Abstract
Antibiotic resistance is often used to describe drug resistance, which occurs when bacteria, viruses, fungi, and parasites develop resistance to drugs intended to treat infections. Recent studies have confirmed that indole derivatives, heterocyclic benzimidazole derivatives and benzotriazole derivatives have antimicrobial activity against various microorganisms. Indole derivatives have attracted wide attention due to their diverse biological and clinical applications. Indole derivatives have antimicrobial activity against various microorganisms, including MRSA. Benzimidazole is a heterocyclic aromatic organic compound that has become an important pharmacophore and preferred structure in medicinal chemistry. Benzimidazole is a very effective compound in terms of antibacterial activity. Biochemical and pharmacological studies show that this molecule is active against several microbial species. Azoles, especially the benzotriazole ring, play an important role in heterocyclic chemistry and medicine. Benzotriazole derivatives are an important class of nitrogen-containing heterocycles believed to have a variety of pharmacological effects, including sedative, analgesic, antibacterial, and antifungal effects. In this review we discusses indoles, benzimidazoles and benzotriazoles for their antibacterial activity.
Keywords
Antimicrobial resistance, Antibiotics, Indole, Benzimidazole, Benzotrialzole.
Introduction
Antimicrobial resistance (AMR) has emerged as one of the major public health challenges of the 21st century, with an increasing number of infections caused by bacteria, parasites, viruses and fungi under the influence of conventional medicine. This threatens effective deterrence. Medicines are used to treat it. The problem of AMR is even more important when it comes to antibiotic resistance in bacteria. Over the decades, bacteria that cause a variety of common and serious infections have developed resistance to every new antibiotic on the market. Considering this fact, measures should be taken to prevent the emergence of a global health crisis [1]. The World Health Organization has long recognized the need to increase and coordinate global efforts to control AMR. In 2001, the WHO Global Strategy for Antimicrobial Resistance provided measures to slow the emergence and spread of antimicrobial resistance [2]. In 2012, the World Health Organization published The Emerging Threat of Antimicrobial Resistance - Options for Action and proposed a combination of strengthening health systems and surveillance measures. Increase the use of antibiotics in hospitals and communities. Prevention and control of infectious diseases. Encourage the development of new drugs and appropriate vaccines. and political interference [3]. A major challenge in combating AMR understands the true burden of resistance, especially in areas where surveillance and data are scarce. There is extensive literature evaluating AMR morbidity, mortality, length of hospital stay, and healthcare costs of pathogen-drug combinations in specific settings [4,5]. However, to our knowledge, there is no comprehensive estimate that covers the entire area. and many different pathogens and pathogen-drug combinations have been published to date. For example, the US Centers for Disease Control and Prevention (CDC) reported AMR infections and 18 AMR threats in the United States, and Cassini et al. published a report in 2019 using observational data. They evaluated drugs. From 2007 to 2015, the creation of the European Union and the European Economic Area. Lim et al. estimated the severity of multidrug resistance in six bacterial pathogens in Thailand in 2010 [6,7] and Temkin et al. Escherichia coli and Klebsiella suspected pneumonia. 2014 [8]. Antimicrobial resistance is often used as a definition of drug resistance, which occurs when bacteria, viruses, fungi, and parasites develop resistance to drugs intended for the treatment of infectious diseases [9,10]. Several strains of methicillin-resistant Staphylococcus aureus (MRSA) cause serious infections in intensive care units, including pneumonia, endocarditis, and skin and soft tissue infections [11,12]. Recent studies have confirmed that indole derivatives, heterocyclic benzimidazole derivatives and benzotriazole derivatives have antimicrobial activity against various microorganisms.
INDOLE DERIVATIVES:
Indole, also known as benzopyrrole, has a benzenoid nucleus, contains 10 ? electrons (2 electrons from the lone pair on nitrogen and 2 electrons from the double bond forming 8 electrons), and is mostly aromatic. Like the benzene ring, indole easily undergoes electrophilic substitution due to ? electron transfer [13]. Indole is an important heterocyclic ring that provides lysergic acid diethylamide (LSD), a plant-derived strychnine alkaloid. Physically, it is mostly colorless crystals with a distinct smell. By adding an indole nucleus, a biologically active pharmacophore, to the drug compound, it has become an important heterocyclic compound with various biological activities [14]. Therefore, researchers have been interested in the synthesis of different indole scaffolds to screen them for different pharmacological activities. Various natural compounds, such as tryptophan, have indole as their nucleus. Indole-3-acetic acid is a plant hormone produced by the cleavage of tryptophan in higher plants. Indole derivatives have attracted wide attention due to their diverse biological and clinical applications. Here, we have summarized the important pharmacological activities of indole derivatives [15].

Figure 1: General chemical structure of Indole derivative.
Indole derivatives have emerged as important drugs in recent years and are known for their important biological activities, such as cytotoxic, antibacterial, anti-diabetic, and anti-inflammatory activities. Many drugs containing indole are available in the market. Some drugs containing indole are shown in Figure 2 [16].

