Abstract
CAR-NK cells are a novel development within cancer immunotherapy that merges the innate cancer killing features of NK cells with the targeted nature of CAR technologies. Unlike CAR-T cells, CAR-NK compels cells among which there are lower side effects and the ability to target cancer cells regardless of MHC expression. These kinds of NK cells are readily available for application in a variety of solid tumors and as well as against hematologic malignancies hence the biology and bioengineering aspects are explained in this CAR-NK review. The ongoing progress in gene editing tools based on the CRISPR/Cas9 system, as well as increased productivity and cloning of these cells, greatly expands their possible applications. New strategies including cytokine support and combination therapy are instrumental in improving the functionality of CAR-NK cells while dealing with challenges including a short persistence time and immunosuppressive tumor microenvironments. Increasingly adaptive therapeutic models and systems are starting to produce positive results in the treatment of solid and hematological malignancies using CAR-NK cells. This is not so far as to claim that CAR-NK cells will self propel in further advance of phase 4 or beyond as it seems as though it is a new pathway for cancer therapeutics that is patient centered
Keywords
CAR-NK cells, immunotherapy, natural killer cells, tumor microenvironment, hematological malignancies.
Introduction
Cancer comes second to heart attacks as the leading cause of death worldwide. There are various approaches to treating cancer; they include Chemotherapy, surgery, and radiotherapy. A major downside to these three methods is that they come with a range of side effects and there is a good chance that they may cause cancer to recur[1,2]. However, the newest and most efficient approach to cancer treatment is immunotherapy. More recent literature has shown that immunotherapy works for several forms of cancer, including lymphomas and myelomas[3,4]. Natural killer (NK) cells, which include effector cells such as cytotoxic T lymphocytes (CTLs), are among the immune response cells that target the primary malignant cell and metastatic cells by blocking tumor cell proliferation and movement[5]. Whether or not NK cells are cytotoxic, they can secrete different cytokines, specifically interferon-? (IFN-?), to help induce regulatory immune responses and other important processes. Most importantly, NK cells can tell the difference between healthy and malignant cells and therefore have less cross reactivity after anti-tumor cytotoxicity and targeting[6,7]. There are numerous examples from both in vitro and in vivo studies that indicate that immunological escape can be prevented using NK cells modified with the chimeric antigen receptor (CAR)[8,9]. The emerging technology of CAR-modified NK cells seems to give a chance of developing a new generation of anti-cancer immunotherapeutic agents[10]. Cancer target cells are destroyed by CAR-NK cells via both NK cell receptors and CAR which is designed to target tumor-associated antigens (TAAs)[7]. This review aims to highlight the importance of the advancement of CAR-NK cell therapy as an advanced solution for cancer immunotherapy and summarize the latest advances in the field of treating human cancers with this therapy.
METHODOLOGY
The scope of the systematic review extends to the following keywords: CAR-NK cells, immunotherapy, tumor microenvironment, and hematologic malignancies. This review covers the period from 2000 to 2024. Articles from the source were collected, analyzed and discussed. It was not necessary to get ethical clearance. The results will be published in academic journals, presented at conferences and researchers.
Chimeric Antigen Receptor Structure
Chimeric antigen receptors are a category of artificial receptors that merge the ability to recognize an antigen with that of cell-activating T-cells (CAR-T) into one single polypeptide[12,13,14]. A typical CAR is composed of four distinct areas: an extracellular structural domain, the hinge region, a membrane-spanning domain and an intracellular T cell activating domain or an endodomain[11,23,24].
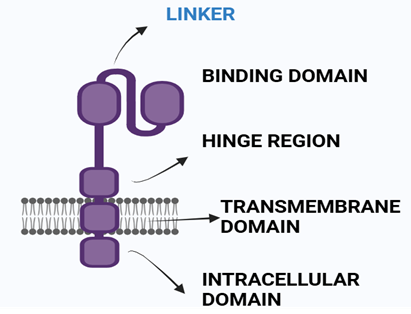
Fig 1 Structure of CAR
Antigen Recognition Domain this domain recognizes antigens present in the receptor's epiderm[15]. This domain is linked together as a single chain of variable fragments[16,17].
