Abstract
In the present study, a simple method was developed to prepare stomach specific drug delivery. The beads were prepared by ionotropic gelation method using calcium chloride as crosslinker and gas forming calcium carbonate (CaCO3) as floating inducer. In vitro release studies of prepared beads were achieved up to 12 h. The creation of floating beads was optimized using 32 full factorial design and analysis of variance.
Keywords
Floating drug delivery system, Metoclopramide HCL, Floating Beads, Poloxamer188- Sodium alginate blending, Gastro-retentive, Ionotropic Gelation, In-vitro release.
Introduction
Gastroretentive dose forms are oral formulations that have the ability to resist fast stomach emptying and stay in the GI system. These systems are perfect for drugs with a limited window of absorption. They are designed as formulations of modified-release drug delivery systems with the ability to modify the release rate and be site-constrained in the gastrointestinal tract (stomach). Several factors affect the effectiveness of gastroretentive drug delivery systems, such as the site of medication absorption, the length of stomach transit, and the impact of food. Oral dose forms with the ability to resist fast stomach emptying are referred to as gastroretentive dosage forms. They are designed to be retained in the GI system. These systems are perfect for drugs with a limited window of absorption. The efficiency of gastroretentive drug delivery systems is influenced by a number of variables, including dietary effects, stomach transit duration, and the medication's location of absorption. The simplicity of medication administration has a significant impact on compliance. When taking medication, a patient is more likely to adjust to it if it does not disrupt regular routines. Oral dose forms continue to be the best method of administration. This is a result of a variety of variables, such as the drug's convenience in storage and transportation, controlled distribution, formulation flexibility, and generally lower cost when compared to other dosage forms. The creation of a systematic drug that may be given in a single dosage form is the common goal of drug delivery systems. Particularly when the patient is required to take the drug in question on a regular basis throughout their lifetime. Reduced frequency of medical administration would also result from an integrated single unit dose form. Also, it should be mentioned. The mechanisms for oral medication administration involve a complicated absorption process. In order for the medication to be absorbed in the stomach, small intestine, or colon, it must be soluble in gastric fluid.
Alginate beads are multi-unit floating dosage forms beads made from freeze-dried calcium alginate. These can be made by dissolving sodium alginate solution into an aqueous solution of calcium chloride and watching calcium alginate precipitate. This process results in the formation of 2.5-mm-diameter spherical beads. Low-methoxylated pectin, cellulose acetate, polycarbonate, calcium alginate, Eudragit, and agar are a few of the polymers that were used to create these systems. By adding sodium alginate solution to a calcium chloride aqueous solution, calcium alginate can be precipitated to create spherical beads. Following the separation of the beads, a porous system that can sustain a floating force for more than 12 hours is created by snap-freezing them in liquid nitrogen and freeze-drying them at 400C for 24 hours.
The Benefits of Floating Drug Delivery System.
- Drugs that are absorbed through the stomach benefit from the gastro-retentive system such as antacids and ferrous salts.
- Beneficial for drugs that act locally effective in the stomach, such as antacids.
- Because of the intestine's alkaline pH, floating medications of all kinds, including capsules and tablets, will remain in the fluid for a considerable amount of time.
- In order to retain the medication in a floating state in the stomach and promote a stronger response, the FDDS formulation is effective in promoting intestinal movement and diarrhoea. In order to increase patient compliance, FDDS decreases the dosing frequency.
- Care for gastrointestinal issues such as gastroesophageal reflux disease.
- Despite the first-pass effect, the plasma drug concentration has little impact on bioavailability.
- Hydrodynamically balanced system / Floating Drug Delivery System (HBS/FDDS) formulations may be helpful for administering aspirin and other medications of a similar nature because they are acidic and irritate the stomach.
- The medication is transported to a specific location. [1,7]
METHODOLOGY
Materials
Table 1: List of Materials
|
Materials
|
Supplier
|
|
Metoclopramide Hydrochloride
|
Ronak Healthcare, Gujrat
|
|
Sodium alginate
|
Loba Chemie Pvt. Ltd
|
|
Calcium carbonate
|
SD Chem Lab India
|
|
Calcium chloride
|
Loba Chemie Pvt. Ltd
|
|
Poloxamer 188
|
Vishal chem, Mumbai
|
Methods:
Identification of Pure Metoclopramide Hydrochloride
Description:
Metoclopramide Hydrochloride white or almost white crystalline powder. Very soluble in water, freely soluble in alcohol.
Solubility:
Solubility of pure Metoclopramide Hydrochloride was checked in water.
Melting point of pure Metoclopramide Hydrochloride:
The sample obtained was characterized for melting of the substance. The melting point was determined by introducing small amount of substance in the capillary attached to graduated thermometer and constant heat was applied with assembly suspended in Thiele’s tube containing paraffin bath. The temperature required to melt the substance completely was noted.
Study of UV spectrum of pure Metoclopramide Hydrochloride: [17]
Accurately weighted 100 mg of Metoclopramide Hydrochloride was transferred in to the 100 ml volumetric flask and volume was made up to 100 ml with 0.1 N HCl. From this solution, 1 ml was withdrawn and added to the 10 ml volumetric flask and diluted to 10 ml with 0.1 N HCl. Finally, volume was scanned in the range of 200-400 nm. The wavelength of the maximum absorption was noted, and UV spectrum was taken.
Study of IR spectrum of pure Metoclopramide Hydrochloride:
IR spectroscopy is one of the most powerful analytical techniques, which offers the possibility of detecting chemical interactions. The purity of the chemical was determined by analyzing the IR spectra of Metoclopramide Hydrochloride. A Fourier transform infrared spectrophotometer was used to record the IR spectra of Metoclopramide Hydrochloride. Utilizing dried potassium bromide, a baseline correction was made.
Standard curve of pure Metoclopramide Hydrochloride in 0.1N HCl [17]
Standard curve of Metoclopramide Hydrochloride in 0.1N HCl:
Accurately weighted 100 mg of Metoclopramide Hydrochloride was added to the 100 ml volumetric flask. Volume was made up to 100 ml with 0.1 N HCl (1000 µg/ml) pH 1.2. From this solution 1 ml was withdrawn and added into 10 ml volumetric flask and volume was made to 10 ml with 0.1 N HCl (100 µg/ml). This solution was used as stock solution. From the stock solution 0.2, 0.4, 0.6, 0.8, 1.0, 1.2, 1.4, 1.6 ml were withdrawn and added to 10 ml volumetric flask and finally diluted to 10 ml with 0.1 N HCl to get the solution with concentration of 1-10 µg/ml respectively. Each solution’s absorbance at 272 nm was measured using a UV-visible spectrophotometer. The graph was plotted for absorbance verses concentration
Compatibility study
IR spectral analysis:
Compatibility of drug with excipients was carried out using IR spectroscopy. The Infrared spectra of formulations recorded with KBr method were captured using Fourier Transform Infrared Spectrophotometer (spectrum one, made by Thermo Fisher Scientific India). A baseline correction was made by using dried potassium bromide. Scanning was done from 500 to 3500cm-1
Differential Scanning Calorimetry (DSC) Thermal analysis:
Differential Scanning Calorimetric analysis (DSC) was used to study the interaction between drug and polymer. Thermo-grams were recorded for pure Metoclopramide Hydrochloride and Metoclopramide Hydrochloride with the excipients to check the compatibility of Metoclopramide Hydrochloride with them using Differential Scanning Calorimeter (KEP Technologies, France) by using setaram software data acquisition. Under nitrogen purge with a constant flow rate of 5 ml/min, thermal analysis was carried out using an empty pan as a reference and a scan rate of 500C per minutes from 20 to 5000C.
Factorial Design:
In statistics, a full factorial experiment is an experiment whose design consists of two or more factors, each with discrete possible values or "levels", and whose experimental units take on all possible combinations of these levels across all such factors. A full factorial design may also be called a fully-crossed design. In this method two or more factors are selected. The selected factors may also be called as variables. The number of selected factors is varied in each formulation at specific predetermined levels keeping the amount of other ingredients constant. These levels are selected with preliminary studies of factors. The levels could be selected as low/ medium/ and high and coded as -1 / 0 / +1. With the help of this method, one could detect the effect of individual variable in each formulation and also the combined or interactive effect of variables in each formulation. Thus, the factorial method is used to design the formulation and to evaluate the effect of factors in the formulation.
A study, in which there are 2 factors with 3 levels, is called a 32 factorial design. A 32 full factorial design (FFD) was constructed where the amounts of Sodium Alginate (X1) and Poloxamer 188 (X2) were selected as the factors. The levels of the two factors were selected based on the preliminary studies carried out before implementing the experimental design. All other formulation and processing variables were kept invariant throughout the study.
Table 2. Amount of variables in 32 factorial design batches
|
Coaded Values
|
Actual Value(mg)
|
|
X 1
|
X2
|
|
-1
|
1%
|
30
|
|
0
|
2%
|
40
|
|
+1
|
3%
|
50
|
Table 3. A 32 full factorial experimental design layout
|
Formulation code
|
Coded Values
|
|
X1
|
X2
|
|
F1
|
1% (-1)
|
30(-1)
|
|
F2
|
1% (-1)
|
40(0)
|
|
F3
|
1% (-1)
|
50(+1)
|
|
F4
|
2% (0)
|
30(-1)
|
|
F5
|
2% (0)
|
40(0)
|
|
F6
|
2% (0)
|
50(+1)
|
|
F7
|
3% (+1)
|
30(-1)
|
|
F8
|
3% (+1)
|
40(0)
|
|
F9
|
3% (+1)
|
50(+1)
|
Method of Preparation of floating beads: [12,19,20]
Ionotropic Gelation Method:

