The number of components in a mixture, the identity and purity of a compound, the status of a reaction, the composition of the solvent used for preparative separations, and the analysis of the fractions obtained from column chromatography are all feasible quickly, sensitively, and affordably with thin layer chromatography (TLC). In TLC, the mobile phase travels upward with the aid of capillary action in the finely separated stationary phase. Despite the use of organic solvents can be perilous, normal phase TLC often uses organic (non-Polar) solvents such as benzene, 1-2 dichloroethane, carbon tetrachloride, acetonitrile, methanol, tetrahydrofuran, telazin, toluene, sulfolane, and N-N dimethylacetamide. The federal Centres for Disease Control and Prevention state that organic solvents have the potential to be neurotoxic, carcinogenic, and potentially hazardous to procreation. Certain organic solvents can be highly costly as well, and getting rid of them from labs, factories, etc. requires a unique approach that adds to the expense and labour of the operation. In order to tackle this issue, we have used mixed hydrotropic mixtures as mobile phases in this study, which forbids the TLC of amino acids using organic solvents. For some amino acids, the mobile phase formed with the mixed hydrotropy idea produced acceptable results. Furthermore, new mixes can be created and utilised as a solvent for the mobile phase. Therefore, this work suggests that environmentally friendly solvents can be made in substitute of toxic and carcinogenic organic solvents.
Amino Acids, Hydrotropy, Thin Layer Chromatography (TLC), Rf Values, Organic Solvents
Thin-layer chromatography (TLC) is a widely utilized analytical technique due to its simplicity, cost-effectiveness, and versatility in qualitative and quantitative analyses. It plays a critical role in the separation and identification of compounds, including amino acids, through the differential migration of analytes on a stationary phase under the influence of a mobile phase. Despite its benefits, the traditional use of organic solvents in TLC has raised significant environmental and health concerns. Organic solvents, such as benzene, toluene, and dichloroethane, are not only costly but also pose hazards, including neurotoxicity, carcinogenicity, and environmental damage due to improper disposal. To address these challenges, the present study explores the use of mixed hydrotropic solutions as alternative mobile phases in TLC. Hydrotropy, characterized by the solubilization of sparingly soluble compounds in water using hydrotropic agents, offers an eco-friendly and safer approach. By utilizing blends of hydrotropic agents, such as sodium benzoate, urea, and sodium salicylate, this research seeks to enhance the efficiency of TLC while reducing reliance on harmful solvents. The primary objective of this work is to evaluate the efficacy of mixed hydrotropic solutions in the TLC of amino acids. This study demonstrates the potential for hydrotropic mixtures to produce satisfactory retention factors (Rf) for amino acids, thereby offering a viable alternative to traditional organic mobile phases. Moreover, it highlights the applicability of such green methodologies to other analytical and preparative chromatography techniques, paving the way for more sustainable laboratory practices..
Materials And Method
MATERIALS
Silica gel 60 F254 pre-coated TLC plates (20*20cm) 2 units obtained from Supelco Aldrich Germany, D.M. Water, Standard amino acids (Glutamine, Histidine, Tryptophan, Arginine, Methionine, Valine, Alanine and Lysine) obtained from Loba Chemie Pvt. Ltd. Mumbai, Maharashtra; Ninhydrin, Sodium Benzoate, Sodium Acetate, Sodium Salicylate, Sodium Citrate, Urea, n-butanol and Glacial Acetic Acid
Preparation of different solvent systems for the mobile phase Sodium Benzoate (2.5% w/v w/v) + Urea (2.5% w/v) (Blend 1) – 1.25 gm of both Sodium Benzoate and Urea was weighed and taken in a 50ml volumetric flask. To the volumetric flask, 20-30ml D.M. Water was added and the flask was shaken until both solutes gets fully dissolved. Then, the volume was made up to the 50ml mark with D.M. Water. Sodium Salicylate (2.5% w/v) + Urea (2.5% w/v) (Blend 2) - 1.25 gm of both Sodium Salicylate and Urea was weighed and taken in a 50ml volumetric flask. To the volumetric flask, 20-30ml D.M. Water was added and the flask was shaken until both solutes gets fully dissolved. Then, the volume was made up to the 50ml mark with D.M. Water. Sodium Acetate (4% w/v) + Sodium Benzoate (4% w/v) + Sodium Salicylate (2% w/v) (Blend 3) - 2 gm of both Sodium Acetate and Sodium Benzoate and 1 gm of Sodium Salicylate was weighed and taken in a 50ml volumetric flask. To the volumetric flask, 20-30ml D.M. Water was added and the flask was shaken until all the solutes gets fully dissolved. Then, the volume was made up to the 50ml mark with D.M. Water. Sodium Salicylate (2% w/v) + Sodium Citrate (1% w/v) + Urea (2% w/v) (Blend 4) - 1 gm of both Sodium Salicylate and Urea and 0.5 gm of Sodium Citrate was weighed and taken in a 50ml volumetric flask. To the volumetric flask, 20-30ml D.M. Water was added and the flask was shaken until all the solutes gets fully dissolved. Then, the volume was made up to the 50ml mark with D.M. Water.