- Hinge Region The hinge region is a small area between the antigen recognition domain and the outer membrane of the T cell[18]. Provides extra flexibility to CARs[19] .
- Transmembrane Domain of CAR Present in between Hinge domain and intracellular signaling domain[20]. Derived from CD3-?, CD4, CD8, and sometimes CD28 molecules.It plays a critical role in the influence of CAR-T-cell effector function[21].
- Intracellular T-cell Signaling Domain In the Endodomain, the intracellular T cell signaling domain is present[22]. When a protein (antigen) binds to the antigen recognition region, CARS receptors cluster together, resulting in the transmission of an signal of activationfor the intracellular T cell signaling domain. Co-stimulatory domains like CD-27, CD-28, CD-134, and CD-137 are successfully used as signaling domains[11].
Evolution Of Cars.
Initial T-cell receptor (TCR) provided by first-generation CARs, but immunotherapy was ineffective due to insufficient T-cell persistence. Second and third-generation CARs incorporate co-stimulatory domains, such as CD28, CD137 (4-1BB), OX40 or ICO improving CAR efficacy. Fourth-generation CARs have additional signals for potency. CAR T-cells bind antibody-like manner, targeting is independent of HLA haplotype or tumor-associated HLA downregulation, and non-protein antigens can be engaged[25,26].
Enumerated Achievements In Car-NK Therapy
The development of CAR T cell therapy began in 1989 with the introduction of effector T cells. It has since been used against prostate cancer, leukemia, and refractory leukemia. CD19 CAR T cell therapy has been successful in treating pediatric Acute Lymphoblastic leukemia. The concept of CAR-NK cell was introduced in 2015. The FDA approved CAR T cell therapy in 2017 and 2018 for Acute Lymphoblastic Leukemia[11].
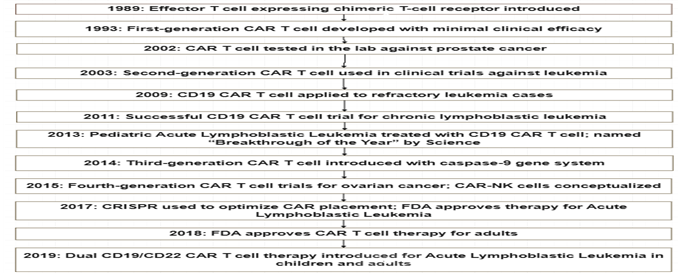
Fig 2. Development of CAR NK therapy
Role Of Nk Cells In Cancer Progression
NK cells are involved in cancer biology, more so in hematological malignancies[27]. According to the preceding literature, they possess synthetic receptors that bind to tumor-associated antigens enabling them to efficiently target cancer cells[28,29,30]. They have a remarkable capacity to detect and destroy abnormal cells with no prior sensitization such that they become ideal tools for therapeutic applications[31]. When activated, they secrete perforin and granzymes, trigger apoptosis and death receptor pathways[32,33,34]. They secrete cytokines such as IFN-? and TNF-? which are also able to activate the immune system[35]. However, there are various challenges hampering their use, including the inability to effectively infiltrate solid tumors and the complex tumor microenvironments (TME)[36]. To eliminate these TME barriers, NK cells have to penetrate through the immunosuppressive factors along with the suppressive immune cells residing in the tumor microenvironment[37]. In spite of these issues, the TME infiltration of NK cells correlates with positive outcomes in several cancers so far studied, including breast cancer, cervical cancer, liver cancer, and melanoma[38,39]. They can kill tumor cells by different ways including the secretion of perforin, granzymes, apoptosis, lymphokines and neoantigens[40]. Further advancing comprehension of activating NK cells, the convoluted TME and new relative tools such as iPSC derived NK cells can help introduce new therapeutic concepts[41,42,43,44].
