Abstract
Benzimidazole derivatives have emerged as a prominent class of heterocyclic compounds due to their diverse pharmacological activities and therapeutic applications. This review endorsed current advancements in the synthesis, bioactivity, and clinical relevance of benzimidazole derivatives, highlighting their potential in combating various diseases, including cancer, infectious diseases, and metabolic disorders. The review examines the synthesis of benzimidazole derivatives, encompassing both traditional and innovative methods. The introduction includes various discussions about the importance of benzimidazole in medicinal chemistry. It delves into the emergence of microwave-assisted synthesis, green chemistry approaches, and solid-phase strategies in chemical production. Recent studies have demonstrated significant progress in the design of benzimidazole-based drugs, showcasing their roles as potent anticancer agents and effective treatments for infections. Furthermore, innovative synthetic methodologies have been developed to streamline the production of these derivatives, emphasizing environmentally friendly practices and high yield processes. This review aims to provide a comprehensive overview of the current landscape of benzimidazole research, focusing on structure-activity relationships, synthetic strategies, and emerging therapeutic applications, thereby paving the way for future investigations in this vital area of medicinal chemistry.
Keywords
Benzimidazole, Medicinal chemistry, Drug discovery, Pharmacological activities, Strategies, Developments.
Introduction
Benzimidazole derivatives have garnered significant attention in medicinal chemistry due to their remarkable pharmacological properties and therapeutic potential. As nitrogenous heterocyclic, these compounds exhibit a diverse range of biological activities, including antimicrobial, antiviral, anticancer, and antihypertensive effects. The structural versatility of benzimidazole allows for extensive modifications, leading to the development of novel agents that target various diseases. Benzimidazole derivatives are pivotal in the development of new pharmaceuticals, leading to the creation of various medications such as pracinostat for cancer, lansoprazole as a proton pump inhibitor, albendazole for anthelmintic purposes, enviroxine as an antiviral, ridinilazole for antibacterial use, flubendazole as an antiparasitic, risperidone for psychosis, and etofylline as a bronchodilator. The broad therapeutic potential of benzimidazole and its derivatives suggests that many new drugs containing this compound are expected to become available in the coming years. Recent advancements in synthetic methodologies have further enhanced the accessibility of these compounds, facilitating the exploration of their structure-activity relationships (SAR) and optimizing their bioactivity profiles. The increasing prevalence of drug resistance and the need for targeted therapies have propelled research into benzimidazole derivatives as promising candidates in precision medicine. Their unique core structure and minimal toxicity make them suitable scaffolds for designing innovative therapeutics aimed at overcoming current treatment challenges[1].To highlight the benefits of employing benzimidazole in drug development, we outline pharmacological active benzimidazole hybrids in this review.
Overview of Benzimidazole
Benzimidazole, characterized by their fused benzene and imidazole ring structure, boast an exceptional potential for structural diversity through selective substitution patterns. This fundamental structural motif lays the groundwork for an array of derivatives, each possessing a unique set of biological characteristics. The incorporation of various substituents lends versatility, highlighting its utility in numerous therapeutic areas.
Structural Characteristics of Benzimidazole
The fused benzene and imidazole ring structure of benzoimidazoles sets them apart, and their many structural nuances are crucial to their pharmacological effects. This section delves into the basic structural properties of benzimidazole, elucidating the structure-activity relationships (SAR) principles that govern their interactions with biological targets and examining the impact of substituent modifications on biological activity.
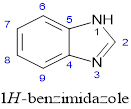
Figure 1: Benzimidazole structure with numbering
The core structure and substituent variations
The core of benzimidazole is a conjugated ring structure made up of an imidazole ring fused with a benzene ring (Figure 1). The molecule's chemical and pharmacological characteristics can be customized by attaching different functional groups to its structure, which serves as a scaffold. By increasing structural diversity through strategic substitutions, compounds with desirable features can be created, as this improves selectivity and affinity for certain target molecules.
Substitution effects on biological activity
The biological activity of benzimidazole derivatives is strongly influenced by the kind and location of the substituent on the core structure. Increased solubility, higher binding affinity, altered pharmacokinetic profiles, and altered metabolic stability are just a few of the effects that substitutions can have. The design and optimization of benzimidazole-based medicines are guided by the careful balance between expected side effects and desired biological activity, as demonstrated by a thorough analysis of substituent effects.