Figure 2: Antimicrobial activities of indole derivatives
Recent studies have confirmed that indole products have antibacterial activity against various microorganisms, including MRSA [17]. Studies have shown that the light efflux pump A [18,19] is one of the main causes of antibiotic resistance in Staphylococcus aureus. Nora can export a variety of non-specific drugs, including fluoroquinolones, ethidium bromide, cetamide, benzalkonium chloride, tetra-phenyl-phosphonium bromide, and acriflavine [20]. Indoles are one of the reported classes of Nor A inhibitors, such as 5-nitro-2-phenylindole, a promising lead structure that represents ciprofloxacin S. It can increase camera sensitivity by 4 times. Tert-butyl (2-(3-hydroxyurido)-2-(1H-indole-3-yl)ethyl) carbamate, which is non-toxic to human cells, was also identified as an active endolic NORA inhibitor [21]. Azole-containing compounds such as fluconazole, ketoconazole, and itraconazole are the most commonly used antifungal drugs in the clinic [22,23]. However, some studies have shown fluconazole to be ineffective against Candida krusei, a potentially multidrug-resistant fungal pathogen [24, 25, 26]. Therefore, there is a need to develop new active compounds against fungal pathogens such as C. krusei. Multidrug-resistant infections are an emerging medical concern and attract the attention of researchers. Synthesis and antifungal activity of indole-conjugated triazole derivatives showed that almost all indole derivatives showed excellent antifungal activity against Candida albicans and Chlamydia cruzi with minimum inhibitory concentration. [27]. Antibacterial activity 1,2,4-triazole and 1,3,4-thiadiazole derivatives show antibacterial activity [29]. In addition, imidazoles and triazoles (azoles) are known to form the largest class of drugs against fungal infections. Indole derivatives are more effective and more suitable for development in antimicrobial drug development research [30]. According to the literature, all synthesized compounds show antibacterial and antifungal effects, and the antifungal activity of these compounds gives hope for the development of new lead compounds that are more effective against C. krusei. About 17% of Candida isolates are resistant to azoles and the widespread use of fluconazole is the main reason for this resistance. C. krusei is a strain that shows resistance to fluconazole [31]. Therefore, the search for new and more effective antifungal agents against C. krusei appears to be increasing [32]. Azole compounds inhibit ergosterol synthesis by inhibiting the cytochrome P-450 enzyme lanosterol 14?-demethylase. Triazoles are widely used in the treatment of fungal infections due to their high affinity for fungal cytochrome P-450 enzymes. It is reasonable to assume that the synthesized indole-triazole derivatives have a similar mechanism of action [33]. The combination of general use of antifungal molecules and inappropriate treatment is responsible for the development of resistance of these microorganisms to the drugs used in treatment. The effects of antibacterial and antifungal activity are thought to be mediated by targeting, as the introduction of substitutions in indole leads to differences in activity [34].
BENZIMIDAZOLE DERIVATIVES:
Benzimidazoles have been reported to have antibacterial properties against bacteria or fungi [35]. Compounds with azetidinone ring show various biological activities, including antibacterial and antifungal activity. The broad therapeutic value of this nucleus led to the synthesis of benzimidazole-derived compounds and the essential oil of Citrus hystrix (Makro Lime) substituted in position 2, which have antibacterial properties. Essential oils from plants may have antibacterial properties due to their synergistic effects [36]. Essential oils usually consist of many components and their mechanism of action involves multiple targets in bacterial cells. Several essential oil compounds have been identified as antimicrobial agents, including carvacrol [2], citral, eugenol, geraniol, perylaldehyde, and thymol. In addition, essential oils have antioxidant, antiseptic, insecticidal, antifungal, antiviral, and anti-parasitic properties [37]. Lime oil has been reported to be effective against 20 serotypes of Salmonella and 5 other enteric bacteria. Essential oils and various plant extracts have long been used in medicine [36].
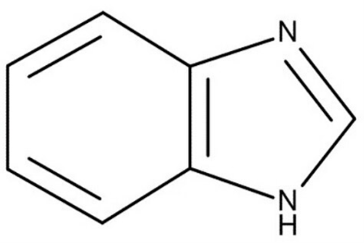
Figure 3: General chemical structure of benzimidazole derivative
This is a source of new drugs, especially against bacterial pathogens. Citrus hystrix DC, commonly known as Makrut orange, is a typical tropical plant of the Rutaceae family found in Southeast Asia [38]. Makrut is a thorny shrub with lime-scented leaves and dark green fruits that are irregularly shaped. The valuable components of lemon lime are the leaves and the skin of the fruit. Makrut lime is an important ingredient in many dishes of Thailand, Cambodia, Indonesia, Laos, Malaysia and the Philippines. Essential oils are used not only as fragrances and fragrances, but also in the preparation of perfumes and medicines. Essential oils usually consist of many components and their mechanism of action involves multiple targets in bacterial cells. Several essential oil compounds have been identified as antimicrobial agents, including carvacrol citral, eugenol, geraniol, perylaldehyde, and thymol [39-44]. Macroroot oil has been reported to be active against 20 Salmonella serotypes and 5 other enteric bacteria [45]. Citrus essential oil has significant antimicrobial activity against bacteria and fungi [46-48]. Citrus essential oil may be a good candidate for increasing the shelf life and safety of low-processed fruit and low-fat dairy products. However, most research has focused on subtropical citrus essential oils. Essential oils from two tropical citrus cultivars, including Citrus hystrix DC and Citrus aurantifolia, have been shown to have antimicrobial activity against Bacillus spp., Staphylococcus aureus, and Salmonella typhi [49]. The structure of benzimidazoles is important in the drug discovery process. Many drugs contain a benzimidazole ring. For example: proton pump inhibitors (lansoprazole, omeprazole), antihistamines (astemizole), antihypertensives (telmisartan, candesartan), anthelmintics (albendazole, flubendazole, mebendazole) (Figure 4). Benzimidazol and others have attracted a lot of attention due to their medicinal properties. Benzimidazole analogs have several therapeutic properties including antibacterial, antioxidant, anthelmintic, antihypertensive, anti-inflammatory, antiprotozoal, anti-hepatitis B virus, anti-viral, anti-fungal, anti-inflammatory, anti-inflammatory, anti-inflammatory and anti-inflammatory swelling Cancer is beneficial in this regard [50]. Amoxicillin, norfloxacin, and ciprofloxacin are the most commonly used drugs against these bacterial infections, but are associated with serious side effects [51]. A steady increase in the number of infections caused by bacteria resistant to one or more types of antibiotics is a serious threat and can lead to treatment failure and complications. Therefore, many efforts have been made by new research groups to find new antimicrobial agents [52,53,54].