Generation Process Of CAR?NK Cells
The ability to edit already formed NK cells or even make new NK cells from different cells (Cord blood, Peripheral blood, hiPS, hES, HSC etc[45,46,47,48,49]) with the use of lentivirus or retrovirus vectors is possible, and then culturing them in NK cell culture medium. Heavily enriched CAR-NK cells are also given intravenously so that tumour cells can be selectively eliminated[7,50].
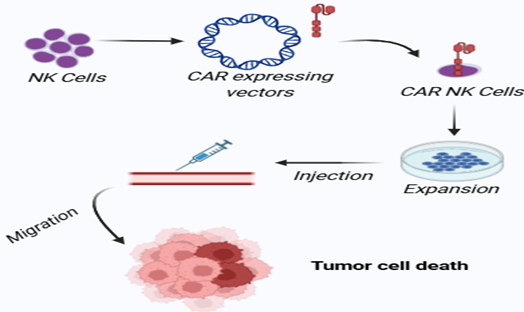
Fig 3. Generation of CAR NK cells
Car?NK Cell?Target Therapy For Human Cancers
CAR-NK therapies target a wide range of tumor antigens across diverse cancers such as leukemia, breast, ovarian, and lung cancers[51,52,53,54,55].
Table 1. CAR?NK cell?target for human cancer therapy
|
Target
|
Tumor
|
|
CD19, CD7, CD5, FLT3
|
Acute lymphocytic leukemia (ALL)
|
|
CD33, CD123, CD4
|
Acute myelocytic leukemia (AML)
|
|
HER2, EpCAM, TF, EGFR, NKG2D
|
Breast cancer
|
|
CD19
|
Chronic lymphocytic leukemia (CLL)
|
|
EpCAM, CEA, NKG2D
|
Colorectal cancer
|
|
GD2
|
Ewing sarcoma
|
|
HER2
|
Gastric cancer
|
|
EGFRvIII, EGFR, CD73, HER2
|
Glioblastoma
|
|
ROBO1
|
Glioma and Neuroblastoma
|
|
GPC3, NKG2D
|
Hepatocellular cancer (HCC)
|
|
c-MET
|
liver cancer
|
|
NKG2D, B7-H3
|
Lung cancer
|
|
CD19, CD4
|
Lymphoma
|
|
GPA7
|
Melanoma
|
|
CD138, CS1, BCMA
|
Multiple Myeloma
|
|
GD2, CD276
|
Neuroblastoma
|
|
aFR, HER2, Mesothelin, GPC3, NKG2D
|
Ovarian cancer
|
|
Mesothelin, ROBO1
|
Pancreatic cancer
|
|
PSMA
|
Prostate
|
|
HER2, EGFR
|
Renal cell carcinoma (RCC)
|
|
PSCA
|
Ladder carcinoma
|
|
HLA-G
|
Renal clear cell and renal papillary cell carcinoma, Pancreatic ductal adenocarcinoma, Thyroid cancer
|
|
CD20
|
Lymphoma, Leukemia cells
|
|
NKG2D
|
Osteosarcoma
|
Ongoing Clinical Trials Targeting Tumors Using Car-NK
Various types of malignancies, such as ovarian, lung, pancreatic, breast, colorectal, and renal cell carcinoma, are the subject of ongoing CAR-NK cell treatment studies. The purpose of these studies, which are mostly in Phase 1 or Phase 1/2 phases, is to evaluate the therapeutic potential, safety, and effectiveness of novel cancer immunotherapy strategies[56,57,58].