Table 1: Common benzimidazole derivatives and their biological activities[2
|
Sr. No.
|
Benzimidazole derivatives
|
Biological activity
|
|
1.
|
Albendazole
|
Antiparasitic, antihelminthic
|
|
2.
|
Astemizole
|
Antihistamine, antiarrhythmic
|
|
3.
|
Albendazole sulfoxide
|
Antiparasitic, antihelminthic
|
|
4.
|
Astemizole oxide
|
Antihistamine, antiarrhythmic
|
|
5.
|
Benzocaine
|
Local anesthetic
|
|
6.
|
Carbendazim
|
Antifungal, antiparasitic
|
|
7.
|
Clopidogrel
|
Antiplatelet
|
|
8.
|
Domperidone
|
Antiemetic
|
|
9.
|
Etofylline
|
Bronchodilator
|
|
10.
|
Flubendazole
|
Antiparasitic
|
|
11.
|
Fenbendazole
|
Anthelmintic, antiparasitic
|
|
12.
|
Itraconazole
|
Antifungal
|
|
13.
|
Ketoconazole
|
Antifungal
|
|
14.
|
Lansoprazole
|
Proton pump inhibitor
|
|
15.
|
Levamisole
|
Immunomodulator, anthelmintic
|
|
16.
|
Lansoprazole sulfide
|
Proton pump inhibitor
|
|
17.
|
Mebendazole
|
Antiparasitic, antihelminthic
|
|
18.
|
Metronidazole
|
Antibacterial, antiprotozoal
|
|
19.
|
Meclizine
|
Antihistamine, antiemetic
|
|
20.
|
Mebendazole oxide
|
Antiparasitic, antihelminthic
|
|
21.
|
Niclosamide
|
Anthelmintic, antiparasitic
|
|
22.
|
Nimorazole
|
Antibacterial, antiprotozoal
|
|
23.
|
Niclofolan
|
Anthelmintic
|
|
24.
|
Omeprazole
|
Proton pump inhibitor
|
|
25.
|
Oxibendazole
|
Antiparasitic, anthelmintic
|
|
26.
|
Oxfendazole
|
Antiparasitic, anthelmintic
|
|
27.
|
Pantoprazole
|
Proton pump inhibitor
|
|
28.
|
Praziquantel
|
Anthelmintic
|
|
29.
|
Rabeprazole
|
Proton pump inhibitor
|
|
30.
|
Risperidone
|
Antipsychotic
|
|
31.
|
Rabeprazole sulfide
|
Proton pump inhibitor
|
|
32.
|
Thiabendazole
|
Antifungal, antiparasitic
|
|
33.
|
Tegaserod
|
Serotonin receptor agonist
|
|
34.
|
Tinidazole
|
Antibacterial, antiprotozoal
|
|
35.
|
Tiabendazole
|
Antiparasitic, antihelminthic
|
|
36.
|
Vardenafil
|
Erectile dysfunction treatment
|
SAR Studies
Deciphering the intricate relationships between benzimidazole structural characteristics and pharmacological actions requires an understanding of their structure-activity relationship (SAR). SAR research offers crucial information on the ideal substituent location and properties to produce desired biological effects. By analyzing the connections between chemical structure and activity profiles, researchers may validate known effects, predict the impacts of changes, and guide the development of molecules with increased efficacy and decreased toxicity[3,4].
Importance Medicinal Chemistry
Benzimidazole’ s wide range of pharmacological actions highlights their significance in medicinal chemistry. Because of their versatility, they can make significant contributions to fields like cancer treatment, antimicrobial defense, and central nervous system regulation. Their capacity to interact selectively with biological targets is a result of the intricate interaction between the core structure and the additional functional groups, making them potential candidates for drug development (Table 1). The objective of the current article is to present a thorough analysis of the various uses, synthetic methods, and potential future developments of benzimidazole in medicinal chemistry. By dissecting their structural characteristics and comprehending their importance in various pharmacological contexts, we seek to offer a thorough grasp of the intricate relationship between structure and activity. Furthermore, we aim to look into how benzimidazole research is evolving, with an emphasis on emerging patterns and potential findings that may have an impact on medicinal chemistry in the future.