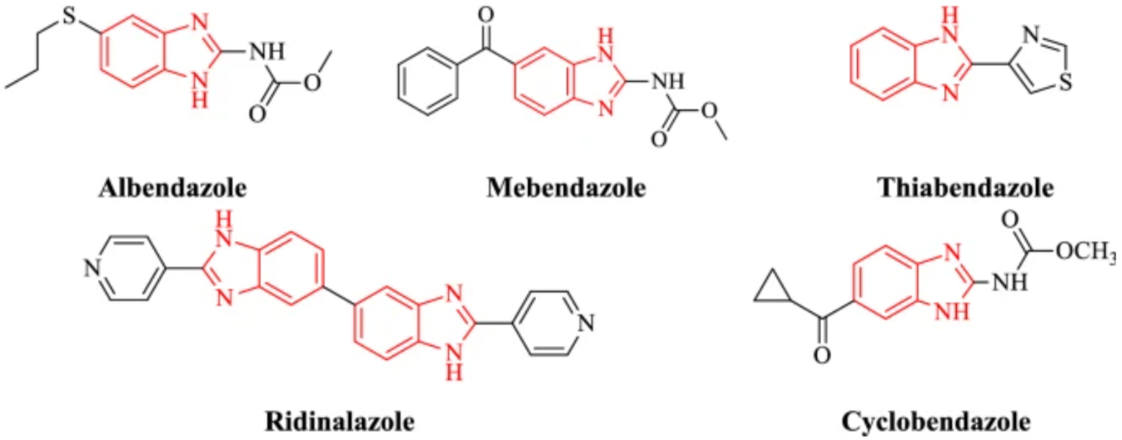
Figure 4: Benzimidazole derivatives for anti-microbial activities (anthelmintics)
Coumarin–benzimidazole hybrids as a potent antimicrobial agent:
It has been widely reported that natural compounds can play an important role as lead structures for the development of synthetic molecules. Coumarin (2H-chromene-2-one) is a natural compound with a wide range of biological activities. Coumarin-based novobiocin and chlorobiocin were identified as potent inhibitors of bacterial DNA gyrase (Figure 5) [55]. Recently, Li et al reported a coumarin-based inhibitor with strong antibacterial activity against ciprofloxacin- and methicillin-resistant Staphylococcus aureus strains. Sahoo and Padshetty reported that cobalt complexes such as 3-aryl-azo-4-hydroxycoumarin have antibacterial, wound healing and antioxidant properties [56]. Paul et al. reported the anticancer activity of a coumarin-benzimidazole hybrid [57]. Al-Majdi et al reviewed previous studies on coumarins in terms of antibacterial activity and reported that coumarin analogs showed stronger antibacterial activity than the parent molecule [58]. These and other studies led to the design and synthesis of coumarin-based hybrid analogs with antimicrobial activity. The molecular hybridization approach allows combining two or more drug units with different or similar biological activities into a molecular scaffold with new or improved biological activity based on the respective drug unit. Hybrids are often reported to exhibit interesting multi-biological activity, high selectivity and favorable pharmacokinetics, avoiding undesirable disadvantages such as side effects and poor oral bioavailability. These advantages make hybrid molecules an attractive resource for drug discovery today. Benzimidazole is an important heterocyclic pharmacophore structure that has great application in the synthesis of drug-like molecules [62,63]. According to the literature review, many benzimidazole derivatives have been successfully developed as clinical drugs and are widely used in the treatment of various diseases. Recent studies have shown that benzimidazole can inhibit the growth of bacteria [64].

Figure 5: General structure of coumarin-benzimidazole complexes synthesized by antibacterial benzimidazole compounds.
The antibacterial potential of benzimidazole derivatives has been studied specifically since the late 1990s and early 2000s [65]. Given the extensive research on the antibacterial properties of benzimidazole derivatives since 2012, the following section summarizes their antiviral, antiulcer, antiprotozoal, and antituberculosis properties, as well as their antibacterial and antifungal activity will focus on the latest information [66].
BENZOTRIAZOLE DERIVATIVES
Azoles, especially the benzotriazole ring, play an important role in heterocyclic chemistry and medicinal chemistry [67]. For example, benzotriazole compounds are widely used in the treatment of various diseases such as anticancer, antibacterial, antifungal, antibacterial, antiprotozoal, antiviral, antifungal and antitubulin agents. [68] Benzofused azoles are a group of heterocyclic compounds that are of great interest due to their properties and applications in medicinal chemistry. Benzimidazole and its derivatives have been studied for many years [69], and drugs containing this heterocyclic reaction as the main component are widely used clinically, for example as anthelmintics in humans [70]. Benzylated azoles containing three heteroatoms, such as bisoxadiazole, benzothiazole, and benzotriazole, have been widely studied due to their various biological activities. However, some reviews have focused on it. Indeed, the purpose of this article is to provide an overview of benzotriazole-based systems and their importance in medicinal chemistry [71,72]. Benzotriazole can be considered a structure of interest for several pharmacological activities. Bt, useful for designing new pharmacologically active compounds, is developing rapidly in the synthesis of heterocycles [73].
