Abstract
The transdermal route offers several advantages over traditional drug delivery methods. These include high bioavailability, avoidance of ?rst-pass liver metabolism, consistent drug levels in the bloodstream, and a non- invasive approach to treatment. Furthermore, transdermal drug delivery systems (TDDS) offer prolonged therapeutic effects, fewer side effects, enhanced bioavailability, improved patient adherence, and easy discontinuation of therapy. TDDS is used not only in pharmaceuticals but also in skincare and cosmetics. Research has shown that the transdermal route causes minimal skin irritation and performs better in various in vivo tests compared to oral administration. This review article provides an in- depth analysis of TDDS, emphasizing its bene?ts over conventional dosage forms, discussing limitations, examining transdermal patch components, exploring various patch types, outlining preparation methods, and identifying ideal TDDS requirements. Additionally, it addresses regulatory factors, physicochemical evaluation methods, therapeutic applications, and recent progress in transdermal drug delivery systems.
Keywords
Tdds; Transdermal Patch; Permeation Enhancer; Partition Coe?cients; Iontophoresis
Introduction
Transdermal drug delivery systems (TDDS), often called patches, offer a unique way to administer medications through the skin. These patches are designed to effectively deliver therapeutic amounts of drugs into the bloodstream, allowing for e?cient treatment and prevention of various health conditions. A transdermal patch is a medicated adhesive that sticks to the skin, releasing precise doses of medication, which can also aid in healing targeted areas. Compared to oral, intravenous, subcutaneous, or transmucosal methods, this delivery route provides more consistent drug levels and reduces side effects, overcoming challenges linked with traditional pills or injections. Transdermal systems are ideal for conditions that need long-term, frequent dosing, as they are less invasive, pain-free, and can be used independently by patients, making them both convenient and cost-effective. These systems are tailored to deliver drugs through the epidermis or dermis, effectively managing skin-related conditions by bypassing ?rst-pass metabolism and allowing controlled release over time. The primary aim is to achieve a steady and predictable drug release rate, reducing patient-to-patient variability. Historically, early transdermal systems used drug-infused patches with natural adhesives to help drugs absorb through the skin. This review highlights recent progress in developing chemical permeation enhancers and carriers, such as gels, emulsions, and vesicular systems, that improve the effectiveness of transdermal drug delivery.
1. Advantages of TDDs
- It allows for a continuous and stable release of medication over a prolonged period, minimizing the chances of side effects and treatment failures that can occur with intermittent dosing.
- These systems enable patients to administer the medication on their own.
- Transdermal delivery prevents the variations in drug levels that occur with peak and trough cycles, permitting extended and less frequent dosing.
- It provides a quicker and more convenient way to administer medication.
- The rate of absorption can be managed through a layered design.
- It avoids issues related to gastrointestinal compatibility.
- Patients are more likely to follow their treatment plans, as they are not required to take multiple doses daily.
- This method allows patients to take control of their medication management independently.
2. Disadvantages of TDDs
- For a drug to be suitable for transdermal delivery, it needs certain physicochemical characteristics to penetrate the stratum corneum. If the required dose is over 10 mg daily, effective transdermal delivery can become di?cult.
- At present, only small, lipophilic drugs can be effectively transported through the skin.
- Transdermal administration offers extended drug release, but it can be costly due to the complex formulations involved.
- Drugs with low solubility, limited stability, short half-lives, or sensitivity to oxidation and hydrolysis present challenges, adding to manufacturing costs.
- There are limitations in the amount of medication that transdermal systems can carry.
- Transdermal delivery might result in lower drug levels in the bloodstream due to variations in skin barrier function, which can be in?uenced by factors like skin location and patient age.