Figure 1. Floating Beads of Metoclopramide Hydrochloride
The floating beads of Metoclopramide Hydrochloride were prepared using the sodium alginate as gelling agent, calcium chloride as crosslinking agent, calcium carbonate as gas forming. Metoclopramide Hydrochloride alginate beads were made using the ionotropic gelation technique. Sodium alginate was dissolved in 100 ml of distilled water and stirred continuously. Next, polymer was added to the solution, shown in Table no. 7 and stirred up to 20 minutes. Metoclopramide Hydrochloride and calcium carbonate were added to the above solution. The mixture was set aside for 25 minutes to allow air bubbles to escape. This mixture was extruded into a solution of calcium chloride using a syringe. The beads were gathered, washed with distilled water, and then set to dry. A 32 factorial design was applied to develop the formulation. Sodium alginate (gelling agent) and poloxamer 188 (polymer) were selected as two factors. The amount of these two factors were varied at 3 different levels (as low, medium and high). Total nine formulations were prepared and the effect of two factors along with their combined effect were studied in the formulation.

Figure 2. Formulated Floating beads of Metoclopramide Hydrochloride
Table 4. Formulation of floating beads of Metoclopramide Hydrochloride.
|
Sr. No
|
Ingredients
|
Formulation
|
|
F1
|
F2
|
F3
|
F4
|
F5
|
F6
|
F7
|
F8
|
F9
|
|
1
|
Metoclopramide Hydrochloride (mg)
|
40
|
40
|
40
|
40
|
40
|
40
|
40
|
40
|
40
|
|
2
|
Sodium alginate
|
1%
|
1%
|
1%
|
2%
|
2%
|
2%
|
3%
|
3%
|
3%
|
|
3
|
Poloxamer 188
|
30
|
40
|
50
|
30
|
40
|
50
|
30
|
40
|
50
|
|
4
|
Calcium chloride
|
3%
|
3%
|
3%
|
3%
|
3%
|
3%
|
3%
|
3%
|
3%
|
|
5
|
Calcium carbonate (gm)
|
1
|
1
|
1
|
1
|
1
|
1
|
1
|
1
|
1
|
In vitro evaluation of formulated floating beads of metoclopramide hydrochloride.
Uniformity of content: [49]
The drug content of the beads was determined by taking 50 mg beads of each formulation. 50 mg from each formulation were accurately weighed and triturated in a mortar and pestle. In the 100 ml of 0.1N HCL, the beads powder was added. It was stirred for 2hours using a magnetic stirrer. From this solution, 1 ml was withdrawn and added to a 10 ml volumetric flask, and finally the volume was made to 10 ml with 0.1 N HCL (10?g/ml), then the solution was filtered with Whatman filter paper, and the absorbance of the resulting solution was measured at 272nm using a UV spectrophotometer.
Drug content = Actual drug content / Total Weight of beads ×100
Entrapment efficiency: [12]
To make a fine powder, the beads were smashed with a mortar and pestle. Approximately 100 mg of powder was properly weighed and quantitatively placed into a 100-ml volumetric flask, and the volume was made up with 0.1 N HCL. After 24 hours, the solution was filtered using Whatman filter paper. 1 ml of filtrate was pipetted out and diluted to 10 ml. The solution was analyzed spectrophotometrically at 272 nm using a UV-visible spectrophotometer (Shimadzu Double beam UV 1800, Japan). The method's precision and accuracy were tested. The entrapment efficiency was determined using the following formula.
Drug entrapment = (Practical drug content / Theoretical drug content) 100
In Vitro buoyancy study: [12, 21]
50 individual floating beads were introduced to 100 ml of simulated stomach fluid (pH 1.2), which was kept at 37.50C and swirled at 100 rpm with a magnetic stirrer. The quantity of floating beads was visually counted every hour for the next 12 hours. The number of beads floated were calculated by the following equation.
% Floating beads = Floating beads / Total beads × 100
In vitro dissolution study: [12]
In vitro dissolution of Metoclopramide Hydrochloride floating beads was studied using the USP Type II paddle dissolution apparatus. Beads equivalent to 40 mg of metoclopramide hydrochloride were suspended in 900 ml of 0.1 N HCL (pH 1.2). The dissolution medium was stirred at 100 rpm and maintained at 37±0.5?C. 5 ml aliquots were withdrawn at a specific time interval with an equal volume of fresh, pre-warmed dissolution medium to maintain the sink condition. The samples were filtered through membrane filter paper, diluted appropriately, and examined at 272 nm with a UV Spectrophotometer (Shimadzu Double Beam UV1800 Japan). All dissolution tests were performed in triplicate.
Scanning electron microscopy (SEM) [21]
The morphological characteristics of metoclopramide hydrochloride floating beads were investigated using JSM 6330 JEOL (Japan) Scanning Electron Microscope. The sample for this investigation was prepared by dusting microsphere powder on two-fold sticky tape that was fastened to an aluminum stub. The analyzer also recorded photomicrographs with a 2000A thickness and gold covering around the stub using a sputter coater.
DATA ANALYSIS:
The effect of formulation variables on the response variables were statistically evaluated by applying ANOVA at 0.05 level using a commercially available software package Design-Expert® version 360 (Stat-Ease Inc.). To describe the response surface curvature, the design was evaluated by quadratic model, which bears the form of equation:
Y = b0 + b1X1 + b2X2 + b3X12 + b4X22 + b5X1X2……………
Where, Y is the response variable, b0 the constant and b1, b2, b3, b4, b5 the regression coefficient. X1 and X2 stand for the main effect; X1X2 are the interaction terms, show how response changes when two factors are simultaneously changed. X12 and X22 are quadratic terms of the independent variables to evaluate the nonlinearity.
The polynomial equation was established by applying ANOVA using the Design-Expert software version 7.1.6. Also, the data were subjected to 3-D response surface graph and contour plots to study the interaction of independent variables i.e., Sodium Alginate (X1) and Poloxamer 188 (X2) on dependent variable.
RESULTS AND DISCUSSION
Characterization of Drug
Description: Metoclopramide Hydrochloride is a white or almost white odorless powder.
Solubility: Freely soluble in alcohol, very soluble in water.
Taste: Metoclopramide Hydrochloride is intensely bitter in taste.
Melting point: Melting point of Metoclopramide hydrochloride was found to be 1020C
UV Spectrophotometry of the Metoclopramide Hydrochloride.
Determination of ? max
The maximum absorbance 0.490 and minimum absorbance 0.075 was found to be at 272 nm.