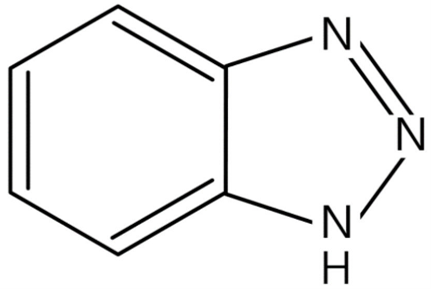
Figure 6: General chemical structure of benzotriazole derivative
Benzotriazoles also act as electron donors or radical precursors or carbanions. It can be easily converted into other chemical structures by a series of reactions such as condensation, addition reactions, and alkylation of benzotriazolyl. Several authors have reported the synthesis of stable nitrene ions using BT as a synthon. Polymer-supported benzotriazoles have also been used as catalysts for the construction of tetra-hydroquinoline libraries [73]. However, suitable substituted benzotriazole derivatives show plant growth regulatory activity, choleretic activity, antibacterial activity, antiprotozoal activity, antiviral activity, antiproliferative activity, and others. can have many different biological properties such as part [73].
Chemistry of Benzotriazole:
The benzotriazole core can have a large conjugated system that allows it to form interactions, and the three nitrogen atoms help form coordination bonds with hydrogen and other atoms. Therefore, benzotriazole derivatives have high affinity for various enzymes and receptors in biological systems, leading to various non-covalent interactions, leading to several biological activities. 1 Inorganic Benzotriazole (C6H3) is a heterocyclic nitrogen product that consists of three nitrogen atoms, each nitrogen atom has a single electron pair that can form a 5-membered ring that can exist in the corresponding tautomer [74]. It is called oxidiazole because of its five-membered ring structure with one oxygen atom and two nitrogen atoms. The arrangement of these atoms can be different, for example in Figure 7.

Figure 7: The compounds 1,2,4-oxadiazole (a), 1,2,5- oxadiazole (b), 1,2,3-oxadiazole (c), and 1,3,4-oxadiazole (d)
Benzotriazole as antimicrobial and antiprotozoal agent:
The antibacterial activity of benzotriazole derivatives has been widely studied since the late 1980s, and the whole azole ring has become one of the most important active ingredients in recent years [75]. The discovery and development of antibiotics in the first half of the 20th century was a major scientific breakthrough. Despite investment in the discovery of antimicrobial drugs, no new class of drugs has been discovered in the last two decades [76]. In addition, the challenge of treating infectious diseases due to increasing antibiotic resistance has highlighted the need to develop new drugs [77]. Over several decades, Speratore et al studied and characterized various nitrogen rings and reported that BT has biological activity, especially antibacterial properties, when it is part of a larger heterocyclic ring system [78-79]. In 1989, Sanna's research group reported the relationship between the benzotriazole reaction of the closely related triazolone [4,5-f]-quinolinone carboxylic acid and oxolinic acid (1) (Figure 7). In vitro, this acid exerts antibacterial activity against E. coli with a minimum inhibitory concentration (MIC) of 12.5–25 ?g/mL. They also showed that cyclization in different positions of the triazole ring, such as triazolo[4,5-h]-quinolinone carboxylic acid, leads to a partial or complete loss of antimicrobial activity [80,81].
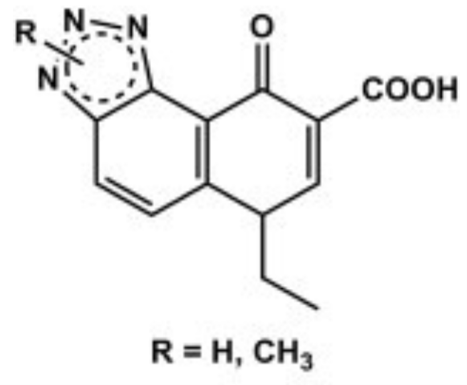
Figure 8: General formula of triazolo[4,5-f]-quinolinone carboxylic acids derivatives.
Benzotriazole as anti-bacterial:
Clinically useful 1,2,3-triazole derivatives include tazobactam, which is used in combination with beta-lactam antibiotics as antibacterial and anticonvulsant agents. Clinically important 1,2,4-triazole derivatives include rizatriptan, the antidepressant trazodone, the miotic dapiprazole (Figure 10), the antiviral drug ribavirin, the antiviral drug Irapfent, and the antidepressant drug Lotrifen (Figure 12). Lilmazafone (Figure 9) is a potent sedative and hypnotic [82,83].