Anatomy And Physiology of Skin:
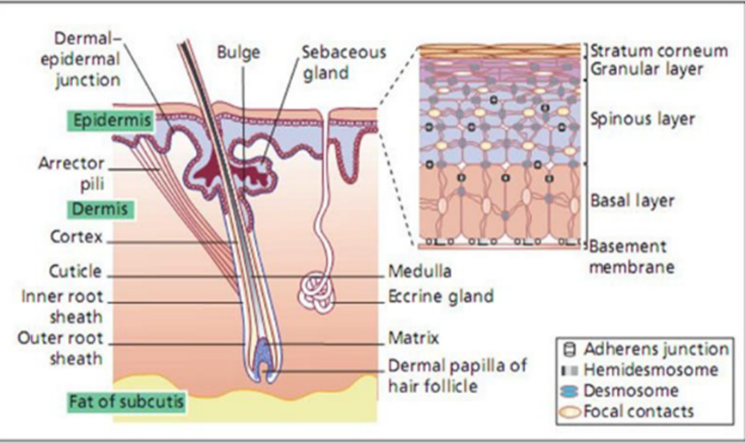
Figure 1 Anatomy and physiology of skin [20]
The skin acts as the body’s main barrier against external elements. As the largest organ, it makes up around 16% of the body’s length, typically covering an area between 1.5 and 2.0 square meters and representing roughly 6-10% of total body weight. Composed of multiple cellular layers, human skin can be classi?ed into two main types: hairless and hair-bearing skin. Hair-bearing skin includes both hair follicles and sebaceous glands.
Layers of skin
Epidermis
The epidermis is the skin’s outermost layer, functioning as a vital protective barrier. It consists of strati?ed epithelial cells and keratinocytes that actively multiply in the suprabasal area, with basal layers showing differentiation. The thickness of the epidermis varies, with regions like the palms and soles reaching about 0.8 mm. It is composed of several layers of epithelial cells, and the lower layers are commonly known as the viable epidermis. Keratinocytes are the dominant cells in this layer.
Dermis
Located below the epidermis, the dermis is a complex, ?bro-elastic layer that provides the skin with structural strength. It contains an extensive network of nerves and blood vessels. Discomfort during parenteral drug administration can result from possible irritation to nerve endings within this layer.
Hypodermis
The hypodermis, or subcutaneous fat layer, supports the epidermis and dermis by storing fat, regulating temperature, and providing cushioning. It contains signi?cant blood vessels and nerves extending to the skin and may house sensory pressure receptors. For transdermal drug delivery, medications must penetrate the epidermis, dermis, and hypodermis to enter the bloodstream, while topical applications focus on permeating the stratum corneum for retention in the skin layers.
Skin and drug permeation
Understanding Transdermal Drug Delivery Systems (TDDS) requires examining the skin’s structure and biochemistry, as these factors in?uence its barrier properties and the rate of drug absorption. Covering roughly 2 square meters in an average adult, the skin is one of the largest organs and receives about one-third of the body’s blood ?ow. The epidermis, the outermost skin layer, is about 150 micrometers thick, formed through the continuous movement of basal epithelial cells migrating to the surface as they differentiate.
Below the epidermis, other layers include the stratum lucidum, stratum granulosum, stratum spinosum, and stratum germinativum, known collectively as the viable epidermis.
The dermis lies beneath the epidermis, acting as the connective tissue foundation and originating from the mesoderm. It consists of a dense network of connective tissues, primarily collagen ?bers, along with some elastic tissue in its upper layers. The dermis also houses blood vessels, lymphatic vessels, nerves, as well as structures like hair follicles, sweat glands, and sebaceous glands.
1. Functions of skin
- Acts as a protective shield against physical, mechanical, and thermal damage, as well as against harmful substances.
- Helps retain moisture to keep the skin hydrated.
- Minimizes the harmful effects of UV radiation from sunlight.
- Serves as a sensory organ, enabling the sensation of touch and temperature changes.
- Aids in temperature regulation by releasing sweat to cool the body as needed.
- Functions as part of the immune system, detecting and responding to potential infections.
- Contributes to vitamin D synthesis when exposed to sunlight.
[These diverse functions highlight the skin’s essential and adaptable role in the human body.]
1. Barrier functions of the skin
The outermost layer of the skin, the stratum corneum, is crucial in preserving the skin’s barrier function. In this layer, tightly packed and overlapping cells provide a strong defense against bacterial invasion while helping to maintain moisture. The stratum corneum is mainly composed of keratinized dead cells and contains less water than other skin layers. To reinforce this barrier, lipids are released by cells from deeper skin layers to the surface, where they form a sturdy, interlocking network like mortar between bricks in a wall.