Table 2. Ongoing clinical trials targeting tumors using CAR-NK
|
NCT Number
|
Tumor Type
|
Phase Stage
|
|
NCT05776355
|
Ovarian Cancer
|
N/A
|
|
NCT05213195
|
Refractory Metastatic Colorectal Cancer
|
Phase 1
|
|
NCT05410717
|
Stage IV Ovarian Cancer, Refractory Testis Cancer, Endometrial Cancer Recurrent
|
Phase 1
|
|
NCT06066424
|
Non-small Cell Lung Cancer, Breast Cancer
|
Phase 1
|
|
NCT05507593
|
Small Cell Lung Cancer
|
Phase 1
|
|
NCT04847466
|
Gastric or Head and Neck Cancer
|
Phase 2
|
|
NCT05922930
|
Ovarian Cancer, Adenocarcinoma, Pancreatic Cancer
|
Phase 1/2
|
|
NCT05703854
|
Advanced Renal Cell Carcinoma, Mesothelioma Osteosarcoma
|
Phase 1/2
|
|
NCT03383978
|
Glioblastoma
|
Phase 1
|
|
NCT03940833
|
Multiple Myeloma
|
Phase 1/2
|
|
NCT02944162
|
Myeloid Leukemia Acute
|
Phase 1/2
|
|
NCT03415100
|
Solid Tumors
|
Phase 1
|
|
NCT04623944
|
AML, AML Relapsed/Refractory, Adult MDS
|
Phase 1
|
|
NCT05020678
|
Lymphoma, Non-Hodgkin, B-cell Acute Lymphoblastic leukemia, Large B-cell Lymphoma
|
Phase 1
|
|
NCT05110742
|
Hematological malignancy
|
Phase 1/2
|
|
NCT03692637
|
Epithelial Ovarian Cancer
|
Phase 1
|
|
NCT03692663
|
Metastatic Castration-resistant Prostate Cancer
|
Phase 1
|
|
NCT03941457
|
Pancreatic Cancer
|
Phase 1/2
|
|
NCT03940820
|
Solid Tumor
|
Phase 1/2
|
|
NCT03931720
|
Malignant Tumor
|
Phase 1/2
|
FDA Approved Car-T Cell Therapies
The FDA has authorised six CAR-T cell treatments for the treatment of haematological malignancies since 2017[59,60,61,62]. They are
- Kymriah (tisagenlecleucel, CD19 CAR-T cells)
- Yescarta (axicabtagene ciloleucel, CD19 CAR-T cells)
- Tecartus (brexucabtagene autoleucel, CD19 CAR-T cells)
- Breyanzi (lisocabtagene maraleucel, CD19 CAR-T cells)
- Abecma (idecabtagene vicleucel B-cell maturation antigen (BCMA) CAR-T cells) and
- Carvykti (ciltacabtagene autoleucel, BCMA CAR-T cells).
Among these, four (Kym-riah, Yescarta, Tecartus, and Breyanzi) are anti-CD19 CAR-T cells and two (Abecma andCarvykti) target BCMA[63,64].
DISCUSSION
A new potential avenue for advancing cancer immunotherapy is CAR-NK cell therapy which has a more of advantages over CAR T therapies that include higher precision, lesser off-targets, and fewer chances of suffering from cytokine release syndrome (CRS)[65,66,67]. Nowadays, more and more practitioners have global access to technology that enhances viral transduction, electroporation, chimeric antigen receptors (CAR), CRISPR/Cas9 gene editing, and effective cell proliferation strategies, all of which might help facilitate the production and characterization of genetically modified NK cells. Those promising inventions assist scientists and clinicians in creating a stronger NK cell for therapeutic applications. In this regard, it must be emphasized that the utilization of a CAR for NK cell rerouting holds great potential for cancer immunotherapy. CAR-NK cells have undergone a range of preclinical and recently clinical trials with targets like high target cell killing with no side effects such as nurotoxicity and cytokine release syndrome that are common with CAR T therapies[68].