Figure 9: Rizatriptan

Figure 10: Rilmazafon
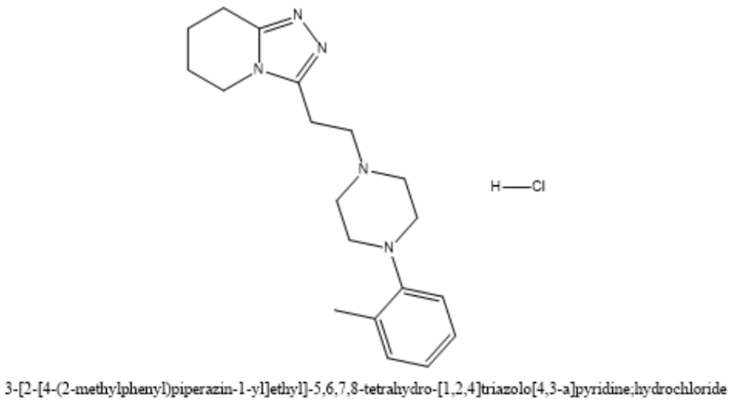
Figure 11: Dapiprazole
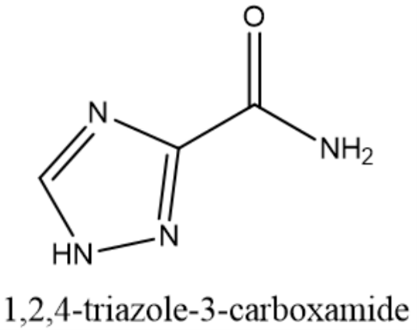
Figure 12: ibivirin
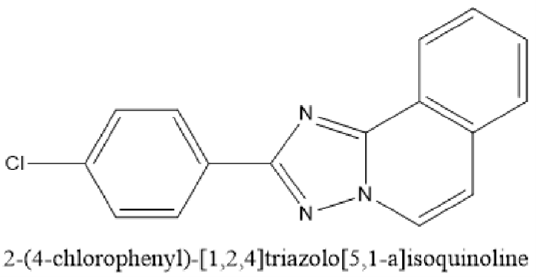
Figure 13: Lotrifen
CONCLUSION:
Indole is one of the most valuable heterocyclic compounds from a synthetic and therapeutic point of view. Indole and its derivatives have many biological properties, including anti-inflammatory, analgesic, antibacterial, anticonvulsant, anthelmintic and antiallergic effects. Considering the various biological activities exhibited by indole-containing compounds, we are investigating their antibacterial activity to save the lives of patients. Benzimidazole and pyrazole compounds have important biological properties such as antibacterial, antiviral, antipyretic, analgesic and anti-inflammatory. In addition, recent literature has suggested the synthesis of several benzimidazole-pyrazole hybrids and their antibacterial and other biological properties. Benzotriazole has anticonvulsant, anti-inflammatory and anti-cancer properties. This suggests that electron-substituted benzimidazole derivatives can be used as more effective antibiotics, antioxidants, anti-inflammatory agents, and analgesics with less gastrointestinal disturbances. It is believed that medicine can be produced. Benzotriazole derivatives are an important class of nitrogen-containing heterocycles believed to have a variety of pharmacological effects, including sedative, analgesic, antibacterial, and antifungal effects.
REFERENCES:
- Prestinaci F, Pezzotti P, Pantosti A. Antimicrobial resistance: a global multifaceted phenomenon. Pathog Glob Health. 2015;109(7):309-18.
- World Health Organization WHO global strategy for containment of antimicrobial resistance. Geneva: WHO; 2001.
- World Health Organization The evolving threat of antimicrobial resistance. Options for action. Geneva: WHO Library Cataloguing-in-Publication Data; 2012.
- O’Neill J. Antimicrobial resistance: tackling a crisis for the health and wealth of nations. London: Review on Antimicrobial Resistance, 2014.
- de Kraker MEA, Stewardson AJ, Harbarth S. Will 10 million people die a year due to antimicrobial resistance by 2050? PLoS Med 2016; 13: e1002184.
- Cassini A, Högberg LD, Plachouras D, et al. Attributable deaths and disability-adjusted life-years caused by infections with antibioticresistant bacteria in the EU and the European Economic Area in 2015: a population-level modelling analysis. Lancet Infect Dis 2019;19:56–66.
- Lim C, Takahashi E, Hongsuwan M, et al. Epidemiology and burden of multidrug-resistant bacterial infection in a developing country. eLife 2016;5:e18082.
- Temkin E, Fallach N, Almagor J, Gladstone BP, Tacconelli E, Carmeli Y. Estimating the number of infections caused by antibiotic-resistant Escherichia coli and Klebsiella pneumoniae in 2014: a modelling study. Lancet Glob Health 2018;6:e969–79.
- Cerniglia CE, Pineiro SA, Kotarski SF. An update discussion on the current assessment of the safety of veterinary antimicrobial drug residues in food with regard to their impact on the human intestinal microbiome. Drug Test Anal. 2016;8:539–548.
- Hahn AW, Jain R, Spach DH. New Approaches to antibiotic use and review of recently approved antimicrobial agents. Med Clin North Am. 2016;100:911–926.
- Liu C, Bayer A, Cosgrove SE, Daum RS, Fridkin SK, Gorwitz RJ, Kaplan SL, Karchmer AW, Levine DP, Murray BE, J Rybak M, Talan DA, Chambers HF. Clinical practice guidelines by the infectious diseases society of America for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children. Clin Infect Dis. 2011;52:18–55.
- Kaku N, Morinaga Y, Takeda K, Kosai K, Uno N, Hasegawa H, Miyazaki T, Izumikawa K, Mukae H, Yanagihara K. Antimicrobial and immunomodulatory effects of tedizolid against methicillin-resistant Staphylococcus aureus in a murine model of hematogenous pulmonary infection. Int J Med Microbiol. 2016;306:421–428.