Figure 3. UV Spectrum of Metoclopramide Hydrochloride
Standard Curve of Metoclopramide Hydrochloride.
Standard calibration curve of Metoclopramide Hydrochloride in 0.1 N HCl was plotted by using the observations recorded in table and as shown in figure.
Table 5. Observation for standard calibration curve of metoclopramide hydrochloride.
|
Sr. No
|
Concentration (mg/ml)
|
Absorbance
|
|
1
|
0
|
0
|
|
2
|
2
|
0.075
|
|
3
|
4
|
0.125
|
|
4
|
6
|
0.181
|
|
5
|
8
|
0.246
|
|
6
|
10
|
0.320
|
|
7
|
12
|
0.372
|
|
8
|
14
|
0.459
|
|
9
|
16
|
0.490
|

Figure 4. Calibration curve of Metoclopramide Hydrochloride in 0.1N HCL
Table 6. Standard curve statistics
|
Sr. No
|
Parameters
|
Observation
|
|
1
|
Absorbance maximum
|
0.490
|
|
2
|
Slope
|
0.031208
|
|
3
|
Intercept
|
0.002333
|
|
4
|
Coefficient of Correlation (r2)
|
0.9965
|
Infrared Spectrum Analysis
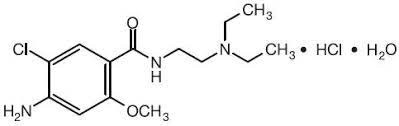
Figure 5. Chemical Structure of Metoclopramide Hydrochloride
The infrared spectrum of the pure drug Metoclopramide Hydrochloride was studied and it was found that all the various functional groups present in the structure of Metoclopramide Hydrochloride were present. Thus, through infrared spectroscopy the purity of compound was identified and presence of any impurities in the sample was overruled. The details of the peak observed and the corresponding functional groups are as in the table given below.
Table 7. Interpretation of IR spectrum of Metoclopramide Hydrochloride and formulation F9
|
Sr. no
|
Wave number (cm-1)
|
Interpretation
|
Peak observed
|
|
cm-1
|
|
1
|
3500 – 3000
|
OH stretching vibration
|
3185.95
|
|
2
|
2000 – 1500
|
NH bending vibration
|
1589.96
|
|
3
|
2000 – 1500
|
C=C stretching
|
1533.66
|
|
4
|
1500 – 1000
|
-OCH3 stretching
|
1254.80
|
|
5
|
1500 – 1000
|
-CN stretching
|
1208.28
|
Thermal Analysis
A sharp endothermic peak was observed at 105º C which corresponds to the melting point of Metoclopramide Hydrochloride. Thus, the purity of the drug was ascertained
Evaluation of floating beads
Compatibility study using Infrared Spectrum Analysis

Figure 6. IR Spectrum of Metoclopramide Hydrochloride

Figure. 7 IR spectrum of formulation F9 batch

Figure 8. IR of Spectrum Pure Metoclopramide Hydrochloride
Table 8. Interpretation of IR spectrum of Metoclopramide Hydrochloride and formulation F9
|
Sr. no
|
Wave number (cm-1)
|
Interpretation
|
Peak observed
|
|
cm-1
|
Drug
|
Formulation
|
|
1
|
3500 – 3000
|
OH stretching vibration
|
3188.15
|
Yes
|
Yes
|
|
2
|
2000 – 1500
|
NH bending vibration
|
1587.82
|
Yes
|
Yes
|
|
3
|
2000 – 1500
|
C=C stretching
|
1533.87
|
Yes
|
Yes
|
|
4
|
1500 – 1000
|
-OCH3 stretching
|
1251.28
|
Yes
|
Yes
|
|
5
|
1500 – 1000
|
-CN stretching
|
1207.48
|
Yes
|
Yes
|
The infrared spectrum of pure drug metoclopramide hydrochloride was studied and it was found that all the important peaks that correspond to various functional groups present in the structure of Metoclopramide Hydrochloride was present. The drug exhibits peaks due to the 3185.95 OH stretching vibration, 1589.96 NH bending vibration (Primary and secondary amide), 15.33 C=C aromatic stretching, 1254.80 -OCH3 stretching, 1208.28 -CN amine stretching. In the IR study, it was found that there was no exhibited interaction between metoclopramide hydrochloride and excipients used. Physical mixture showed band at 3188.15 OH stretching vibration, 1587.82 NH bending vibration, 1533.87 C=C stretching, 1251.28 -OCH3 stretching, 1207.48 -CN stretching. [38]

