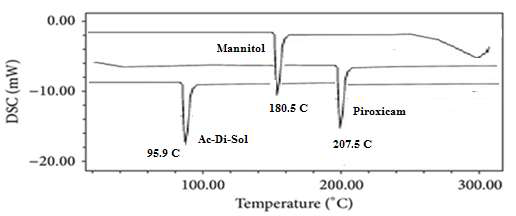
Figure 12: DSC for physical mixture of Piroxicam Orodispersible Tablets.
Differential Scanning Calorimetry (DSC) is a thermal analysis technique used to study the thermal behavior of materials, including their melting points, glass transition temperatures, crystallization, and thermal stability. Here's a general overview of what you might observe in a DSC analysis of Piroxicam, Mannitol, and Ac-Di-Sol:
Piroxicam: Melting Point: Piroxicam typically exhibits a sharp endothermic peak corresponding to its melting point, which is around 207.5°C. Mannitol: Melting Point: Mannitol typically shows a well-defined endothermic peak around 180.5 °C, corresponding to its melting point. Ac-Di-Sol: Melting Point: Ac-Di-Sol generally exhibits a broad endothermic peak around 90.5°C, corresponding to its melting point.
Compatibility Study Piroxicam Orodispersible Tablets: The compatibility study of Piroxicam Orodispersible Tablets formulation, where the drug and excipients were mixed in equal proportions and stored under different environmental conditions (40°C/RH 60% and 40°C/RH 75%) for 3 month, revealed no interaction between the drug and excipients. Weekly analysis of the samples for curcumin content using UV-visible spectrophotometer showed consistent levels, indicating stability of the formulation under the tested conditions. Additionally, no significant changes in the physical appearance of the samples were observed throughout the study period. Furthermore, the FT-IR spectrum recorded for Piroxicam Orodispersible Tablets formulation F9 confirmed the absence of any notable interactions between the drug and excipients, supporting the overall compatibility of the formulation.

Figure 13: FT-IR spectra of drug with excipients in Piroxicam Orodispersible Tablets formulation.
Stability Studies Piroxicam Orodispersible Tablets: 20-22
Stability study of Piroxicam Orodispersible Tablets formulations for 3 month at different temperatures 40, 250 C /RH 60%, and 400C /RH 75%Samples of the formulations stored at various conditions of temperature and humidity were taken out at weekly intervals and the concentration of piroxicam in these were determined using UV-Visible spectrophotometer. The samples were dissolved in water and suitably diluted to read absorbance against water blank at 354 nm. Formulation was subjected to stability studies at 40 oC ± 2 oC / 75% RH ± 5% for 3 months.
The product was evaluated for following parameters:
- Weight variation
- Hardness
- Friability
- Drug content
- Dissolution analysis
Storage condition at 40°C ± 2°C/75 %RH ± 5%:

Table no. 13: Stability Study of Optimized Batch of F9 Piroxicam Orodispersible Tablets:
Each data represents Mean ±SD (n=3).
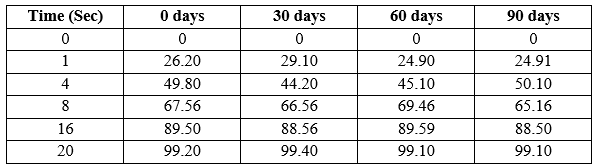
Table 14: % drug release during Stability Study of Optimized Batch of F9 Piroxicam Orodispersible Tablets by dissolution testing:

Figure 14: % drug release during Stability Study of Optimized Batch of F9 Piroxicam Orodispersible Tablets by dissolution testing.
The stability studies for optimized formulation F9 was carried out based accelerated stability conditions and study of various parameters carried out at 0, 30, 60, 90 days of intervals and the results found satisfactorily and that reveals that the optimized formulation was stable under accelerated condition.
DISCUSSION:
The study on Piroxicam Orodispersible Tablets (ODTs) focuses on improving patient compliance, particularly for those with swallowing difficulties. Key points include: Objective: Develop ODTs of Piroxicam to aid patients with swallowing issues, especially the elderly and mentally ill. Material Procurement and Confirmation: Excipients and Piroxicam were procured and their identities confirmed using UV-visible and FT-IR spectroscopy. Formulation Development: Multiple formulations were created and optimized for rapid disintegration and dissolution. Formulation F6 was identified as optimal. Optimization: Experimental designs identified formulation F9 as the best due to its superior disintegration properties and composition. Preformulation Studies: Assessed parameters like tablet appearance, melting point, taste, size, weight uniformity, hardness, friability, wetting time, and disintegration time. Dissolution Test: Conducted using the USP paddle method to determine the dissolution profile of the ODTs.
Compatibility Study: Confirmed the stability of the drug and excipients under various conditions. Physical Characterization: Employed UV-spectrophotometric methods and FT-IR spectroscopy for analysis. Stability Studies: Conducted over three months at different temperatures and humidity levels to assess shelf-life. Overall, the study provides a detailed understanding of the formulation, characterization, and stability of Piroxicam ODTs, highlighting their potential to improve patient adherence and medication effectiveness.
CONCLUSION:
In conclusion, the development and evaluation of Piroxicam Orodispersible Tablets (ODTs) present a significant advancement in addressing swallowing difficulties, particularly among elderly and mentally ill patients. The systematic formulation and optimization process focused on achieving rapid disintegration and dissolution, ultimately enhancing patient compliance with medication regimens. Key highlights of this study include confirming ingredient identity, optimizing composition through Preformulation studies, conducting dissolution testing, compatibility studies, and physical characterization. Among the formulations tested, Formulation F9 emerged as promising due to its favorable disintegration properties and composition. Stability studies conducted over a period of three months confirmed the robustness of Formulation F9, further validating its potential for clinical use. Overall, this study provides valuable insights into the development of Piroxicam ODTs, which can significantly improve patient adherence and medication delivery. It serves as a foundation for further advancements in ODT development, ultimately enhancing patient care and treatment outcomes.
CONFLICT OF INTEREST:
Authors don’t have any conflict of interest
ACKNOWLEDGEMENT:
I would like to express my heartfelt gratitude to KVPS’s Institute of Pharmaceutical Education, Boradi, including all teaching and non-teaching staff, and to Principal Dr. Vikas V Patil for their support throughout this research. A special thanks to my research guide, Prof. Kalpeshkumar S. Wagh, for his invaluable guidance and encouragement. Without their collective assistance, this work would not have been possible
REFERENCE
- Yourong Fu, Hicheng Yang, Seong Hoon Jeong, Susumu Kimura. Orally Fast Disintegrating Tablets: Developments, Technologies, Taste-Masking and Clinical Studies. Critical Reviews in Therapeutic Drug Carrier Systems, 2004; 21(6):433–475.
- Arya Arunand Chandra Amrish. Fast Drug Delivery Systems: A Review. Scholars Research Library, 2010; 2 (2):350-361.
- Dali Shukla, Subhashis Chakraborty, Sanjay Singh, Brahmeshwar Mishra. Mouth Dissolving Tablets: An Overview of Formulation Technology. Scientia Pharmaceutia, 2009; 76:309–326.
- P. Ashish, M.S. Harsoliya, J.K. Pathan, S. Shruti. A Review- Formulation of Mouth Dissolving tablet. International Journal of Pharmaceutical and Clinical Science, 2011;1(1):1-8.
- Sharma Deepak, Kumar Dinesh, Singh Mankaran, Singh Gurmeet, Rathore M.S. Fast disintegrating tablets: A new era in novel drug delivery system and new market opportunities. Journal of drug delivery & therapeutics, 2012; 2(3):74-86.
- Alokkumar Gupta, Anuj Mittal and Prof. K. K. Jha. Fast dissolving tablet- a review. The PharmaInnovation, 2012; 1: 2-5.
- Tapankumar Giri, Dulal Krishna Tripathi And Rana Majumdar. Formulation aspects in the development of Orodispersible tablets: an overview. International journal of pharmacy and pharmaceutical sciences, 2010; 2(3):38-42.
- Pooja Arora, Vandana Arora Sethi. Orodispersible tablets: A comprehensive review. International Journal of Research and Development in Pharmacy and Life Sciences, 2013; 2:270-284.
- Malay Kumar, B Chotaliya. Overview of Oral Dispersible Tablets. International Journal of Pharm Tech Research, 2012; 4:1712-20.
- S TejvirKaur, Bhawandeep Gill, Sandeep Kumar, G.D. Gupta. Mouth dissolving tablets: A novel approach to drug delivery. International journal of current pharmaceutical research, 2011; 3(1)1-7.
- Priyanka Nagar, Kusum Singh, Iti Chauhan, Madhu Verma. Orally disintegrating tablets: formulation, preparation techniques and evaluation. Journal of Applied Pharmaceutical Science, 2011; 1(4):35-45.
- Abhay Asthana, Swati Aggarwal, Gayti Asthana. Oral Dispersible Tablets: Novel Technology and Development. International. Journal of Pharmaceutical Science, 2013; 20(1):193-199.
- Md. Nehal Siddiqui, Garima Garg, Pramod Kumar Sharma. Fast dissolving tablets: preparation, characterization and evaluation. An overview. International Journal of Pharmaceutical Sciences Review and Research, 2010; 4(2):187-189.
- Kamal Saroha, Pooja Mathur, Surender Verma, Navneet Syan. Mouth dissolving tablets: An overview on future compaction in oral formulation technologies. Pelagia Research Library, 2010; 1(1):179-187.
- K.P.R. Chowdary, K. Ravi Shankar and B. Suchitra, Recent research on orodispersible tablet: A review. International Research Journal of Pharmaceutical and Applied Sciences, 2014; 4(1):64-73.
- V. N. Deshmukh. Mouth Dissolving Drug Delivery System: A Review. International Journal of Pharm Tech Research, 2012; 4(1):412-421.
- Panigrahi D, Baghel S and Mishra B. Mouth dissolving tablets: An overview of preparation techniques, evaluation and patented technologies. J Pharm Res, 2005; 4(3):35?38
- Dr. Amin FA, Shah T, Bhadani M and Patel M. Emerging trends in development of orally disintegrating tablet technology. pharminfo.net.
- anmoy Ghosh, Amitava Ghosh and Devi Prasad. A Review on new generation orodispersible tablets and its future prospective. International Journal of Pharmacy and Pharmaceutical Sciences, 2011; 3(1):1-7.
- Abdul Sayeed, Mohd. Hamed Mohinuddin. Mouth dissolving tablets: An overview. Int. Journal of Research in Pharmaceutical and Biomedical Sciences, 2011; 3(2):959-970.
- Yang S, Fu Y, Jeong SH, Park K. Applications of poly (acrylic acid) Superporoushydrogel microparticles as a super disintegrants in fast disintegrating tablets. J Pharma Pharmacol, 2004; 56:429-36.
- Ozeki T, Yasuszawa Y, Katsuyama H, Takshima Y, Kasai T, Eguchi T. Design of rapidly disintegrating oral tablets using acid treated yeast cell wall: A technical note. APPS Pharma sci Tech, 2003; 4:42-47


 Mr. Kunal D. Patil*
Mr. Kunal D. Patil*
 Kalpeshkumar Wagh
Kalpeshkumar Wagh

























 10.5281/zenodo.12787375
10.5281/zenodo.12787375