- Lakhdar S, Westermaier M, Terrier F, Goumont R, Boubaker T, Ofial AR, Mayr H. Nucleophilic reactivities of indoles. J Org Chem 2006;71:9088–9095.
- Sharma V, Pradeep K, Devender P. Biological importance of the indole nucleus in recent years: a comprehensive review. J Heterocycl Chem 2010;47:491–502.
- Kaushik NK, Kaushik N, Attri P, Kumar N, Kim CH, Verma AK, Choi EH (2013) Biomedical Importance of Indoles. Molecules 2013;18:6620–6662.
- Kaur H., Singh J. & Narasimhan B. Indole hybridized diazenyl derivatives: synthesis, antimicrobial activity, cytotoxicity evaluation and docking studies. BMC Chemistry 2019;13:65.
- Al-Qawasmeh RA, Huesca M, Nedunuri V, Peralta R, Wright J, Lee Y, Young A. Potent antimicrobial activity of 3-(4,5-diaryl-1H-imidazol-2- yl)-1H-indole derivatives against methicillin-resistant Staphylococcus aureus. Bioorg Med Chem Lett. 2010;20:3518–3520
- Lepri S, Buonerba F, Goracci L, Velilla I, Ruzziconi R, Schindler BD, Seo SM, Cruciani G. Indole based weapons to fight antibiotic resistance: a structure-activity relationship study. J Med Chem. 2016;59:867–891.
- Costa SS, Viveiros M, Amaral L, Couto I. Multidrug Efflux Pumps in Staphylococcus aureus: an Update. Open Microbiol J. 2013;7:59–71.
- Fridkin SK, Jarvis WR. Epidemiology of nosocomial fungal infections. Clin Microbiol Rev. 1996;9:499–511.
- Che X, Sheng C, Wang W, Cao Y, Xu Y, Ji H, Dong G, Miao Z, Yao J, Zhang W. New azoles with potent antifungal activity: design, synthesis and molecular docking. Eur J Med Chem. 2009;44:4218–4226.
- Samaranayake YH, Samaranayake LP. Candida krusei: biology, epidemiology, pathogenicity and clinical manifestations of an emerging pathogen. J Med Microbiol. 1994;41:295–310.
- Deorukhkar SC, Saini S. Echinocandin susceptibility profile of fluconazole resistant Candida species isolated from blood stream infections. Infect Disord Drug Targets. 2016;16:63–68.
- Pfaller MA, Diekema DJ, Gibbs DL, Newell VA, Nagy E, Dobiasova S, Rinaldi M, Barton R, Veselov A. Global Antifungal Surveillance Group. Candida krusei, a Multidrug-Resistant Opportunistic Fungal Pathogen: Geographic and Temporal Trends from the ARTEMIS DISK Antifungal Surveillance Program, 2001 to 2005. J Clin Microbiol. 2008;46:515–521
- Na YM. Synthesis and activity of novel indole linked triazole derivatives as antifungal agents. Bull Korean Chem Soc. 2010;31:3467–3470.
- Ryu CK, Lee JY, Park RE, Ma MY, Nho JH. Synthesis and antifungal activity of 1H-indole-4,7-diones. Bioorg Med Chem Lett. 2007;17:127–131.
- Chen H, Li Z, Han Y. Synthesis and fungicidal activity against rhizoctoniasolani of 2-alkyl (alkylthio)-5-pyrazolyl-1,3,4-oxadiazoles (thiadiazoles) J Agric Food Chem. 2000;48:5312–5315.
- Ahmed S, Zayed MF, El-Messery SM, Al-Agamy MH, Abdel-Rahman HM. Design, synthesis, antimicrobial evaluation and molecular modeling study of 1,2,4-Triazole-based 4-Thiazolidinones. Molecules. 2016;21.
- Odds FC, Brown AJ, Gow NA. Antifungal agents: mechanisms of action. Trends Microbiol. 2003;11:272–279.
- Varvaresou A, Tsantili-Kakoulidou A, Siatra-Papastaikoudi T, Tiligada E. Synthesis and biological evaluation of indole containing derivatives of thiosemicarbazide and their cyclic 1,2,4-triazole and 1,3,4- thiadiazole analogs. Arzneimittelforschung. 2000;50:48–54.
- Kanafani ZA, Perfect JR. Antimicrobial resistance: to antifungal agents: mechanisms and clinical impact. Clin Infect Dis. 2008;46:120–128.
- Gill K, Kumar S, Xess I, Dey S. Novel synthetic anti-fungal tripeptide effective against Candida krusei. Indian J Med Microbiol. 2015;33:110–116.
- Asami T, Mizutani M, Shimada Y, Goda H, Kitahata N, Sekimata K, Han SY, Fujioka S, Takatsuto S, Sakata K, Yoshida S. Triadimefon, a fungicidal triazole-type P450 inhibitor, induces brassinosteroid deficiency-like phenotypes in plants and binds to DWF4 protein in the brassinosteroid biosynthesis pathway. Biochem J. 2003;369:71–76.
- Shirinzadeh H, Süzen S, Altanlar N, Westwell AD. Antimicrobial Activities of New Indole Derivatives Containing 1,2,4-Triazole, 1,3,4-Thiadiazole and Carbothioamide. Turk J Pharm Sci. 2018 Dec;15(3):291-297.
- David W., Zhijan K., & Christine C. Synthesis of dicationic extended bis-benzimidazoles. Molecules, 2004;9:158-163.