Figure. 9 DSC of Metoclopramide Hydrochloride
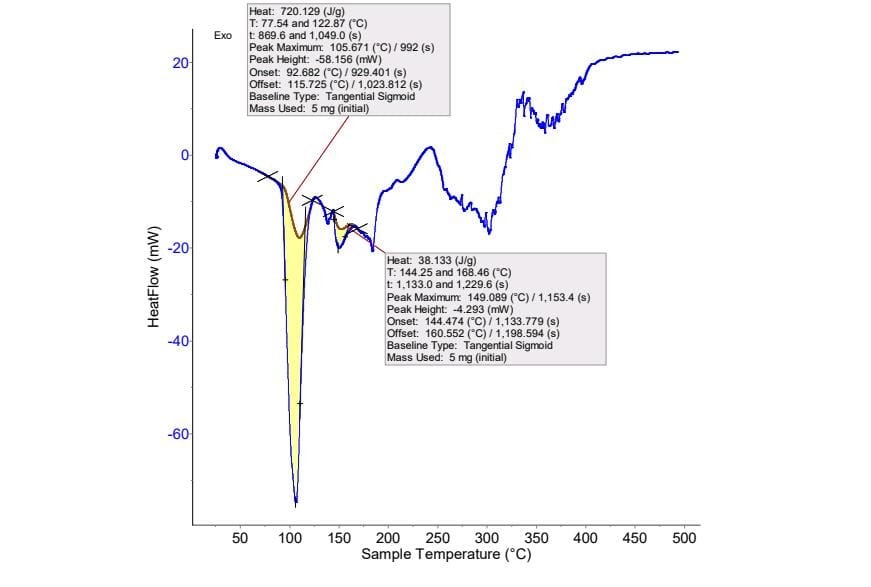
Figure.10 DSC of Formulated F9 batch
Thermal Analysis
A sharp endothermic peak was observed at 105º C of Metoclopramide hydrochloride. Supporting evidence for compatibility between drug and excipients was obtained from DSC studies. In DSC thermograms of pure Metoclopramide hydrochloride (Fig.), and formulations (Fig.), no any shift in the endothermic peaks of drug was found, which suggested that there was no any interaction between drug and formulation mixture. [69]
Characterization of formulated floating Beads of Metoclopramide Hydrochloride
Particle size:
|
Formulations
|
Percentage Yield
|
|
F1
|
88.32±0.98%
|
|
F2
|
91.84±1.09%
|
|
F3
|
86.76±1.23%
|
|
F4
|
94.98±0.76%
|
|
F5
|
98.47±1.56%
|
|
F6
|
94.98±1.32%
|
|
F7
|
97.63±0.62%
|
|
F8
|
94.76±0.98%
|
|
F9
|
98.20±1.26%
|
Table 9: Particle Size Analysis
All formulations were round in particle shape with smooth surface. The mean particle size of beads of metoclopramide hydrochloride ranged from 91.12 to 113.00 µm (Table 9). The mean diameter of prepared beads was increased with an increase in sodium alginate concentration (F1-F9) due to increased viscosity of solution which influence the interaction between disperse phase and dispersion medium that affect the size distribution of particle. the sodium alginate concentration to 1% W/V resulted in clumping of the beads after drying whereas high concentration alginate (2% to 3%W/V) resulted free flowing beads. This could be attributed to an increase in relative viscosity at higher concentration of polymer and formation of larger particle. [38,69]
Percentage Yield
|
Formulation
|
Particle Size (µm)
|
|
F1
|
87.14±0.34
|
|
F2
|
91.86±0.24
|
|
F3
|
87.36±0.31
|
|
F4
|
91.12±0.21
|
|
F5
|
97.32±0.15
|
|
F6
|
99.00±0.21
|
|
F7
|
101.18±0.09
|
|
F8
|
112.28±0.18
|
|
F9
|
113.00±0.34
|
Table 10. Percentage yield
The studies were conducted, and the maximum percentage yield was found to be 98.20% with F9 batch and minimum of 86.76% with batch F3. [68]
Drug Content
The data for drug content of floating beads of Metoclopramide hydrochloride is as shown below.
Table 11: Drug Content
|
Formulations
|
Drug content (%)
|
|
F1
|
72.49±0.25
|
|
F2
|
69.28±0.39
|
|
F3
|
75.46±0.17
|
|
F4
|
79.57±0.08
|
|
F5
|
81.56±0.17
|
|
F6
|
85.01±0.10
|
|
F7
|
91.73±0.23
|
|
F8
|
93.83±0.11
|
|
F9
|
95.09±0.17
|
Determination of drug content was carried out to quantify the amount Metoclopramide Hydrochloride in prepared beads, it was found to be in range 69.28% to 95.05%. The all nine batches’ results are shown in Table 11, indicating drug content improved with an increase in sodium alginate and poloxamer 188 concentrations. Enhanced drug content with an increase in sodium alginate concentration could be due to enhanced availability of the calcium binding site in anionic liner polysaccharide chain and consequently, an additional degree of crosslinking by raising the sodium alginate fraction. [69
Table No 12: Entrapment Efficiency
|
Formulations
|
Entrapment Efficiency (%)
|
|
F1
|
80.27±0.075
|
|
F2
|
78.42±0.78
|
|
F3
|
79.07±0.20
|
|
F4
|
88.20±0.05
|
|
F5
|
82.00±0.22
|
|
F6
|
86.42±0.16
|
|
F7
|
91.10±0.07
|
|
F8
|
90.60±0.34
|
|
F9
|
96.12±0.23
|
All values are expressed as mean ± SD (n=3)
The determined encapsulation efficiency of prepared metoclopramide HCl beads is elaborated in Table.15. The effect of polymer content on encapsulation efficiency was studied. The encapsulation efficiency in 0.1 N HCl ranged from 78.42 to 96.12%. On examination of the results, an increase in percent entrapment efficiency from 78.42 to 96.12 as the polymer concentration increased was observed. It is evident that with the increase in polymer concentration, entrapment efficiency also increased. Additionally, the increased viscosity, which is directly related to polymer concentration, hindered drug mobility, which had an impact on entrapment efficiency. [67, 68]
In Vitro Buoyancy Study
Table 13. In vitro buoyancy study
|
Formulations
|
Floating lag time (min)
|
Floating Time
|
In vitro Buoyancy Study (%)
|
|
F1
|
2:10±0.34
|
?12
|
100±0.43
|
|
F2
|
1:39±0.12
|
?12
|
99±0.28
|
|
F3
|
1:52±0.43
|
?12
|
99.66±0.11
|
|
F4
|
2:21±0.29
|
?12
|
99.34±0.37
|
|
F5
|
2:12±0.98
|
?12
|
98.66±0.45
|
|
F6
|
2:20±0.61
|
?12
|
99±0.81
|
|
F7
|
1:33±0.55
|
?12
|
100±0.68
|
|
F8
|
1:29±0.23
|
?12
|
99±0.39
|
|
F9
|
1:14±0.58
|
?12
|
100±0.47
|
All values are expressed as mean ± SD (n=3)
The table no 13 shows the in vitro buoyancy data of Metoclopramide Hydrochloride beads in simulated gastric fluid pH 1.2. all formulation had a lower density than simulated gastric fluid. The in vitro floating study revealed that all formulation had excellent floating ability. due to the formation of air bubbles during preparation, the beads containing gas producing agent floated for longer than 12 hours. [12]

Figure 13. Floating beads Initial Time (F1, F2, F3)

Figure 14. Floating beads after 12hr (F7, F8, F9)
Scanning electron microscopy (SEM)
The scanning electron microscopy (SEM) photographic images of optimized formulation F9. Drug loaded Metoclopramide Hydrochloride beads are represented in figure A demonstrated that round in shape. Surface morphology of dried beads figure B and C demonstrated rough surface, visible wrinkles, and rough appearance caused by embedded metoclopramide HCl. The magnification capacity of microscopic fields allows for a clear and concise examination of various images. The image 1B at 200x magnification indicating and figure. C shows the tidy material at 500x magnification with inner penetration of sodium alginate and metoclopramide.