Pharmacological Diversity of Benzimidazole
Due to their wide range of pharmacological properties, benzimidazoles are an important class of compounds with a wide range of medicinal uses. Their diverse roles and effects, are discussed in this section[5].
Anticancer properties
Benzimidazole have demonstrated potential as strong anticancer drugs, making them intriguing oncology options. This section explores their distinct contributions to cancer treatment, highlighting current clinical applications and focusing on the basic mechanisms of their anticancer effect.
Anticancer activity mechanisms
Numerous anticancer activities of benzimidazole compounds demonstrate their ability to target a wide range of disease indicators. By disrupting the dynamics of microtubules, certain substances prevent the development of mitotic spindles and result in cell cycle arrest or death. Others, such as DNA repair, angiogenesis, and proliferation, obstruct vital enzymes or processes that contribute to the development of cancer. Because of their intricate mechanisms of action, benzimidazole may be able to target a variety of cancer types[6].
Anti-microbial properties
As antibacterial agents, benzimidazole have shown exceptional efficacy, showcasing their versatility and strength. Their roles in antibacterial, antifungal, and antiparasitic activities are the main topic of this section[7].
Antibacterial activity
Given their strong antibacterial properties, some benzimidazole compounds exhibit potential for treatments against bacterial illnesses. By interfering with vital biochemical processes through interactions with significant bacterial enzymes or cellular constituents, these substances hinder the development and survival of bacteria. Benzimidazole-based antibacterial medications have demonstrated promise against a range of infections, including both gram-positive and gram-negative bacteria.
Antifungal activity
In the field of antifungal medicines, benzimidazole have become medications that can combat fungal infections. By altering fungal cell division, membrane integrity, or the production of vital components, benzimidazole derivatives can have fungicidal or fungistatic effects on a range of fungal species. They are helpful instruments in the development of antifungal medications due to their ability to target both superficial and systemic fungal infections [8].
Antiparasitic activity
Benzimidazole are essential components of antiparasitic treatments, offering efficient options for treating a range of parasitic diseases. By disrupting the microtubule dynamics of parasites, they hinder their ability to divide cellularly and result in structural damage. Consequently, benzimidazole derivatives play a significant role in the global effort to manage parasitic diseases by effectively battling parasitic infections like nematodes and cestodes[9].
Anti-inflammatory activity
Benzimidazole derivatives have shown significant anti-inflammatory activity, which is beneficial for therapeutic uses. Recent research has revealed that certain benzimidazole compounds can effectively suppress inflammatory responses by acting on different pathways and receptors associated with inflammation. For example, compounds like MBNHYD and MBPHYD have yielded encouraging results in diminishing inflammation in animal studies, through the inhibition of cyclooxygenases (COXs) and the modulation of transient receptor potential channels. Moreover, structure-activity relationship studies have shown that specific substituents on the benzimidazole ring can increase their anti-inflammatory effectiveness. These discoveries point to the potential of benzimidazole derivatives as innovative anti-inflammatory drugs for a variety of inflammatory conditions [10,11,12].
Current clinical applications
Benzimidazole derivatives are currently utilized in a wide array of therapeutic applications due to their diverse pharmacological properties. They are prominent in the development of anticancer agents, such as Bendamustine and Binimetinib, which target specific cancer pathways and demonstrate minimal toxicity to healthy cells. Additionally, benzimidazole compounds serve as effective antimicrobial agents, with several derivatives showing activity against both bacterial and fungal infections. They are also employed as proton pump inhibitors (e.g., omeprazole) for treating gastrointestinal disorders, and as anthelmintics (e.g., albendazole) for parasitic infections. Furthermore, ongoing research is exploring their potential in anti-inflammatory, antiviral, and antihypertensive therapies, highlighting the structural versatility of benzimidazole that allows for the design of novel compounds targeting multiple biological pathways[13,14,15].