Future Prospectives
Strategies to enhance the CAR-NK therapy include the use of cytokines like IL-15 to improve in vivo persistence, improvement in gene editing approaches such as CRISPR/Cas9, and the introduction of viral vectors that are both cheap and easy to administer. Such engineering can also help in combatting TGF-? which is crucial to engage a hostile tumor microenvironment. Like CAR T cells, larger-scale production of CAR NK cells should rely on iPSCs that would make CAR-NK cells widely available and affordable due to their universal “off the shelf” properties. Furthermore, CAR-NK cells can be used along with checkpoint inhibitors and other modalities, and this approach has great promise in achieving a combined effect.
CONCLUSION
Because of their properties CAR-NK cells will surely improve cancer treatments by addressing the limitations that other modified approaches face. There is assurance for continuous development in treating solid tumors and blood cancer even in the early stages of clinical development. For CAR-NK cells to become mainstream therapies, there is a need to work on enhancing their performance, durability, and affordability for maximum acceptance in surgeries.
ACKNOWLEDGMENT:
We would like to take this opportunity to express my heartfelt thanks to everyone who directly and indirectly, helped throughout the my work.
REFERENCES
- Fitzmaurice C, Dicker D, Pain A, Hamavid H, Moradi-Lakeh M, MacIntyre MF, et al. The global burden of cancer 2013. JAMA Oncol. 2015;1(4):505-27.
- Nies YH, Ali AM, Abdullah N, Islahudin F, Shah NM. A qualitative study among breast cancer patients on chemotherapy: experiences and side-effects. Patient preference and adherence. 2018:1955-64.
- Muenst S, Soysal SD, Tzankov A, Hoeller S. The PD-1/PD-L1 pathway: biological background and clinical relevance of an emerging treatment target in immunotherapy. Expert opinion on therapeutic targets. 2015;19(2):201-11.
- Ahmad U, Khan Z, Ualiyeva D, Amissah OB, Noor Z, Khan A, et al. Chimeric antigen receptor T cell structure, its manufacturing, and related toxicities; A comprehensive review. Adv Cancer Biol Metastasis. 2022;4:100035.
- Sivori S, Pende D, Quatrini L, Pietra G, Della Chiesa M, Vacca P, Tumino N, Moretta F, Mingari MC, Locatelli F, Moretta L. NK cells and ILCs in tumor immunotherapy. Mol Asp Med. 2021;80:100870.
- Cheng M, Zhang J, Jiang W, Chen Y, Tian Z. Natural killer cell lines in tumor immunotherapy. Front Med. 2012;6(1):56?66.
- Marofi F, Abdul-Rasheed OF, Rahman HS, Budi HS, Jalil AT, Yumashev AV, et al. CAR-NK cell in cancer immunotherapy; A promising frontier. Cancer Sci. 2021;112(9):3427-36.
- Burger MC, Zhang C, Harter PN, Romanski A, Strassheimer F, Senft C, et al. CAR-engineered NK cells for the treatment of glioblastoma: turning innate effectors into precision tools for cancer immunotherapy. Front Immunol. 2019;10:2683.
- Hambach J, Riecken K, Cichutek S, Schütze K, Albrecht B, Petry K,et al. Targeting CD38-expressing multiple myeloma and burkitt lymphoma cells in vitro with nanobody-based chimeric antigen receptors (Nb-CARs). Cells. 2020;9(2):321.
- Siegler EL, Zhu Y, Wang P, Yang L. Off-the-shelf CAR-NK cells for cancer immunotherapy. Cell stem cell. 2018;23(2):160-1.
- Ahmad U, Khan Z, Ualiyeva D, Amissah OB, Noor Z, Khan A, et al. Chimeric antigen receptor T cell structure, its manufacturing, and related toxicities; A comprehensive review. Adv Cancer Biol Metastasis. 2022;4:100035.
- Javdan SB, Deans TL. Design and development of engineered receptors for cell and tissue engineering. Curr Opin Syst Biol.2021;28:100363.
- Hong M, Clubb JD, Chen YY. Engineering CAR-T cells for next-generation cancer therapy. Cancer cell. 2020;38(4):473-88.
- Rivière I, Sadelain M. Chimeric antigen receptors: a cell and gene therapy perspective. Mol Ther. 2017;25(5):1117-24.