Figure. Scanning electron microscopic photograph of drug loaded floating beads (magnification X45, X95, X200, X500)
In vitro Dissolution Study:

Figure 16. Dissolution test apparatus
Table 14: In vitro dissolution profile of formulation F1, F2, F3
|
Time
|
Cumulative % Drug Released
|
|
|
F1
|
F2
|
F3
|
|
0
|
0
|
0
|
0
|
|
2
|
42.7±0.63
|
37.08±0.98
|
36.17±0.45
|
|
4
|
69.56±0.24
|
64.07±0.78
|
63.28±0.56
|
|
6
|
76.32±0.28
|
71.26±0.5
|
69.14±0.49
|
|
8
|
84.56±0.66
|
76.32±0.92
|
72.60±0.23
|
|
10
|
86.38±052
|
84.8±0.61
|
77.40±0.39
|
|
12
|
92.02±0.37
|
86.79±0.57
|
81.75±0.16
|
All values are expressed as mean ± SD (n=3)
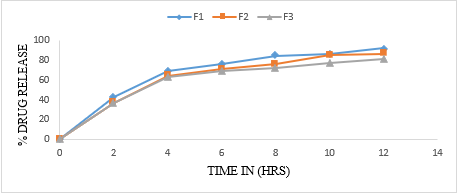
Figure 17. In vitro dissolution profile of F1-F3
Table 15: In vitro dissolution profile of formulation F4, F5, F6
|
Time
|
Cumulative % Drug Released
|
|
|
F4
|
F5
|
F6
|
|
0
|
0
|
0
|
0
|
|
2
|
41.12±0.12
|
38.12±0.39
|
39.98±0.26
|
|
4
|
64.96±0.43
|
59.83±0.67
|
54.12±0.49
|
|
6
|
75.67±0.47
|
73.18±0.61
|
67.97±0.23
|
|
8
|
79.09±0.81
|
73.43±0.28
|
71.12±0.63
|
|
10
|
82.16±0.32
|
80.35±0.97
|
78.4±0.22
|
|
12
|
89.23±0.29
|
83.31±0.97
|
82.28±0.76
|
values are expressed as mean ± SD (n=3)
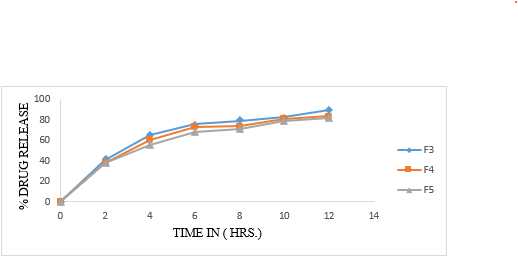
Figure 18. In Vitro-dissolution profile of F4-F6
Table 16: In vitro dissolution profile of formulation F7, F8, F9
|
Time
|
Cumulative % Drug Released
|
|
|
F7
|
F8
|
F9
|
|
0
|
0
|
0
|
0
|
|
2
|
38.67±0.34
|
35.12±0.11
|
33.82±0.34
|
|
4
|
68.19±0.45
|
65.7±0.34
|
61.16±0.54
|
|
6
|
74.3±0.29
|
71.49±0.86
|
69.46±0.25
|
|
8
|
79.96±0.22
|
75.06±0.28
|
72.18±0.96
|
|
10
|
89.12±0.17
|
79.98±0.22
|
76.4±0.89
|
|
12
|
92.32±0.38
|
84.12 ±0.26
|
79.87±0.52
|
All values are expressed as mean ± SD (n=3)

Figure 19. In Vitro-dissolution profile of F7-F9
The floating beads were subjected to in-vitro release using paddle type 2 dissolution apparatus in 900ml of 0.1 N HCl medium. The dissolution profile of pure metoclopramide hydrochloride alginate beads given in table 17, 18 and 19. The release of all formulation observed was between 63.12% to 73.98%. The polymer concentration (1%W/V sodium alginate) shows release 71.73, 68.61 and 73.98 respectively. From F4 to F6, the values are 63.12, 64.98 and 62.66. From formulation F7 to F9 shows the release 66.44, 69.34, and 63.18 respectively. It was chosen as an ideal formulation showing an extended drug release over a period of 8 hours with acceptable floating property.
Data Analyses of Formulations:
Traditional designing of the pharmaceutical formulations is based on time consuming approach of changing one variable at a time which does not take into consideration the joint effect of independent variables. Thus, factorial design can serve as an essential tool to understand the complexity of the pharmaceutical formulations. The results can be expressed either as simple linear or second order polynomial equation to statistically evaluate the responses obtained after experiments. A 32 full factorial design was selected, and the 2 factors were evaluated at 3 levels. The amount of Sodium alginate (X1) and Poloxamer 188 (X2) were selected as independent variables and the dependent variables were rel12hr. The data obtained was treated using Stat-Ease Design Expert 360 software and analyzed statistically using analysis of variance (ANOVA)
Table 17. Design Summary
|
Factor
|
Name
|
Unit
|
Type
|
Coded Level
|
Actual Level
|
|
Low
|
Medium
|
High
|
Low
|
Medium
|
High
|
|
X1
|
Sodium alginate
|
%
|
Numerical
|
-1
|
0
|
+1
|
1%
|
2%
|
3%
|
|
X2
|
Poloxamer 188
|
Mg
|
Numerical
|
-1
|
0
|
+1
|
30
|
40
|
50
|
Table 18. Response Summary
|
Response
|
Name
|
Unit
|
Observations
|
Analysis
|
Minimum
|
Maximum
|
|
Y1
|
Rel12hr
|
Hrs.
|
9
|
Polynomial
|
79.87
|
92
|
Table 19. The responses of all formulations
|
Responses
|
X1
|
X2
|
Rel12hr (%)
|
|
F1
|
1% (+1)
|
30 (+1)
|
92.02
|
|
F2
|
1% (+1)
|
40 (0)
|
86.76
|
|
F3
|
1% (+1)
|
50 (-1)
|
81.75
|
|
F4
|
2% (0)
|
30 (+1)
|
89.23
|
|
F5
|
2% (0)
|
40 (0)
|
83.31
|
|
F6
|
2% (0)
|
50 (-1)
|
82.28
|
|
F7
|
3% (-1)
|
30 (+1)
|
92.32
|
|
F8
|
3% (-1)
|
40 (0)
|
87.50
|
|
F9
|
3% (-1)
|
50 (-1)
|
79.87
|
The data clearly indicates that Rel12hrs are strongly dependent on the selected independent variables. The equations related with responses of rel 12hrs to transformed factors are shown below.
Drug Rel12hrs = +85.25+0.3400X1-4.04X2-0.5675X1X2+2.42X12+0.2800X22 [R2=0.8446]…………………….(1)
[ Adjusted R2 = 0.5856, Predicted R2 = - 0.5899, F= 3.26, P = 0.1797]
From the response surface model plot of drug release it is observed that change in concentration of sodium alginate affects the drug release. The information, the equation conveyed was the basis to study the effects of variables. The regression coefficient values are the estimates of the model fitting. The R2 was high indicating the adequate fitting of the quadratic model.
All the polynomial equations were found to be statistically significant (P< 0>
7.8. ANOVA Study:
Evaluation and interpretation of research findings are utmost important, and P-value serves a valuable purpose in these findings. ANOVA and Multiple regression analysis were done using Stat-Ease Design Expert 360 software. Table represents ANOVA for the dependent variables rel12hrs. The coefficient of X1 and X2 were found to be significant at P values is greater than 0.1000 indicate the model term is not significant effect of the variables on the selected responses. Increasing the amount of poloxamer 188 resulted in the decrease in the release of Metoclopramide hydrochloride. Overall, both the variables caused significant change in the responses.
Table 20: Analysis of variance for Rel12hr.
|
Source
|
Sum of
Squares
|
Degree of
Freedom
|
Mean
Square
|
F Value
|
P Value
|
Model
Significant/
Nonsignificant
|
|
Model
|
111.94
|
5
|
22.39
|
3.26
|
0.1797
|
Not significant
|
|
X1
|
0.6936
|
1
|
0.6936
|
0.1010
|
0.7714
|
|
|
X2
|
98.09
|
1
|
98.09
|
14.29
|
0.0324
|
|
|
X1X2
|
1.29
|
1
|
1.29
|
0.1876
|
0.6941
|
|
|
(X1)2
|
11.71
|
1
|
11.71
|
1.71
|
0.2826
|
|
|
(X2)2
|
0.1568
|
1
|
0.1568
|
0.0228
|
0.8895
|
|
|
Residual
|
20.60
|
3
|
6.87
|
|
|
|
|
Core Total
|
132.54
|
8
|
|
|
|
|