Synthetic Strategies for Benzimidazole Derivatives
A vital component of the medicinal chemistry research is the production of benzimidazole derivatives. This section explores the many synthetic strategies, such as solid phase synthesis techniques, modern methodology, and conventional methodologies, that are employed to produce these compounds [16].
Conventional synthetic approaches
Traditional methods for synthesizing benzimidazole derivatives mainly consist of condensing ortho-phenylenediamines (OPD) with aldehydes or carboxylic acids. The Phillips reaction, a common technique, involves OPD reacting with carboxylic acids in acidic conditions, usually necessitating heat to facilitate cyclization and dehydration. The Weidenhagen reaction, another established method, includes the reaction of OPD with aldehydes or ketones. While these processes typically provide good to excellent outcomes, they often demand severe conditions and can produce considerable waste, leading to environmental concerns.
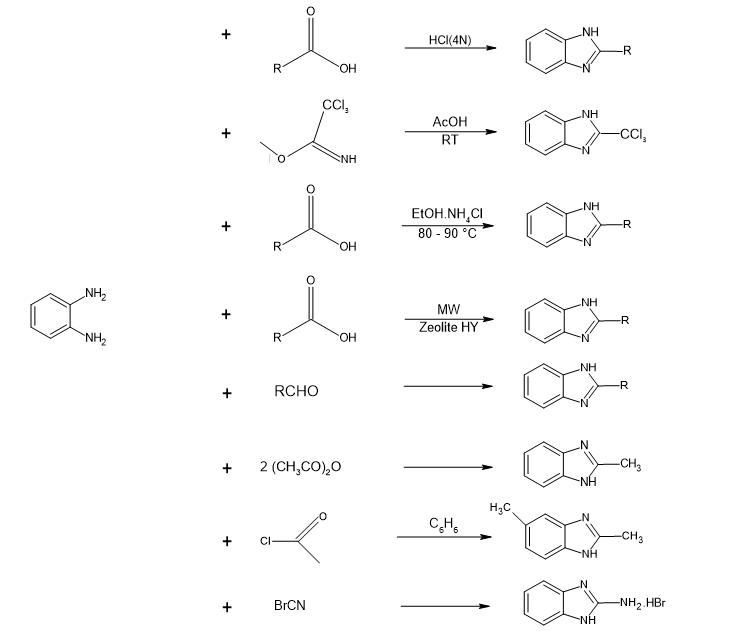
Figure 2: Different synthetic scheme for benzimidazole from o-Phenylenediamine
Recent developments have introduced more sustainable alternatives, such as the use of environmentally friendly catalysts like zinc triflate or the adoption of solvent-free conditions, which improve yields and minimize environmental impact, all while preserving the efficacy of conventional techniques[17,1].
Synthetic methods of today
- Microwave assisted synthesis
The microwave-assisted synthesis of benzimidazole derivatives represents a significant advancement in organic chemistry, offering a more efficient and eco-friendly method. Utilizing microwave irradiation, this technique speeds up chemical reactions, cuts down on reaction times, and boosts yields beyond what traditional heating achieves. It typically involves condensing ortho-phenylenediamines with carboxylic acids or aldehydes under microwave conditions, often without solvents or with green solvents. Early studies have shown successful synthesis with solid supports and microwave energy, leading to quick cyclization and fewer byproducts. This method not only improves the synthetic process's efficiency but also adheres to the principles of green chemistry by minimizing hazardous waste and energy use, making it an attractive option for researchers developing new benzimidazole compounds with various biological activities [18].
- Green chemistry synthesis
The synthesis of benzimidazole has also been influenced by green chemistry concepts, which emphasize sustainability and reducing environmental impacts. Green synthetic pathways are facilitated by catalytic processes, solvent-free or water-based reactions, and bio-inspired transformations. These techniques provide the efficiency needed for drug development operations while also being in line with the increased emphasis on environmentally friendly approaches to chemical synthesis [17,19].
- Benzimidazole solid phase synthesis
A high throughput technique to produce benzimidazole derivatives is solid phase synthesis. By attaching the starting components to a solid substrate, researchers can speed up reactions and simplify purification processes. The demands of parallel synthesis and combinatorial chemistry are well satisfied by this approach, which makes it possible to produce a variety of chemical libraries for rapid screening.