- Zhang C, Liu J, Zhong JF, Zhang X. Engineering car-t cells. Biomark Res. 2017;5:1-6.
- Nakajima M, Sakoda Y, Adachi K, Nagano H, Tamada K. Improved survival of chimeric antigen receptor?engineered T (CAR?T) and tumor?specific T cells caused by anti?programmed cell death protein 1 single?chain variable fragment?producing CAR?T cells. Cancer Sci.. 2019;110(10):3079-88.
- Chandran SS, Klebanoff CA. T cell receptor?based cancer immunotherapy: emerging efficacy and pathways of resistance. Immunol Rev. 2019;290(1):127-47.
- Alabanza L, Pegues M, Geldres C, Shi V, Wiltzius JJ, Sievers SA, Yang et al. Function of novel anti-CD19 chimeric antigen receptors with human variable regions is affected by hinge and transmembrane domains. Mol Ther. 2017;25(11):2452-65.
- Darowski D, Kobold S, Jost C, Klein C. Combining the best of two worlds: highly flexible chimeric antigen receptor adaptor molecules (CAR-adaptors) for the recruitment of chimeric antigen receptor T cells. MAbs. 2019;11(4):621-31.
- Morales L, Paramio JM. Cell therapies in bladder cancer management. International Journal of Mol Sci. 2021;22(6):2818.
- Julamanee J, Terakura S, Umemura K, Adachi Y, Miyao K, Okuno S, et al. Composite CD79A/CD40 co-stimulatory endodomain enhances CD19CAR-T cell proliferation and survival. Mol Ther. 2021;29(9):2677-90.
- Lanitis E, Coukos G, Irving M. All systems go: converging synthetic biology and combinatorial treatment for CAR-T cell therapy. Current opinion in biotechnology. 2020;65:75-87.
- Sadelain M, Brentjens R, Rivière I. The basic principles of chimeric antigen receptor design. Cancer discovery. 2013;3(4):388-98.
- McErlean EM, McCarthy HO. Non-viral approaches in CAR-NK cell engineering: connecting natural killer cell biology and gene delivery. J Nanobiotechnology. 2024;22(1).552.
- Van Der Stegen SJ, Hamieh M, Sadelain M. The pharmacology of second-generation chimeric antigen receptors. Nat Rev Drug Discov. 2015;14(7):499-509.
- Halim L, Ajina A, Maher J. Pre-clinical development of chimeric antigen receptor T-cell immunotherapy: Implications of design for efficacy and safety. Best Practice & Research Clinical Haematology. 2018;31(2):117-25.
- Bald T, Krummel MF, Smyth MJ, Barry KC. The NK cell–cancer cycle: advances and new challenges in NK cell–based immunotherapies. Nat Immunol. 2020;21(8):835-47.
- Rezvani K, Rouce R, Liu E, Shpall E. Engineering natural killer cells for cancer immunotherapy. Mol Ther. 2017;25(8):1769-81.
- Zhang C, Hu Y, Xiao W, Tian Z. Chimeric antigen receptor-and natural killer cell receptor-engineered innate killer cells in cancer immunotherapy. Cell Mol Immunol. 2021;18(9):2083-100.
- Wilkins O, Keeler AM, Flotte TR. CAR T-cell therapy: progress and prospects. Human gene therapy methods. 2017;28(2):61-6.
- Shimasaki N, Jain A, Campana D. NK cells for cancer immunotherapy. Nat Rev Drug Discov. 2020;19(3):200-18.
- Zenere G, Olwenyi OA, Byrareddy SN, Braun SE. Optimizing intracellular signaling domains for CAR NK cells in HIV immunotherapy: a comprehensive review. Drug discovery today. 2019;24(4):983-91.
- Sievers NM, Dörrie J, Schaft N. CARs: beyond T cells and T cell-derived signaling domains. Int J Mol Sci. 2020;21(10):3525.