Figure 20: Response 3-D surface plot for DR
CONCLUSION
The objectives of the study were developed sodium alginate beads for intragastric delivery of Metoclopramide Hydrochloride. The beads was prepared by the gelation technique using calcium chloride as a crosslinker and calcium carbonate as a gas forming floating inducer. The entrapment efficiency of alginate beads was significantly improved after the inclusion of sodium alginate was significantly improved after the inclusion of sodium alginate in the matrices and by increasing the poloxamer ration. Various aspects of the formulation, the characterization, Fourier Transform Infrared Spectroscopy, Differential Scanning Calorimetry, in vitro drug release, in vitro floating behavior studies, and scanning electron microscopy was carried out and evaluated. Scanning electron microscopy proved that the rough appearance and visible wrinkles of floating beads exhibit great buoyancy in simulated gastric fluid. The best formulation from batches F1-F9, found to be excellent yield, percent entrapment and release of drug, was batch F9 with drug release of 81.78%.
REFERENCES
- Chaudhary Sachin, Dua J.S., Prasad D.N. (2022) Recent Development in Floating Drug Delivery System: An Overview. Journal of Drug Delivery and Therapeutics, 12(01), 185-193.
- Anuradha A. Birajdar, Madhuri T. Deshmukh, Rajkumar, V. Shete (2021) A Review on Gastro-Retentive Floating Microspheres. Journal of Drug Delivery and Therapeutics, 1(11), 131-138
- Krishna, Abhishek Kumar, Rajat Srivastava. (2021) In Vivo In Vitro Studies on floating microsphere for Gastroretentive Drug Delivery System. Asian Journal of Pharmaceutical and Clinical Research, 14.1, 13-26.
- Nur Sena Basarr, Burcu Mesut, Yildiz Ozsoy. (2020) A Review On Current Perspective Of Gastroretentive Drug Delivery Syetem Prioritising Floating Dosage Forms, Journal of Advanced Research in Health Science, 43- 64.
- Mohamed Ibrahim, Youssef W Naguib, Hatem A Sarhan, Hamdy Abdelkader (2019) Gastro-retentive oral drug delivery systems: a promising approach for narrow absorption window drugs. Journal of Advanced Biomedical and Pharmaceutical Sciences, 02, 98-110.
- Beena Kumari (2018) recent development in floating drug delivery system: a review. Asian Journal of Pharmacy and Pharmacology. 14(01), 131-139.
- Uttam Kumar, Mandal, Bappaditya Chatterjee, Faria Gias Senjotis (2016) Gastro-retentive drug delivery systems and their in vivo success: A recent update. Asian Journal of Pharmaceutical Sciences,02, 575-584
- Shivram Shinde, Imran Tadwee, Sadhana Shahi (2011) Gastro retentive Drug Delivery System: A Review. International Journal of Pharmaceutical Research & Allied Science, 01(01), 01-13.
- Ahmed N. Allam, Mohammed M. Mehanna. (2015) Formulation, Physicochemical Characterization and in-vivo evaluation of ion sensitive metformin loaded biopolymeric beads. Drug development and Industrial Pharmacy, 1-9.
- Eng-Seng Chan, Hui-Peng Lim, Chien-Wei Ooi, Beng-Ti Tey (2017). Controlled delivery of oral insulin aspart using pH-responsive alginate / k-carrageenan composite hydrogel beads. Reactive and Functional Polymer, 120, 20-29.
- Jitendra Naik, Gokul Khairnar, Vinod Mokale, Arun Mujumdar (2019). Development of nanoparticulate Sustained release Oral drug delivery system for the antihyperglycemic with antihypertensive drug. Material Technology Advanced Performance material, 1-13.
- Nikhil Bhiswas, Rajan Kumar Sahu (2015), Tropica Starch blended alginate mucoadhesive-floating beads for intragastric delivery of Metaprolol Tartrate. International journal of biological macromolecule, 83, 61-70.
- Nitin Rajendra Shirsath, Ajaygiri kamalgiri Goswami (2020). Vildagliptin-loaded gellan gum mucoadhesive beads for sustained drug delivery: design, optimisation and evaluation. Material Technology, 1-12.
- Pankaj Dangre, Swapnil Dudhkohar, Shailesh chalikwar (2019). Development of Alginate Neusilin US2 (Magnesium almino-metasilicate) micro composite hydrogel beads for oralsustained release of cilnidipine: a statistical optimization. Polymer Plastic Technology and Material, 1-9
- Yan Hendrika, Julia Reveny, Sumaiyah Sumaiyah (2017). Formulation and In vitro evaluation of gastroretentive floating beads of amoxicillin using pectin from banana peel (Musa Balbisiana ABB). Asian Journal of Pharmaceutical and Clinical Research, 11(4), 72-77.
- Zang Tong, Zang Yue, Zang Xi-Tong, Zang Qi, Wang Bing (2018). Formulation development and evaluation of gastroretentive floating beads with Brucea Javanica oil using ionotropic gelation technology. Chinese Journal of Natural Medicine, 16 (4), 293-302.
- Raymond C Rowe, Paul J Sheskey, Marian E Quinn, (2009) Handbook of Pharmaceutical Excipients Pharmacist Press and American Pharmacist association Royal Pharmacist Association of Great Britain, SIX edition, 1-917
- Vinay Wamorkar, Manjunath S. Y, M.Mohan Varma. (2011). Development and Validation of UV Spectroscopic method for determination of metoclopramide Hydrochloride in bulk and tablet formation. International Journal of pharmacy and pharmaceutical science, 3(3), 171 – 174.
- Huimin Yao, Huijuan Yao, Junyi Zhu, Junlin Yu, Lifan Zhang, (2012). Preparation and evaluation of a novel gastric floating alginate / poloxamer inner porous beads using foam solution. International Journal of Pharmaceutics, 422(1-2), 211-219.
- Md.Lutful Amin, Tajnin Ahmed, Md Abdul Mannan, (2016). Development of floating mucoadhesive microsphere for site specific release of metronidazole. Advance Pharmaceutical Bulletin, 6 (2), 195-200.