Basic components of TDDs
- Drug
- Polymer matrix
- Permeation enhancers
- Adhesives
- Backing membrane
- Release Linear [28]
1. Drug
For effective transdermal absorption, drugs must have speci?c physicochemical properties, such as low irritation potential, molecular weights under 1000 Daltons, low melting points, short half-lives, and a balance a?nity for both lipophilic and hydrophilic environments. Selecting suitable drugs for TDDS is crucial to
successful system development [30].
1. Polymer matrix
Polymers play a vital role in TDDS by controlling the drug’s release rate. The polymer matrix can incorporate the drug in a solid or liquid form. In intramuscular drug delivery, biodegradable polymers, either natural or synthetic, are essential for matrix formation, where the drug is dispersed. For targeted injectable delivery, the polymer must be stable and compatible with the drug and other system components, ensuring a safe and controlled release. In TDDS, various polymers are utilized, including:
Synthetic elastomers: polybutadiene, polyisobutylene, silicone rubber, etc.
Synthetic polymers: polyvinyl alcohol, polyvinyl chloride, polyethylene, etc.
Natural polymers: cellulose derivatives, waxes, gums, and eudragits, among others [30].
2. Permeation enhancers
These agents can temporarily modify the structure of the stratum corneum, which enhances drug penetration from the skin into the bloodstream [31]. They work by disrupting the organized lipid layers in the stratum corneum, either by inserting amphiphilic molecules or removing lipids. This temporary change lowers the skin’s barrier resistance, promoting better drug absorption.
An ideal permeation enhancer should be Inert, non-toxic, non-allergenic, non-irritating, and function in a one-
way manner. It should also be compatible with both the drug and other components in the formulation. The effectiveness of these enhancers depends on the drug type, skin properties, and concentration used [26]. A diffusion cell is used to measure how much drug penetrates the skin [32]. These compounds increase stratum corneum permeability to achieve therapeutic drug levels by directly interacting with the skin barrier [33].
3. Adhesives
Unlike multi-layer or single-layer adhesive systems, the reservoir transdermal system has a unique drug reservoir within a compartment made from a drug-impermeable metallic laminate, featuring a rate-controlling membrane on one side. To keep layers separated, a specialized adhesive, such as polyacrylates, polyisobutylenes, or silicone derivatives, is used to secure the system in place [31].
4. Backing membrane
The backing layer in a transdermal patch serves to protect the system from external factors. This layer is impermeable to both drugs and penetration enhancers, providing structural support for the patch and shielding the drug reservoir from environmental exposure. Common backing materials include polyester, aluminized polyethylene terephthalate, and siliconized polyethylene terephthalate [34]. These backing laminates are crucial for supporting the patch [35], preventing drug loss through the top layer, and allowing for printing on the patch [36].
5. Release Linear
To protect the transdermal patch during storage, a liner is placed over it and removed just before application. While not an integral part of the drug delivery system, this liner serves as primary packaging [37]. Common materials for release liners in transdermal systems include polyester foil and metalized laminate [38].
• Transdermal systems can be divided into two-layer systems:
o The single-layer drug in adhesive
o The multi-layer drug in adhesive
1. Single Layer Drug in Adhesive
In the Single-Layer Drug-in-Adhesive system, the drug is contained in a single layer that adheres directly to the skin. A typical transdermal patch has three main layers: the backing membrane, an adhesive layer with the drug, and a protective liner [39]. The adhesive layer lies between the temporary liner and the backing layer, ensuring proper adhesion to the skin [40].
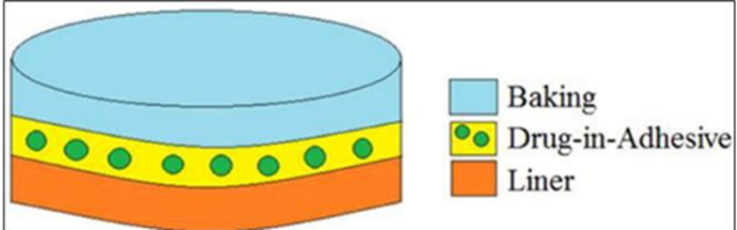
Figure 1 Single layer drug in adhesive [39]
2. Multi-layer drug in adhesive

Figure 2 Multi-layer drug in adhesive [39]
Like the single-layer system, the multi-layer drug-in-adhesive patch delivers the drug through both adhesive layers. It includes one layer for immediate drug release and another layer for sustained release from a reservoir.