Modern and effective technologies have replaced conventional multistep methods for the synthesis of benzimidazole derivatives. In addition to making these compounds more accessible, the application of solid phase procedures, green chemistry principles, and microwave-assisted techniques has made it easier to assess them as potential therapeutic agents in medicinal chemistry research (Table 2)[20].
Table 2:Comparative analysis of classical and modern synthesis approaches for benzimidazole derivatives
|
Synthetic approach
|
Advantages
|
Disadvantages
|
|
Classical methods
|
Established protocols widely available starting materials
|
Multistep procedures Limited regioselectively longer reaction time
|
|
Modern methods
|
Microwave assisted synthesis
Rapid reaction rates
High yields
Energy efficient
Green chemistry approaches
Reduced environmental impact
Solvent free or water-based reactions
Catalytic processes
|
Limited to specific types of reactions
Equipment requirements
Reaction optimization Challenges
Limited to certain reaction types
Reaction optimization challenges
|
|
Solid phase synthesis
|
Parallel synthesis capability
Enhanced purification Faster reaction rates
|
Limited to specific compounds
Requires solid phase equipment
Complexity in library synthesis
|
Current Development in Benzimidazole
Recent advancements in benzimidazole chemistry underscore its increasing importance in medical applications, especially owing to its structural adaptability and extensive pharmacological activities. Benzimidazole, a fused heterocyclic compound consisting of benzene and imidazole rings, has become a notable framework for drug development, especially in oncology and infectious disease areas. Its derivatives demonstrate a broad spectrum of biological activities, such as antibacterial, antiviral, anticancer, anti-inflammatory, and antitubercular effects [21,22]. The simplicity of synthesizing benzimidazole derivatives has captured researchers' attention, spurring innovative methods that amplify their therapeutic capabilities. Recent investigations have delved into diverse synthetic strategies, encompassing green processes, to advance the creation of new compounds with heightened effectiveness and diminished toxicity. Investigations into the structure-activity relationship (SAR) are vital for comprehending how alterations at particular sites on the benzimidazole ring affect biological activity, thus directing the logical development of novel drug candidates[21,23].
Computational techniques for designing benzimidazole
The integration of computational methods is crucial in the development of benzimidazole derivatives, greatly improving the drug discovery process. Techniques like molecular docking, molecular dynamics simulations, and quantitative structure-activity relationship (QSAR) analyses are commonly used to forecast how these compounds will interact with biological targets. Molecular docking, for example, enables scientists to see how benzimidazole derivatives align within protein active sites, aiding in the selection of the most promising candidates for advancement [24,25].
Additionally, molecular dynamics simulations offer valuable perspectives on the stability and conformational transitions of benzimidazole compounds within biological settings, essential for grasping their pharmacodynamics and pharmacokinetics [26]. QSAR models assist in establishing a correlation between chemical structures and biological activities, thereby guiding the rational design of new compounds by forecasting their potential efficacy through structural modifications [24,27].
Targeted drug development
The development of targeted drugs using benzimidazole has attracted considerable interest for their potential in cancer treatment, particularly in selectively halting the growth of cancer cells. These molecules share structural features with nucleosides, which enables them to effectively target and interact with key biological markers involved in the advancement of cancer. Recent research has been directed towards creating new benzimidazole derivatives aimed at specific cellular pathways, thereby improving their therapeutic potential and reducing harm to healthy cells. For example, certain benzimidazole derivatives have been engineered to suppress vital proteins such as Bcl-2, which is instrumental in controlling cell death in cancerous cells. These specially designed compounds have been effective in significantly reducing Bcl-2 levels, resulting in enhanced cell death in breast cancer cell lines, including MCF-7[28]. The strategic modification of benzimidazole structures not only improves their binding affinity but also enhances selectivity towards cancer cells, addressing the common challenge of resistance associated with traditional chemotherapy. This targeted approach is further supported by computational methods, including molecular docking and dynamics simulations, which aid in predicting the interactions of these compounds with their targets and optimizing their design for clinical applications [29].