- Shweta Agarwal, Abhilasha Thakur, Abhishek Sharma. (2022). Development and evaluation of ketoprofen loaded floating microsphere for sustained deliver. Materials Today: Proceedings, 68, 647-652. (httpts://doi.org/10.1016/j.matpr.2022.05.299.)
- www.hospira.com. Active ingredient Metoclopramide. This medication guide approved by the U. S. Food and Drug Administration. Distributed by Hospira. (2018).
- A.G. Stosik, H.E. Junginger, S. Kopp, K.K. Midha, V.P. Shah, S. Stavchansky, J.B. Dressman, D.M. Barends. (2007). Biowaiver monographs for immediate release solid oral dosage forms: metoclopramide hydrochloride. Journal Of Pharmaceutical Sciences, 97 (9), 3700-3708. DOI 10.1002/jps.2127.
- Dili Bhai Gajapathy, U Ubaidulla, Priyanka Sinha, Grace Rathnam. (2022). Gastroretentive Floating Beads – An Emerging Trend In Drug Delivery. International Journal Of Pharmaceutical Research And Application, 7(2), 1510-1520
- British Pharmacopoeia, Volume 3, 1485- 1489.
- Indian Pharmacopoeia, (2014), volume1, M130- M135
- Mohd Abdul Hadi, A. Srinivasa Rao, Srinivas Martha, Y. Sirisha, P. Udaya Chandrika (2013). Development of a floating multiple unit-controlled release beads of zidovudine for the treatment of AIDS. Journal of pharmacy research, 6, 78 -8 3.
- Sumeet Dwivedi1, Hemant Swami, Ashish M. Rathi, Manish G. Baheti , Amit Gangwal and Meenu Shukla, (2013). Formulation and Evaluation of Piperine Alginate Beads for StomachSpecific Delivery. Latin American Journal of Pharmacy (formerly Acta Farmacéutica Bonaerense), 73-75.
- Gayathridevi M, J. Adlin Jino Nesalin and T. Tamizh Man, floating microsphere: a review floating microsphere: a review. International journal of research in pharmacy and chemistry, 6(3), 501-510.
- Hinal Prajapati, Keyur Patel, Arun Kumar Gupta, formulation and evaluation of floating microspheres of baclofen. International journal of pharmaceutical science and research, Vol. 12(3): 1482-1494
- Abdul Sayeed, Dr. Pawan Kumar, Dr. Syed Areefulla Hussainy, (2021). Formulation and evaluation of mouth dissolving tablets of metoclopramide hydrochloride using natural and synthetic super disintegrants. Indo American journal of pharmaceutical sciences, 08 (10), 126-141.
- B. Nagamani, J. Hindu Manognya, K. Naga Raju, B. Deepika, T. Regupathi, K. N. Vrao, K. Rajeswar Dutt, (2017). Formulation and characterisation of floating microspheres containing cinnarizine. Innovate International Journal of Medical & Pharmaceutical Sciences, 2(s1), 2456-8694.
- Deepti, Kapil Kumar, Gurleen Kaur and Deepak Teotia, (2020) Preparation and characterization of floating alginate beads of lafutidine as a gastroretentive dosage form. International journal of Pharmaceutical and Research, 11(6), 2752-2760.
- Sunita Adhikari, Kul Prasad Gharti and Shiva Pandeya (2019), formulation and in-vitro evaluation of metoclopramide hydrochloride orodispersible tablets, 10(4), 1916-1921.
- T. S. Shinde, a. N. Barhate, (2019), A review on floating microspheres. International Journal of Pharmaceutical and Biological Science Archive, 7(3), 87-92
- Wajid Ahmad, Rihan Jawed (2022), Design and Characterization of Sustained Released Alginate Beads of Meclizine Hydrochloride. Research Journal of Pharmaceutical Dosage Forms and Technology, 14(03), 199-205. DOI: 10.52711/0975-4377.2022.00032.
- Monika Setia, Kapil Kumar, Deepak Teotia, Development and evaluation of gastro-retentive floating beads of dicycloverine hydrochloride. Journal of Drug Delivery and Therapeutics, 8(4), 346-355.
- Tathagata Roy, Tapan Kumar Chatterjee, (2023), Formulation and evaluation of microspheres of anti-inflammatory drug diacerein prepared by ionotropic gelation method. Palestinian Medical and Pharmaceutical Journal (PMPJ). 8(1): 00-00.
- Ankit Anand Kharia, Akhlesh Kumar Singhai, (2013). Controlled Release Drug Delivery System with Stomach Specific Mucoadhesive Nanoparticles. Indian Journal of Nanoscience, 1(2), 2320-9674
- P.G Sindhumol, Dr. C.R Sudhakaran Nair and Dr. Jyoti Harindran, (2018), Formulation and evaluation of floating alginate: Chitosan microspheres of cefixime. The Pharma Innovation Journal, 7(4), 919-928.
- Behzad Mokhtare, Meltem Cetin, Rukiye Sevinc Ozakar and Hatice Bayrakceken. (2017). In vitro and in vivo evaluation of alginate and alginate chitosan beads containing metformin hydrochloride. Tropical Journal of Pharmaceutical Research,16 (2), 287-296
- Basak Rumpa, Mohanta Tanmay, Das Sujit, Bhattacharya Suhasis, (2021). Recent advances in the development of floating microspheres for the treatment of hypertension. World Journal of Pharmacy and Pharmaceutical Science, 10 (8), 1299-1313.
- Sujit Kakade, Aviraj Kakade, Shilpa Gawade, (2018). Formulation and Evaluation of Floating Microspheres of Piroxicam. Current Pharma Research, 8(2), 2280-2298.
- Raghad Sinan, Sadeem S. Abed, (2009). Spectrophotometric determination of metoclopramide hydrochloride in tablets by diazotization and coupling reaction with phenol. Iraqi Journal of Science, 50(2), 136-143.
- Thakur Atulkumar Ranvirsingh, Basappa Veerbhadraiah Basavaraj, Srinivasan Bharath1, Rajamanickam Deveswaran1, Varadharajan Madhavan, (2013), Formulation and Evaluation of Floating Alginate Beads of an antiulcer Drug. International Journal of Pharmaceutical Sciences Review and Research, 21(2), 120-124
- Eda Gökbulut, ?mran Vural, Makbule A??ko?lu, Nurten Özdemir (2018). Floating drug delivery system of itraconazole: Formulation, in vitro and in vivo studies. Journal of Drug Delivery Science and Technology, https://doi.org/10.1016/j.jddst.2018.12.019.