The multi-layer system differs by having an additional drug-in-adhesive layer, often separated by a membrane. Known as the Multi-Layer Drug-in-Adhesive system, it also includes a permanent backing layer and a removable liner [41].
5.2.1. Reservoir
In transdermal drug delivery, reservoir systems are characterized by a compartment that holds a drug solution or suspension, separated from the release liner by a semi-permeable membrane and adhesive layer. The adhesive, which secures the patch to the skin, may form a continuous layer between the membrane and the liner or be arranged concentrically around the membrane [42].
A key feature of reservoir systems is their ability to provide zero-order drug release, delivering the drug at a steady and predictable rate throughout the treatment period [43].
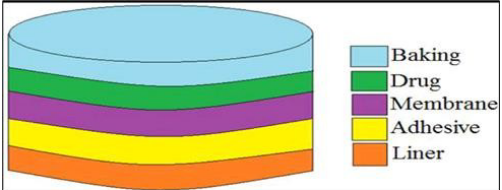
Figure 3 Reservoir [39]
5.2.2. Matrix
A basic transdermal patch design includes three main elements: the drug, an adhesive, and a structural support layer for the patch. In this setup, the drug is embedded within a polymer matrix, simplifying the manufacturing process. Unlike reservoir systems, this design does not include a rate-controlling membrane. However, these patches may be less ?exible compared to reservoir systems. In this design, the drug release rate is mainly governed by the permeability of the skin [44].
3. Route of drug penetration across skin
When a molecule meets unbroken skin, it encounters various substances such as cellular debris, microorganisms, and sebum. This interaction provides the diffusant with three potential pathways to access viable tissue: it can enter through the hair follicles and their associated sebaceous glands, navigate through sweat ducts, or pass through the intact stratum corneum that separates these skin structures [45].
5.3.1. Transcellular Route
Drugs that penetrate the skin via the transcellular route move through the corneocytes. These corneocytes, abundant in hydrated keratin, create a water-rich environment that facilitates the passage of hydrophilic drugs. The transcellular pathway requires not only partitioning into and diffusing through the keratin "bricks" but also in?ltrating and traversing the intercellular lipids [46].
5.3.2. Intercellular Route
The intercellular route allows drugs to diffuse through a continuous lipid matrix. Solutes navigate through the lipid regions by diffusing between the horny cells of the stratum corneum, the viable cells of the epidermis, and into the dermis [47].
This pathway presents notable challenges for two primary reasons:
[I] According to the "bricks and mortar" model of the stratum corneum, the interlocking structure of the corneocytes creates a complex pathway for drugs to permeate intercellularly, unlike the more straightforward transcellular route.
[ii]The intercellular area consists of alternating structured bilayers, meaning a drug must go through repeated cycles of partitioning and diffusing between both aqueous and lipid domains.
This route is generally regarded as the most common for small, uncharged molecules to penetrate the skin [48].
5.3.3. Trans appendageal Route
Also known as the "shunt pathway," this route allows drug molecules to pass through hair follicles, travel along the sebaceous pathways of the pilosebaceous apparatus, or use the aqueous pathways of the eccrine sweat glands. However, this trans appendageal pathway is considered less signi?cant due to its small surface area, which constitutes less than 0.1% of the overall skin surface [49].
Kinetics of Transdermal Permeation
A thorough understanding of transdermal dynamics is essential for the effective development of transdermal devices. This understanding encompasses several critical components:
• "Horny Layer Absorption": This term refers to the uptake of drugs through the stratum corneum, which is the outermost skin layer [50].
• "Drug Absorption Across Skin Layers": This involves the process of drug molecules passing through various skin layers, such as the epidermis and dermis, to enter the bloodstream [50].
• "Absorption in the Epidermal-Dermal Papillae": This focuses on how drugs are absorbed within structures called epidermal-dermal papillae, found within the epidermis where it interfaces with the dermis [50].
Each of these elements plays a vital role in the overall process of transdermal drug delivery.