N-Butanol + Glacial Acetic Acid + Water (4:1:1 v/v) (Organic Phase) – 33.3 ml of n- butanol and 8.3ml Glacial Acetic Acid was pipetted and taken in a 50 ml volumetric flask. After mixing the volume was made up to 50 ml mark with D.M. Water. Ninhydrin Detecting agent (0.25% w/v in ethanol) - 0.25 gm of Ninhydrin was weighed and taken in a 50ml volumetric flask. To the volumetric flask, 20-30ml D.M. Water was added and the flask was shaken until solute gets fully dissolved. Then, the volume was made up to the 50ml mark with D.M. Water.
Preparation Of Standard Solutions for Spotting
Glutamine – 200 mg of glutamine + 10 ml D.M. Water 1 min shaking for complete dissolution.
Histidine – 200 mg of histidine + 10 ml D.M. Water 1 min shaking for complete dissolution.
Tryptophan – 200 mg of tryptophan + 10 ml D.M. Water, 1 min shaking for complete dissolution.
Arginine – 200 mg of arginine + 10 ml D.M. Water 1 min shaking for complete dissolution.
Methionine – 200 mg of methionine + 10 ml D.M. Water 1 min shaking for complete dissolution.
Valine – 200 mg of valine + 10 ml D.M. Water 1 min shaking for complete dissolution.
Alanine – 200 mg of alanine + 10 ml D.M. Water 1 min shaking for complete dissolution.
Lysine – 200 mg of lysine + 10 ml D.M. Water 1 min shaking for complete dissolution.
After preparation of solutions the Solutions were taken in an ignition tube.
Procedure
Development of the TLC Chamber: TLC development containers are specially built beakers with a watch glass on top. The solvent was poured into the chamber to a depth of about 0.5 cm. The filter paper was used to line the interior of the beaker to aid in the saturation of the TLC chamber with solvent vapours. The beaker was covered with a watch glass and kept aside. Preparation of TLC Plates: Silica gel 60 F254 pre-coated TLC plate of dimension 20*20 cm was cutted into small TLC plates of 5*2.5 cm by glass TLC plate cutter.
Spots on TLC Plates: The microcapillary was dipped into the amino acid solution. Then, gently spotting was done at the proper location on the TLC plate at a distance of 1cm from the bottom, and then the plate was air dried at room temperature for 5 minutes.
Development of TLC plate: The prepared TLC plate was placed in the beaker and covered with the watch glass. Then it was left undisturbed. The solvent was allowed to rise up the TLC plate by capillary action. It was made sure that the solvent level did not cover the spot. The plate was allowed to develop until the solvent was about half a centimetre below the top of the plate. Then the plate was removed from the beaker and the solvent front was immediately marked with a pencil. Then the plate was allowed to dry at 50 °C for 20 minutes.
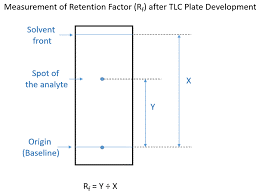
Spot identification and observation: After development and drying of TLC plate (0.25% w/v) Ninhydrin Reagent was sprayed on the TLC plate and was allowed to dry at 100°C for 5 minutes. After 5 minutes violet-coloured spots on the TLC plates were observed. With the help of the spots the distance travelled by the amino acids was measured and the Rf Value was calculated.
Observations
Rf Values are also known as Retention Factor of the solute with respect to the solvent. On the basis of Rf value we can check whether our solute have higher affinity towards mobile phase or with the stationary phase. Rf Values are generally lies between 0 to 1. If Rf Value is less than 0.5 then the solute has more affinity towards stationary phase and if Rf value is more than 0.5 then solute have more affinity towards the mobile phase.
In our experiment first we have applied TLC of Amino Acids using mixed hydrotropy mixtures as a mobile phase. The Rf Values calculated after the TLC using hydrotropy mixtures are given in the following Table 1
Similarly, again we have applied TLC of amino acid by using organic phase as a mobile phase. Here we have taken N-Butanol + Glacial Acetic Acid + Water (4:1:1 v/v) mixture as a mobile phase. The Rf Values calculated after the TLC using organic mixture are given in the following Table 2
RESULTS AND DISCUSSION
It is well observed that the Rf values obtained after employing the proposed methods using the mixed hydrotropic solutions as mobile phases were satisfactory. The selected mobile phases (mentioned in Table 1) gave good results with almost negligible tailing effect. Ideally the Rf Values lie in the range of 0.10 – 0.85. In Organic Phase all Rf Values are lying in this range but in hydrotropy phase some amino acids like Glutamine and Tryptophan have Rf value more than 0.85. It means in hydrotropy phase Glutamine and Tryptophan are having more affinity towards the mobile phase. It may be because of polarity of Glutamine as glutamine is a polar amino acid which have a partial positive and negative charge which make it hydrophilic (water-loving). Tryptophan is a non-polar amino acid which do not contain any type of partial charge but here the components present in the solvent act as a co-factor for Tryptophan by which Tryptophan is showing higher affinity towards solvent. As evident from Table 1, different blends were seen to be suitable for different amino acids.
CONCLUSION
Hence it can be interpreted from the results above that the methods applied are simple, economically feasible, eco-friendly, and safe. Its major advantage is that it precludes the usage of organic solvents. Also, the proposed methods can also be successfully used in the TLC of other drugs or compounds as well. The proposed methods can also be employed in the HPTLC analysis in future to eliminate the use of expensive and toxic organic solvents which are also harmful for the environment.


 Anirudh Padiyar* 1
Anirudh Padiyar* 1
 Rajesh Kumar Maheshwari 2
Rajesh Kumar Maheshwari 2

 10.5281/zenodo.14244007
10.5281/zenodo.14244007