- Rosalba Alonso-Campero, Roberto Bernardo-Escudero, María Teresa de Jesús Francisco-Doce , Myriam Cortés-Fuentes , Gilberto Castañeda-Hernandez, Mario I. Ortiz, (2011). Bioequivalence Study of Metoclopramide Hydrochloride 10 mg Tablets in Healthy Male Volunteers, Journal of Bioequivalence & Bioavailability, 3(10), 222-227.
- Nana Chena, Jing'e Niua , Qian Lia , Jianyin Lia , Xinping chena , Yuan Renb , Guotai Wub , Yingqian Liua , Yanbin Shia (2019). Development and evaluation of a new gastroretentive drug delivery system: Nanomicelles-loaded floating mucoadhesive beads, Journal of Drug Delivery Science and Technology, 51, 485-492.
- K.Varun Kumar, P. Srikanth Choudary, Ajaykumar. B. (2013). Design and Evaluation of Stomach-Specific Drug Delivery of Domperidone using Floating Pectin Beads, International Journal of Drug Development & Research, 5(1), 219-228.
- Sana Roohi, J. Samatha, S. Hymavathi, MD. Muzaffar-Ur-Rehman and Akhila Alladi. (2017) development and characterization of gastro-retentive solid dosage form of anti-hypertensive drug, European Journal of Pharmaceutical and Medical Research, 4(10), 246-254.
- Kajale Archana D, Chandewar A.V. (2016). Formulation and evaluation of oral floating beads of tramadol hydrochloride. Journal of Drug Delivery and Therapeutics, 6(4), 7-16.
- Dharmendra Rai, Durga Pandey, Nishi Prakash Jain, Surendra Kumar Jain, (2019), Formulation Development and Evaluation of Floating Microsphere of Famotidine for the Treatment of Peptic Ulcer. Journal of Drug Delivery & Therapeutics, 9(4-s):426-431.
- Amit Porwal, Harinath Dwivedi, Kamla Pathak. (2017). Decades of research in drug targeting using gastroretentive drug delivery systems for antihypertensive therapy. Brazilian Journal of Pharmaceutical Sciences, 53(3), 73. http://dx.doi.org/10.1590/s2175-97902017000300173
- Khalifa My, Shaikh Siraj N, (2019). Formulation and characterization of floating beads of antibiotic by emulsion gelation technique. Asian Journal Pharm Clinical Research, 12(5), 2019, 57-62.
- Hiba M. Suza Ali, Eman B. H. Al-Khedairy, (2013), Formulation and Evaluation of Prednisolone -Loaded Alginate Beads for Taste Masking. The Egyptian Journal of Hospital Medicine, 90 (2), 2178-2186.
- S. Pushpa Latha1, Dr. Kota Chaitanya Sravanthi, L. Mohan Krishna, (2020), Formulation and Evaluation of Bilayered Floating Tablets of Metoclopramide HCL. Journal Of Pharmacy and Biological Sciences, 15(5), 27-31
- Bharat W Tekade, Umesh T Jadhao, Vinod M Thakare, Kundan P Chaudhari, and Harshal B Kedare. (2014), Formulation and Evaluation of Metoclopramide Hydrochloride Sustained Release Microsphere. Journal of pharmaceutical Science. 2320-1215
- Dili bhai Gajapathy, U Ubaidulla, Priyanka Sinha, Grace Rathnam (2022), Gastroretentive Floating Beads – An Emerging Trend In Drug Delivery. International Journal of Pharmaceutical Research, 7 (2), 1510-1520.
- Anamika Saxena, Santosh Kitawat, Kalpesh Gaur, Virendra Singh, (2016), Formulation, Development and Characterization of Floating Beads of Diltiazem Hydrochloride, International Journal of Drug Delivery Technology, 6(1), 1-6
- Giovanni Falcone, Juan P. Real, Santiago D. Palma, Rita P. Aquino, Pasquale Del Gaudio, Emilia Garofalo, Paola Russo, (2022), Floating Ricobendazole Delivery Systems: A 3D Printing Method by Co-Extrusion of Sodium Alginate and Calcium Chloride, International Journal of molecular science, 23, 1280.
- Monika Kumbhar D., Pournima Morey H., Manisha Karpe S., Vilasroa Kadam J., (2018), Development and characterization of gastroretentive floating microsphere for controlled release of metoclopramide hydrochloride, Indian Journal of Drugs, 6(4), 189-200
- Kishore Narra, Unnikrishnan Dhanalekshmi, Govindaraj Rangaraj, Devendiran Raja, Celladurai Senthil Kumar, Pully Neelakanta Reddy, Asit Baran Manda, (2012), Effect of Formulation Variables on Rifampicin Loaded Alginate Beads. Iranian Journal of Pharmaceutical Research, 11 (3), 715-721
- Walaa Wagih, Abdel Razaak Abdel Mageed Mohamed, Gamal El Din Abdel Fattah El Gendy, Dina Fathalla Mohamed, (2022), Formulation and Assessment of Celecoxib Floating Beads in Capsule using 23 Full Factorial Design, Journal of Advanced Biomedical and Pharmaceutical Sciences, 5 ,101-112.
- NFI- National Formulary of India, (2021), Indian Pharmacopoeia Commission, 114-115
- Shivakalyani Adepu, Seeram Ramakrishna, (2021), Controlled Drug Delivery Systems. Current Status and Future Directions Molecules, 26, 5905. https://doi.org/10.3390/molecules26195905
- Yu-Tung Hsu, Chen-Yu Kao, Ming-Hua Ho, and Shiao-Pieng Lee, (2021), To control floating drug delivery system in a simulated gastric environment by adjusting the Shell layer formulation. Biomaterials Research, 25-31 https://doi.org/10.1186/s40824-021-00234-6
- B. Arun Kumar, K. Mahalakshmi, V. Uma Maheshwara Rao, (2015) Formulation and evaluation of gastro retentive floating microbeads of Sumatriptan. International Journal of Life Science and Review. 1(2).41-47.
- Arun Kumar, Silpi Chanda, Shriya Agarwal, Manisha Singh, Saurabh Sharma, Gunjan Vasant Bonde, Raj Kumar Tiwari (2021) Formulation and evaluation of Gastroretentive Tinidazole loaded floating microsphere for sustained release. Material Today: Proceedings. xxxx. https://doi.org/10.1016/j.matpr.2021.05.616.
- Mohammed F Aldawasari, Mohammed Muqtader Ahmed, Farhat Fatima, Md. Khalid Anwer, Prakash Katakam, Abdullah Khan, (2021). Marine Drugs. (19),467.


 Pooja bodade*
Pooja bodade*
 Dr. Yogesh Gurav
Dr. Yogesh Gurav


















 10.5281/zenodo.14383333
10.5281/zenodo.14383333