Different generations of TDDS
There are Four generations of TDDS according to the advancement of the TDDS, which are as follows.
? First Generation
? Second Generation
? Third Generation
? Fourth Generation
5.5.1. First Generation
The ?rst generation of basic transdermal patches began to appear in the early 1970s. Following the initial approval from the U.S. Food and Drug Administration (FDA) for the scopolamine patch intended for motion sickness, around 19 different types of patches have been commercially produced, including those for nicotine, menthol, and estradiol. However, the range of drugs that can be effectively formulated into patches is still limited due to the physiological barriers of the epidermis. Most of the transdermal drugs from this initial generation are highly lipophilic, exhibiting partition coe?cients greater than 10^4, having small particle sizes, and molecular weights below 400 Daltons. Research in this period focused on tailoring the physicochemical characteristics of these chemical compounds. Essentially, drugs designed for transdermal delivery were either speci?cally selected or modi?ed to have a high partition coe?cient and a low molecular weight to enhance their ability to diffuse through the skin barrier [56].
5.5.2. Second Generation
The second generation of Transdermal Drug Delivery Systems (TDDSs) was created to enhance the ability to deliver small molecule drugs through the skin. These systems are based on two key principles:
• Modi?cation of Drug Properties: Drugs are altered to exhibit optimal characteristics, such as an appropriate logarithm of the partition coe?cient (log P), which aids in their absorption through the skin.
• Structural Changes in the Stratum Corneum (SC): The structure of the SC is modi?ed or pore channels are created within it using various physicochemical techniques. These changes create an additional driving force for the drug to penetrate the skin, thereby improving the e?ciency of transdermal drug delivery.
Although second-generation TDDSs established a foundation for enhancing drug delivery e?ciency, they encountered certain limitations. A major challenge was achieving an optimal balance between maximizing drug absorption through the SC while safeguarding the underlying tissues from potential harm. This challenge paved the way for the development of third-generation TDDSs, which likely sought to resolve these issues and further enhance transdermal drug delivery systems. [57]
5.5.3. Third Generation
The third generation of Transdermal Drug Delivery Systems (TDDS) is distinguished by its minimally invasive techniques that involve disrupting the stratum corneum (SC) to enable the absorption of larger molecule drugs and even vaccines through the skin. Two primary methods used in this generation of TDDS are electroporation and microneedling. [58, 59]. Electroporation employs electric pulses to temporarily disrupt the structure of the SC, creating transient pores. This approach enhances the effectiveness of transdermal drug distribution while protecting deeper tissues. In contrast, microneedling utilizes tiny needles to form microchannels in the SC, which aids in the delivery of drugs into the skin. These techniques provide the opportunity to administer a broader array of medications and vaccines through the skin while reducing invasiveness and ensuring the safety of underlying tissues. [60]
5.5.4. Fourth Generation
Personalized therapy marks a notable shift from traditional medical approaches as it customizes treatment based on each person's speci?c pathophysiological conditions. Implementing personalized therapy requires careful management of the dosage given, informed by real-time tracking of the patient's physiological parameters. This method allows for a tailored assessment of disease progression and drug e?cacy.