- Daher M, Melo Garcia L, Li Y, Rezvani K. CAR-NK cells: the next wave of cellular therapy for cancer. Clin Transl Immunology. 2021;10(4):1274.
- Zhang C, Oberoi P, Oelsner S, Waldmann A, Lindner A, Tonn T, Wels WS. Chimeric antigen receptor-engineered NK-92 cells: an off-the-shelf cellular therapeutic for targeted elimination of cancer cells and induction of protective antitumor immunity. Front Immunol. 2017;8:533.
- Cózar B, Greppi M, Carpentier S, Narni-Mancinelli E, Chiossone L, Vivier E. Tumor-infiltrating natural killer cells. Cancer discov. 2021;11(1):34-44.
- Ni J, Wang X, Stojanovic A, Zhang Q, Wincher M, Bühler L, et al. Single-cell RNA sequencing of tumor-infiltrating NK cells reveals that inhibition of transcription factor HIF-1? unleashes NK cell activity. Immunity. 2020;52(6):1075-87.
- Smyth MJ, Crowe NY, Godfrey DI. NK cells and NKT cells collaborate in host protection from methylcholanthrene-induced fibrosarcoma. Int Immunol. 2001;13(4):459-63.
- Smyth MJ, Thia KY, Street SE, Cretney E, Trapani JA, Taniguchi M,et al. Differential tumor surveillance by natural killer (NK) and NKT cells. J Exp Med. 2000;191(4):661-8.
- Bald T, Krummel MF, Smyth MJ, Barry KC. The NK cell–cancer cycle: advances and new challenges in NK cell–based immunotherapies. Nat Immunol. 2020;21(8):835-47.
- Li Y, Hermanson DL, Moriarity BS, Kaufman DS. Human iPSC-derived natural killer cells engineered with chimeric antigen receptors enhance anti-tumor activity. Cell stem cell. 2018;23(2):181-92.
- Woan KV, Kim H, Bjordahl R, Davis ZB, Gaidarova S, Goulding J, et al. Harnessing features of adaptive NK cells to generate iPSC-derived NK cells for enhanced immunotherapy. Cell Stem Cell. 2021;28(12):2062-75.
- Klingemann H. Are natural killer cells superior CAR drivers?. Onco immunology. 2014;3(4):e28147.
- Cheng M, Zhang J, Jiang W, Chen Y, Tian Z. Natural killer cell lines in tumor immunotherapy. Front Med. 2012;6(1):56–66.
- Mehta RS, Shpall EJ, Rezvani K. Cord blood as a source of natural killer cells. Front Med. 2016;2:93.
- Verneris MR, Miller JS. The phenotypic and functional characteristics of umbilical cord blood and peripheral blood natural killer cells. Br J Haematol. 2009;147(2):185-91.
- Luevano M, Madrigal A, Saudemont A. Generation of natural killer cells from hematopoietic stem cells in vitro for immunotherapy. Cell Mol Immunol. 2012;9(4):310-20.
- Knorr DA, Ni Z, Hermanson D, Hexum MK, Bendzick L, Cooper LJ, Lee DA, Kaufman DS. Clinical-scale derivation of natural killer cells from human pluripotent stem cells for cancer therapy. Stem Cells Transl Med. 2013;2(4):274-83.
- Woll PS, Martin CH, Miller JS, Kaufman DS. Human embryonic stem cell-derived NK cells acquire functional receptors and cytolytic activity. J Immun. 2005;175(8):5095-103.
- Moscarelli J, Zahavi D, Maynard R, Weiner LM. The Next Generation of Cellular Immunotherapy: Chimeric Antigen Receptor-Natural Killer Cells. Transpl cell ther. 2022;28(10):650–6.
- Zhang L, Meng Y, Feng X, Han Z. CAR-NK cells for cancer immunotherapy: from bench to bedside. Biomark Res. 2022;10(1):12.
- Xu J, Niu T. Natural killer cell-based immunotherapy for acute myeloid leukemia. J hematol oncol. 2020;13(1):167.