- Vimol S., Chanwit T., Veena N., Nuntavan B., Kulkanya C., Siwimol P., et al. Antibacterial activity of essential oils from Citrus hystrix (makrut lime) against respiratory tract pathogens. Science Asia, 2012;38:212-217.
- Uthumporn K., Ratchaneeporn R. The Study of Antibacterial Activity of Benzimidazole Derivative Synthesized from Citronellal. IJBBB. 2015;5:280-287.
- Doreen S.H., Rose L.C., Suhaimi H., Mohamad H., Rozaini M.Z.H., & Tai M. Preliminary evaluation onthe antibacterial activities of Citrus hystrix oil emulsionsstabilized by tween 80 and span 80. International Journal of Pharmacy and Pharmaceutical Sciences, 2011;3(Suppl 2):209-211.
- Burt, S. Essential oils: Their antibacterial properties and potential applications in food - a review. International Journal of Food Microbiology, 2004;94:223-253.
- Kim J., Marshall M.R., & Wei C.I. Antibacterial activity of some essential oil components against five foodborne pathogens. Journal of Agricultural and Food Chemistry, 1995;43:2839-2845.
- Lambert R.J., Skandamis P.N., Coote P.J., & Nychas G.J. A study of the minimum inhibitory concentration and mode of action of oregano essential oil, thymol and carvacrol. Journal of Applied Microbiology, 2001;91:453-462.
- Prabuseenivasan S., Jayakumar M., & Ignacimuthu S. In vitro antibacterial activity of some plant essential oils. BMC Compl Alternative Med, 2006;6:39-47.
- Sabulal B., Dan M., Pradeep N.S., Valsamma R.K., & George V. Composition and antimicrobial activity of essential oil from Amomum cannicarpum. Acta Pharmaceutica, 2006;56:473-480.
- Murugulla A., Donthabhakthuni, S. Room temperature synthesis of benzimidazole derivatives using reusable cobalt hydroxide (II) and cobalt oxide (II) as efficient solid catalysts. Tetrahedral Letter, 2011;52:5575-5580.
- Nanasombat S., & Lohasupthawee P. Antibacterial activity of crude ethanolic extracts and essential oils of spices against salmonellae and other enterobacteria. KMITL Science and Technology, 2005;5:527-538.
- Lanciotti R., Gianotti A., Patrignani F., Belletti N., Guerzoni E.M., & Gardini F. Use of natural aroma compounds to improve shelf-life and safety of mini-mally processed fruits. Trends Food Science and Technology, 2004;15:201-208.
- Dabbah R., Edwards M.V., & Moats A.W. Anti-microbial action of some citrus fruit oils on selected food borne bacteria. Journal of Applied Microbiology, 1970;19:27-31.
- Caccioni D.R.L., Guizzardi M., Biondi D.M., Renda A., & Ruberto G. Relationship between volatile components of citrus fruit essential oils and antimicrobial action on Penicillium digitatum and Penicillium italicum. International Journal of Food Microbiology, 1998;43:73-79.
- Chaisawadi S., Thongbute D., Methawiriyaslip W., Pitak-worarat N., Chaisawadi A., Jaturonrasamee K., Khemkhaw J., & Thnuthumchareon W. Pre-liminary study of antimicrobial activities on medicinal herbs of Thai food ingredients. Acta Hort., 2003;75:111-114.
- Deepak K, Pankaj A. Review on: benzimidazole derivatives as potent biological agent. J Curr Pharma Res 2018;9(1):2676–2694.
- Majed H, Askar F. Synthesis and biological evaluation of new benzimidazole derivatives. Al-Mustansiriyah J Sci 2018;29(1):107.
- Alasmary F, Snelling A, Zain M, Alafeefy A, Awaad A, Karodia N. Synthesis and evaluation of selected benzimidazole derivatives as potential antimicrobial agents. Molecules. 2015;20(8):15206–15223.
- Anand K, Wakode S. Synthesis, characterization and biological evaluation of benzimidazole derivatives. Int J Pharm Sci Res 2018;9(2):617–624.
- Yoon Y, Ali M, Wei A, Choon T, Khaw K, Murugaiyah V et al. Synthesis, characterization, and molecular docking analysis of novel benzimidazole derivatives as cholinesterase inhibitors. Bioorg Chem 2013;49:33–39.
- Singh L., Avula S., Raj S. et al. Coumarin–benzimidazole hybrids as a potent antimicrobial agent: synthesis and biological elevation. J Antibiot 2017;70:954–961.
- Li B. et al. Coumarin-based inhibitors of Bacillus anthracis and Staphylococcus aureus replicative DNA helicase: chemical optimization, biological evaluation, and antibacterial activities. J. Med. Chem. 2012;5:10896–10908.
- Sahoo J. & Paidesetty S.K. Antimicrobial activity of novel synthesized coumarin based transitional metal complexes. J. Taibah Univ. Med. Sci. 2017;12:115–124.
- Paul K., Bindal S. & Luxami V. Synthesis of new conjugated coumarin-benzimidazole hybrids and their anticancer activity. Bioorg. Med. Chem. Lett. 2013;23:3667–3672.
- Al-Majedy Y.K., Kadhum A.H., Al-Amiery A.A. & Abu Bakar Mohamad, A. B. Coumarins: the antimicrobial agents. Sys. Rev. Pharm. 2017;8:62–70.