In response to the increasing demand for individualized treatments, advanced transdermal delivery systems augmented by soft bioelectronics have emerged as a promising avenue for future drug delivery techniques. These systems can offer more precise and ?exible drug administration, effectively addressing the unique requirements of each patient. [61]
Microneedle patch
Microneedles (MNs) are tiny needles ranging from tens to hundreds of micrometers that can penetrate the outer skin layers, enhancing drug absorption. However, due to their small size, MNs have limited capacity for drug loading. One approach to address this is the “poke and patch” method. Yang et al. developed a touch-MN array patch (TMAP) for insulin delivery using this strategy. The TMAP consists of PMMA MNs and an insulin- loaded sponge. When pressed against the skin, the MNs puncture both the sponge and skin, allowing insulin to diffuse into the skin only while pressure is maintained. This method can provide up to 12 hours of insulin delivery, signi?cantly better than standard injections, while also lowering the risk of hypoglycemia. However, matching the insulin dosage to a patient's blood glucose level remains challenging. Another method, “coated and poke,” involves pre-coating the MN tips with drugs for rapid release in small doses. For instance, Choi et al. created HA MNs coated with a canine in?uenza vaccine, which remained effective even at high temperatures and generated a strong immune response in guinea pigs, outperforming traditional injections. Similarly, Wang et al. designed mesoporous silica-coated HA MNs for delivering siRNA, which protected the RNA from degradation and successfully delivered it into skin cells.The “poke and ?ow” method utilizes hollow MNs to administer drugs through their inner cavities. Bolton et al. produced a hollow silicon MN array that minimizes pain during insertion, while Resnik developed a hollow MN patch powered by a micro pump, allowing for controlled drug release rates. Yeung created a hollow MN patch with a micro?uidic chip for delivering various drugs, with dosage adjustable through ?ow rate control. They demonstrated this with high-dosage insulin delivery, achieving full release in four hours. Li et al. introduced a rapid-separation MN made from a PLGA/PLA composite for sustained contraceptive release, which can be adjusted for up to 40 days of delivery. The latest advancement is a glucose-responsive delivery system for insulin and glucagon, designed for continuous blood sugar management. This system adjusts the release of insulin and glucagon based on blood glucose levels in a hyperglycemic mouse model. Some of these innovative MN designs for insulin delivery are currently undergoing clinical trials.
Wearable patches for active delivery
5.1 Electricity stimulated patch

A wearable patch equipped with integrated electronics enables precise drug delivery through electric signaling. The microprogrammed control unit (MCU) provides detailed management of drug application. For instance, Xu et al. developed a wearable, software-controlled device for wound treatment that aids healing by assessing and treating infections. The ?exible printed circuit, with a built-in Near Field Communication (NFC) antenna, remotely controls the drug-loaded adhesive patch and biosensing modules. This system monitors factors like temperature, pH, and uric acid at the infection site. Hydrogels are also effective in building wearable devices that respond to electrical stimulation. Lim et al. created a hydrogel patch containing impedance sensors, an electric nerve stimulator, a transcutaneous oxygen pressure sensor, and iontophoretic electrodes. The PEDOT: PSS/PAAM hydrogel structure provided a skin-friendly interface, allowing electrochemical sensing and drug delivery through iontophoresis. This design reduced impedance by about 20% at low frequencies, and the integrated AgCl cathode and Zn anode delivered a stable 200 mA current for over 100 minutes. Tests showed that the self-powered iontophoretic patch increased current density signi?cantly, achieving greater skin penetration for the drug (Rhodamine B) compared to passive diffusion.
5.2 Ultrasound stimulated patch

The ultrasound-responsive patch includes components sensitive to low-frequency (<100>100 kHz and MHz range) ultrasound. Since the 1990s, ultrasound has been known to enhance the permeability of agents into cells and tissues, primarily through thermal and non-thermal effects. Thermal effects arise from the absorption of acoustic energy in tissues, while non-thermal effects are produced by ultrasound pressure, acoustic streaming, microjets, and cavitation. In wearable patches, the ultrasonic ?eld can be generated by external devices or embedded components, facilitating the release of drugs from reservoirs. Soto et al. developed a gel-based wearable patch for delivering lidocaine through acoustic droplet vaporization (ADV). In this design, lidocaine was mixed with a per?uorocarbon emulsion and stored within a soft array of microholes. When exposed to an ultrasound pulse (25 ms, 2.25 MHz, 12 V), the emulsion vaporized, creating pressure to penetrate the skin’s barriers and form micropores for lidocaine absorption. This method allowed a lidocaine dosage 4- and 2-fold higher than passive diffusion and ultrasound alone. For the device’s potential in chronic pain therapy, large-scale clinical trials targeting various nerve depths are needed. Additionally, miniaturizing portable ultrasound probes could increase this technology's potential as a clinical point-of-care device. Choi et al. identi?ed a reversible metal-tannic acid (TA) coordination responsive to ultrasound. They coated porous SiO2 nanoparticles with a Fe-TA network (TA-FeIII/MSN) and loaded them into a sodium alginate hydrogel, creating a skin patch. Applying low-frequency ultrasound (50 Hz for 2 minutes) weakened the Fe-TA bonds, loosening the network and releasing the drug onto the skin.