Future Possibilities and Recent Developments
Emergent breakthroughs that could change drug discovery and therapeutic approaches are driving the changing landscape of benzimidazole medicinal chemistry. This section examines these patterns and makes predictions about how they could have an impact on benzimidazole research in the future. The rise of precision medicine has positioned benzimidazole-based drugs as key players, allowing for treatments tailored to individual genetic profiles and molecular signatures, thus enhancing therapeutic outcomes while minimizing side effects [22].
Applications of Nanotechnology in Drug Discovery
The transfer of benzimidazole compounds to their active sites could be greatly enhanced by nanotechnology. Nanocarrier and nanoparticle-based drug delivery methods offer better targeting, controlled release, and enhanced bioavailability. These methods change the pharmacokinetics and effectiveness of medications based on benzimidazole while simultaneously optimizing therapeutic effects and reducing potential side effects[30].
Investigating Natural Sources for Benzimidazole
The variety of chemicals found in nature is still abundant. Novel benzimidazole derivatives with unique properties and activities might be found through the investigation of natural products and bioactive compounds. These compounds could provide new treatment techniques and act as inspiration for the development of medications.
The medicinal chemistry of benzimidazole has an exciting future ahead of it because to these recent advancements. By adopting personalized medicine, applying multitarget strategies, utilizing nanotechnology, addressing resistance, and drawing on natural sources, researchers can open up new therapeutic development avenues that will eventually result in the development of cutting-edge benzimidazole-based treatments for a range of illnesses [31].
Safety and Toxicity Concerns
The development of benzimidazole derivatives as therapeutic agents must prioritize safety and toxicity considerations. Although these compounds have shown promising pharmacological activity, there is a risk of adverse reactions due to potential off-target effects. It is crucial in preclinical research to assess cytotoxicity, genotoxicity, and organ-specific toxicity to understand their safety profiles and determine appropriate dosage regimens. Despite most benzimidazole derivatives being safe, a minority have shown adverse effects, underlining the need for comprehensive toxicological evaluations prior to clinical use. Clinical reports reveal that about 5% of benzimidazole treatments resulted in negative effects, emphasizing the need for ongoing safety surveillance during drug development.
Challenges and Limitations
Although benzimidazole offer a lot of promise as therapeutic agents, a number of obstacles and limitations need to be removed before their full potential in clinical settings can be achieved. Some of the biggest obstacles that developers and researchers face while dealing with benzimidazole compounds are examined in this section.
Pharmacokinetic Difficulties
The clinical application and effectiveness of benzimidazole derivatives are often compromised by various pharmacokinetic challenges. Poor solubility is a primary issue, significantly limiting the oral bioavailability of these compounds. This is compounded by their low permeability across biological membranes, leading to inadequate absorption and distribution within the body. Additionally, the rapid metabolism of many benzimidazole derivatives results in a short half-life, hindering sustained therapeutic effects. The metabolic stability of benzimidazole varies with structural modifications, making some more susceptible to enzymatic degradation. Addressing this variability is crucial for optimizing their pharmacokinetic properties during drug development. Furthermore, as benzimidazole derivatives may modify the metabolism of pharmaceuticals taken together, changing their efficacy or increasing their toxicity, the possibility of drug-drug interactions needs to be carefully taken into account.
CONCLUSION
In medicinal chemistry, benzimidazole compounds have shown great promise and versatility. Their diverse pharmacological action and structural adaptability make them a valuable tool for drug development. The many aspects of benzimidazole have been carefully investigated in this work, with particular attention paid to their structural characteristics, pharmacological diversity, synthetic approaches, recent successes, new directions, and issues. The fundamental structure and substituent modifications that characterize benzimidazole were examined in this review, along with their roles in antibacterial activity, anticancer potential, and central nervous system function modulation. We observed and examined their synthesis procedures, talked about recent developments including high throughput screening and target-based drug design, and investigated the computational tools guiding the creation of benzimidazole. Various strategies were emphasized, nanotechnology, natural sources for discovery, Safety and toxicity concerns and pharmacokinetic difficulties. Benzimidazole compounds hold the potential to transform treatment strategies across various medical conditions, such as neurological disorders, cancer, and infectious diseases. Their distinctive modulatory mechanisms and structural features enable the development of tailored drugs that offer fewer side effects and greater effectiveness. The idea of devising benzimidazole-based therapies is gaining momentum as further studies uncover their complex interactions with biological targets. Hence, we conclude that the diverse biological activities of benzimidazole have revolutionized medicinal chemistry. Through the exploration of various methods to treat numerous diseases, scientists have succeeded in creating advantageous hybrids within this core structure. Molecules featuring benzimidazole nuclei with increased molecular space will aid researchers globally in developing pharmacologically active drugs for various targets.