- Marofi F, Saleh MM, Rahman HS, Suksatan W, Al-Gazally ME, Abdelbasset WK, et al. CAR-engineered NK cells; a promising therapeutic option for treatment of hematological malignancies. Stem cell research & therapy. 2021;12(1):374.
- Liu E, Tong Y, Dotti G, Shaim H, Savoldo B, Mukherjee M, Orange J, Wan X, Lu X, Reynolds A, Gagea M. Cord blood NK cells engineered to express IL-15 and a CD19-targeted CAR show long-term persistence and potent antitumor activity. Leukemia. 2018;32(2):520-31.
- Geller MA, Cooley S, Judson PL, Ghebre R, Carson LF, Argenta PA, Jonson AL, Panoskaltsis-Mortari A, Curtsinger J, McKenna D, Dusenbery K. A phase II study of allogeneic natural killer cell therapy to treat patients with recurrent ovarian and breast cancer. Cytotherapy. 2011;13(1):98-107.
- Zhong Y, Liu J. Emerging roles of CAR-NK cell therapies in tumor immunotherapy: current status and future directions. Cell Death Discovery. 2024;10(1):318.
- W?odarczyk M, Pyrzynska B. CAR-NK as a rapidly developed and efficient immunotherapeutic strategy against cancer. Cancers. 2022 Dec 24;15(1):117.
- Li T, Niu M, Zhang W, Qin S, Zhou J, Yi M. CAR-NK cells for cancer immunotherapy: recent advances and future directions. Front Immunol. 2024;15:1361194.
- Chen YJ, Abila B, Mostafa Kamel Y. CAR-T: what is next?. Cancers. 2023;15(3):663.
- Gill S, Brudno JN. CAR T-cell therapy in hematologic malignancies: clinical role, toxicity, and unanswered questions. American Society of Clinical Oncology Educational Book. 2021;41:246-65.
- Almåsbak H, Aarvak T, Vemuri MC. CAR T cell therapy: a game changer in cancer treatment. J Immunol Res. 2016;2016(1):5474602.
- Thirumalaisamy R, Vasuki S, Sindhu SM, Mothilal TM, Srimathi V, Poornima B, et al. FDA-Approved Chimeric Antigen Receptor (CAR)-T Cell Therapy for Different Cancers-A Recent Perspective. Mol Biotechnol. 2024;1:1-5
- Young RM, Engel NW, Uslu U, Wellhausen N, June CH. Next-generation CAR T-cell therapies. Cancer discov. 2022;12(7):1625-33.
- Shin MH, Oh E, Kim Y, Nam DH, Jeon SY, Yu JH, Minn D. Recent advances in CAR-based solid tumor immunotherapy. Cells. 2023;12(12):1606.
- Schmidt D, Ebrahimabadi S, Gomes KR, de Moura Aguiar G, Cariati Tirapelle M, Nacasaki Silvestre R, et al. Engineering CAR-NK cells: how to tune innate killer cells for cancer immunotherapy. Immunotherapy adv. 2022;2(1):ltac003.
- Peng L, Sferruzza G, Yang L, Zhou L, Chen S. CAR-T and CAR-NK as cellular cancer immunotherapy for solid tumors. Cell Mol Immunol. 2024;21(10):1089-108.
- Liu F, Miao X, Han L, Song X. Advances in CAR-NK cell therapy for lung cancer: is it a better choice in the future?. Front oncol. 2024;14:1390006.
- Gong Y, Klein Wolterink RG, Wang J, Bos GM, Germeraad WT. Chimeric antigen receptor natural killer (CAR-NK) cell design and engineering for cancer therapy. J hematol oncol. 2021;14(1):73.


 Subrahmanya Pradeep P.*
Subrahmanya Pradeep P.*
 Yuktha S. K.
Yuktha S. K.



 10.5281/zenodo.14745164
10.5281/zenodo.14745164