5.3 Light Stimulated Patches
The light-responsive system offers precise control over light intensity and coverage, which allows for accurate dosing. Near-infrared (NIR) light is widely used due to its ability to deeply penetrate skin tissue with minimal tissue damage. Dhal and colleagues employed NaYF4 upconversion particles that emit light to produce reactive oxygen species (ROS) when triggered by NIR for treating skin cancer. They first prepared oleogels as a drug reservoir using soybean oil as a solvent, stearic acid as a gelling agent, and additional agents to enhance permeation. Traditional ROS therapies based on upconversion nanoparticles often struggle to localize, which complicates targeting deep-seated skin lesions like melanoma. However, the oleogel can effectively transport nanoparticles to deeper skin layers, enabling ROS production in targeted areas and improving bioavailability. In laboratory tests, nanoparticles delivered via oleo gels reached subcutaneous and intradermal layers up to 9 mg/cm?3; within 48 hours, whereas direct nanoparticle application showed minimal penetration. The 1,3-Diphenylisobenzofuran (DPBF) assay, used to measure ROS generation upon light exposure, showed that the oleo gel approach led to a higher ROS level (about 55% DPBF consumption), compared to almost no ROS production in the direct application group. Similarly, Wang and colleagues created a phase-change polycaprolactone (PCL) microneedle (MN) patch containing the NIR-II fluorophore Flav7 and the anticancer drug doxorubicin (DOX) for chemo-thermal tumor treatment. Flav7 has excellent photothermal properties and imaging capability. When exposed to laser light, Flav7 converted light to heat, melting the PCL matrix and releasing DOX. In animal studies, DOX release from the MN patch led to significant tumor cell death. As a proof-of-concept, researchers noted that further optimization of structural parameters, such as needle tip length, is necessary for future applications.
REFERENCES
- Scheuplein RJ. Mechanism of percutaneous adsorption: I. routes of penetration and the influence of solubility. J Invest Dermatol 1965;45: 334e46.
- Blank IH. Penetration of low-molecular-weight alcohols into skin: I. effect of concentration of alcohol and type of vehicle. J Invest Dermatol 1964; 43:415e20.
- Michaels AS, Chandrasekaran SK, Shaw JE. Drug permeation through human skin: theory and in vitro experimental measurement. AIChE J 1975; 21:985e96.
- Bagherifard S, Tamayol A, Mostafalu P, Akbari M, Comotto M, Annabi N, et al. Dermal patch with integrated flexible heater for on demand drug delivery. Adv Healthc Mater 2016; 5:175e84.
- Amjadi M, Sheykhansari S, Nelson BJ, Sitti M. Recent advances in wearable transdermal delivery systems. Adv Mater 2018; 30:1704530.
- Padmanabhan RV, Phipps JB, Lattin GA, Sawchuk RJ. In vitro and in vivo evaluation of transdermal iontophoretic delivery of hydromorphone. J Control Release 1990; 11:123e35.
- Hsieh CH, Ku YA, Chiu LH, Young TH, Huang YY. A transdermal drug delivery system containing deferoxamine mesylate for the treatment of b-thalassaemia major. Biomed Eng 2011; 23:29e35.
- Zhang Y, Wang D, Gao M, Xu B, Zhu J, Yu W, et al. Separable microneedles for near-infrared light-triggered transdermal delivery of metformin in diabetic rats. ACS Biomatter Sci Eng 2018; 4:2879e88.
- Yu J, Wang J, Zhang Y, Chen G, Mao W, Ye Y, et al. Glucoseresponsive insulin patch for the regulation of blood glucose in mice and minipigs. Nat Biomed Eng 2020; 4:499e506.
- Li X, Huang X, Mo J, Wang H, Huang Q, Yang C, et al. A fully intargeted closed-loop system based on mesoporous microneedlesiontophoresis for diabetes treatment. Adv Sci 2021; 8:2100827.
- Mali AD, Bathe R, Patil M. An updated review on transdermal drug delivery systems: advancements and applications. Int J Pharm Sci Rev Res 2023; 77:45-55


 Devesh Kolte*
Devesh Kolte*






 10.5281/zenodo.14452546
10.5281/zenodo.14452546