REFERENCES
- Chung NT, Dung VC, Duc DX. Recent achievements in the synthesis of benzimidazole derivatives. RSC advances. 2023;13(46):32734-71.
- Brishty SR, Hossain MJ, Khandaker MU, Faruque MR, Osman H, Rahman SA. A comprehensive account on recent progress in pharmacological activities of benzimidazole derivatives. Frontiers in pharmacology. 2021 Nov 3;12:762807.
- Feng LS, Su WQ, Cheng JB, Xiao T, Li HZ, Chen DA, Zhang ZL. Benzimidazole hybrids as anticancer drugs: An updated review on anticancer properties, structure–activity relationship, and mechanisms of action (2019–2021). Archiv der Pharmazie. 2022 Jun;355(6):2200051.
- Moghadam Farid S, Noori M, Nazari Montazer M, Khalili Ghomi M, Mollazadeh M, Dastyafteh N, Irajie C, Zomorodian K, Mirfazli SS, Mojtabavi S, Faramarzi MA. Synthesis and structure–activity relationship studies of benzimidazole-thioquinoline derivatives as ?-glucosidase inhibitors. Scientific Reports. 2023 Mar 16;13(1):4392.
- Bansal Y, Silakari O. The therapeutic journey of benzimidazoles: A review. Bioorganic & medicinal chemistry. 2012 Nov 1;20(21):6208-36.
- Chalkappa PK, Aralihalli S, Sudileti M, Aithal SJ, Praveen BM, Birjadar K. The medicinal panorama of benzimidazoles and their scaffolds as anticancer and antithrombotic agents: A review. Archiv der Pharmazie. 2023 Oct;356(10):2300206.
- Marinescu M. Benzimidazole-triazole hybrids as antimicrobial and antiviral agents: A systematic review. Antibiotics. 2023 Jul 22;12(7):1220.
- Moghimi S, Shafiei M, Foroumadi A. Drug design strategies for the treatment azole-resistant candidiasis. Expert Opinion on Drug Discovery. 2022 Aug 3;17(8):879-95.
- Hernández-López H, Tejada-Rodríguez CJ, Leyva-Ramos S. A panoramic review of benzimidazole derivatives and their potential biological activity. Mini Reviews in Medicinal Chemistry. 2022 May 1;22(9):1268-80.
- Moharana AK, Dash RN, Mahanandia NC, Subudhi BB. Synthesis and anti-inflammatory activity evaluation of some benzimidazole derivatives. Pharmaceutical Chemistry Journal. 2022 Nov;56(8):1070-4.
- Veerasamy R, Roy A, Karunakaran R, Rajak H. Structure–activity relationship analysis of benzimidazoles as emerging anti-inflammatory agents: An overview. Pharmaceuticals. 2021 Jul 11;14(7):663.
- Walia R, Hedaitullah M, Naaz SF, Iqbal K, Lamba HS. Benzimidazole derivatives–an overview. Int. J. Res. Pharm. Chem. 2011;1(3):565-74.
- Brishty SR, Hossain MJ, Khandaker MU, Faruque MR, Osman H, Rahman SA. A comprehensive account on recent progress in pharmacological activities of benzimidazole derivatives. Frontiers in pharmacology. 2021 Nov 3;12:762807.
- Lee YT, Tan YJ, Oon CE. Benzimidazole and its derivatives as cancer therapeutics: The potential role from traditional to precision medicine. Acta Pharmaceutica Sinica B. 2023 Feb 1;13(2):478-97.
- Chung NT, Dung VC, Duc DX. Recent achievements in the synthesis of benzimidazole derivatives. RSC advances. 2023;13(46):32734-71.