- Sashidhara K.V. et al. Designing, synthesis of selective and high-affinity chalcone-benzothiazole hybrids as Brugia malayi thymidylate kinase inhibitors: in vitro validation and docking studies. Eur. J. Med. Chem. 2015;103:418–428.
- Sashidhara K.V. et al. Design, synthesis and anticancer activity of dihydropyrimidinone-semicarbazone hybrids as potential human DNA ligase 1 inhibitors. Med. Chem. Commun. 2016;7:2349–2363.
- Viegas-Junior C., Danuello A., da Silva Bolzani V., Barreiro E.J. & Fraga C.A. Molecular hybridization: a useful tool in the design of new drug prototypes. Curr. Med. Chem. 2007;14:1829–1852.
- Narasimhan, B., Sharma, D. & Kumar, P. Benzimidazole: a medicinally important heterocyclic moiety. Med. Chem. Res. 2012;21:269–283.
- Zhang D. et al.. Design, synthesis and antibacterial activity of novel actinonin derivatives containing benzimidazole heterocycles. Eur. J. Med. Chem. 2009;44:2202–2210.
- Ozkay Y., Tunali Y., Karaca H., and Is?kdag I. Antimicrobial Activity of a New Series of Benzimidazole Derivatives. Arch. Pharm. Res. 2011;34:1427–1435.
- Alaqeel S.I. Synthetic Approaches to Benzimidazoles from O-Phenylenediamine: A Literature Review. J. Saudi Chem. Soc. 2017;21:229–237.
- Bajaj K., Sakhuja R. BenzotriazoleP: Much More Than Just Synthetic Heterocyclic Chemistry. In The Chemistry of Benzotriazole Derivatives; Springer, 2015;5:235–283.
- Hall C.D., Panda S.S. The Benzotriazole Story. In Advances in Heterocyclic Chemistry; Elsevier, 2016;119:1–23.
- Barot K.P., Nikolova S., Ivanov I., Ghate M.D. Novel research strategies of benzimidale derivatives: a review. Mini Rev. Med. Chem. 2013;13:1421–1447.
- McKellar Q.A., Scott E.W. The benzimidazole anthelmintic agents – a review. J. Vet. Pharmacol. Ther. 1990;13:223–247.
- Piccionello A.P., Guarcello A. Bioactive compounds containing benzoxadiazole, benzothiadiazole, benzotriazole. Curr. Bioact. Compd. 2010;6:266–283
- Suma B.V., Natesh N.N., Madhavan V. Benzotriazole in medicinal chemistry: an overview. J. Chem. Pharm. Res. 2011;3:375–381.
- Briguglio I, Piras S, Corona P, Gavini E, Nieddu M, Boatto G, Carta A. Benzotriazole: An overview on its versatile biological behavior. Eur J Med Chem. 2015 Jun 5;97:612-48.
- Sease C. Benzotriazole: a review for conservators. Studies in Conservation. Catherine Sease. 1978;23(1):76–85.
- Peng X.M., Cai G.X., Zhou C.H. Recent developments in azole compounds as antibacterial and antifungal agents. Curr. Top. Med. Chem. 2013;13:1963–2010.
- Chopra I. The 2012 Garrod Lecture: discovery of antibacterial drugs in the 21st century. Antimicrob. Chemother. 2013;68:496–505.
- Boucher H.W., Talbot G.H., Bradley J.S., Edwards J.E., Gilbert D., Rice L.B., Scheld M., Spellberg B., Bartlett J. Bad bugs, no drugs: no ESKAPE! An update from the Infectious Diseases Society of America. Clin. Infect. Dis. : official Publ. Infect. Dis. Soc. Am. 2009;48:1–12.
- Sparatore F., Pagani F. Aminoalkyl derivatives of benzotriazole. Farm. Sci. 1962;17:414–429.
- Savelli F., Pau A., Boido A., Sparatore F. Aminoalkyl derivatives of benzotriazole and naphthotriazole. Boll. Chim. Farm. 1988;127:144–147.
- Nuvole A., Sanna P., Paglietti G., Juliano C., Zanetti S., Cappuccinelli P. 1,2,3-triazolo[4,5-f]quinolines. II. Preparation and antimicrobial evaluation of 6-ethyl-6,9-dihydro-1(2)(3)-R-1(2) (3)H-triazolo [4,5-f]quinolin-9-one-8-carboxylic acids as anti-infectives of the urinary tract (1) Farm. Sci. 1989;44:619–632.
- Sanna P., Carta A., Paglietti G., Zanetti S., Fadda G. 1,2,3-Triazolo[4,5-h]quinolines. III. Preparation and antimicrobial evaluation of 4-ethyl-4,7-dihydro-1(2)H Triazolo [4,5-h]quinolin-7-one-6-carboxylic acids as anti infectives of the urinary tract. Farm. Sci. 1992;47:1001–1019.
- Pandey VK, Shukla A. Synthesis and biological activity of isoquinolinyl benzimidazoles. Chm. 1999;41(1):1381.
- Yadav S, Jain PK, Mishra AP. Pharmacological activities and activation as well as comparison of benzotriazole with other groups. Indian Journal of Pharmacy and Pharmacology 2022;9(2):103–114.


 Vishal Yalij*
Vishal Yalij*
 Mayur Suryawanshi
Mayur Suryawanshi
 Amol Shirode 3
Amol Shirode 3













 10.5281/zenodo.10937937
10.5281/zenodo.10937937