- Alaqeel SI. Synthetic approaches to benzimidazoles from o-phenylenediamine: A literature review. Journal of Saudi Chemical Society. 2017 Feb 1;21(2):229-37.
- Asif M. Green synthesis of benzimidazole derivatives: an overview on green chemistry and its applications. Chemical Methodologies. 2019;3(6):620-31.
- Küçükbay H. Part I: microwave-assisted synthesis of benzimidazoles: an overview (until 2013). Journal of the Turkish Chemical Society Section A: Chemistry. 2017 Sep 1;4(1):1-22.
- Nardi M, Cano NC, Simeonov S, Bence R, Kurutos A, Scarpelli R, Wunderlin D, Procopio A. A review on the green synthesis of benzimidazole derivatives and their pharmacological activities. Catalysts. 2023 Feb 11;13(2):392.
- Ebenezer O, Oyetunde-Joshua F, Omotoso OD, Shapi M. Benzimidazole and its derivatives: Recent Advances (2020–2022). Results in Chemistry. 2023 Jan 1;5:100925.
- Monga J, Ghosh NS, Rani I, Singh R, Deswal G, Dhingra AK, Grewal AS. Unlocking the Pharmacological Potential of Benzimidazole Derivatives: A Pathway to Drug Development. Current Topics in Medicinal Chemistry. 2024 Feb 1;24(5):437-85.
- Lee YT, Tan YJ, Oon CE. Benzimidazole and its derivatives as cancer therapeutics: The potential role from traditional to precision medicine. Acta Pharmaceutica Sinica B. 2023 Feb 1;13(2):478-97.
- Keri RS, Hiremathad A, Budagumpi S, Nagaraja BM. Comprehensive review in current developments of benzimidazole?based medicinal chemistry. Chemical biology & drug design. 2015 Jul;86(1):19-65.
- Keri RS, Hiremathad A, Budagumpi S, Nagaraja BM. Comprehensive review in current developments of benzimidazole?based medicinal chemistry. Chemical biology & drug design. 2015 Jul;86(1):19-65.
- Aroso RT, Guedes RC, Pereira MM. Synthesis of computationally designed 2, 5 (6)-benzimidazole derivatives via Pd-Catalyzed reactions for potential E. coli DNA gyrase B inhibition. Molecules. 2021 Mar 2;26(5):1326.
- Noureldin NA, Richards J, Kothayer H, Baraka MM, Eladl SM, Wootton M, Simons C. Design, computational studies, synthesis and in vitro antimicrobial evaluation of benzimidazole based thio-oxadiazole and thio-thiadiazole analogues. BMC chemistry. 2021 Dec;15:1-9.
- DADHICH IA, PANCHAL NB, DADHICH KA, DADHICH CS. Exploring Benzimidazole Chemistry: Synthesis, Biological activity, and Molecular Docking Studies for Alzheimer's Treatment. Oriental Journal of Chemistry. 2024 Aug 1;40(4).
- Abbade Y, Kisla MM, Hassan MA, Celik I, Dogan TS, Mutlu P, Ates-Alagoz Z. Synthesis, Anticancer Activity, and In Silico Modeling of Alkylsulfonyl Benzimidazole Derivatives: Unveiling Potent Bcl-2 Inhibitors for Breast Cancer. ACS omega. 2024 Feb 14;9(8):9547-63.
- Tahlan S, Kumar S, Kakkar S, Narasimhan B. Benzimidazole scaffolds as promising antiproliferative agents: A review. BMC chemistry. 2019 Dec;13:1-6.
- El-Sayed A, Abu-Bakr S, Swelam S, Khaireldin N, Shoueir K, Khalil A. Applying nanotechnology in the synthesis of benzimidazole derivatives: a pharmacological approach. Biointerface Research in Applied Chemistry. 2022;12:992-1005.
- Lungu CN, Mangalagiu I. Editorial for Special Issue—‘’Research Progress and Applications of Natural Products”. Molecules. 2023 Jul 17;28(14):5449.


 Dhananjay Tidke*
Dhananjay Tidke*
 Dinesh Kawade
Dinesh Kawade


 10.5281/zenodo.14351158
10.5281/zenodo.14351158