Abstract
Adverse drug reactions are also known as side effects. Adverse drugs reactions (ADRs), are toxic, unintended, and undesirable impacts which occur as result of drug treatment. These reactions occur due to self-medication or due to intake of over dose of medicines without prescription. The prescribed drugs may produce undesirable effects along with main effect which leads to adverse drug reactions. Most of the adverse drug reactions are preventable. Hence, in order to avoid adverse drug reactions one should take only properly prescribed drugs. This review article explores the pharmacokinetics and mechanism of action of paracetamol, highlighting its side effects. It discusses the importance of accurately calculating the appropriate dose to ensure optimal efficacy while minimizing the risk of toxicity, dosing recommendations, such as age groups (infants, children, adolescents, adults, and older adults), weight categories are thoroughly examined.
Keywords
Adverse, Drug, Reaction, Paracetamol, IV, Case Study
Introduction
Adverse drug reactions (ADR) represent one of the prime topics to be assessed in the modern times[1][2]. According to world health organisation (WHO), an ADR can be defined as any response of a drug which is noxious and unintended, that occurs at doses used in humans for the prophylaxis, diagnosis or therapy of disease; or for the modification of physiologic function purposely excludes therapeutic failures, overdoes, drug abuse, non compliance, and medication errors [3][4]. Moreover, it has been regarded as an appreciable harmful reaction which results from an intervention related to the use of a medical products. An adverse effect, which occurs as overstate of the desired therapeutic effect, forms a part of ADR, whereas, side effects are generally related to the therapeutic activities of a drug which may be beneficial as well as harmful [5][6][7]. Thus, it may be suggested that an ADR is a harmful reaction or unwanted reaction that is followed by the administration of a medicinal product or a combination of drugs under normal conditions of use. ADRs may occur following a single dose or prolonged administration of a drug or result from the combination of two or more drugs. The meaning of this expression differs from the meaning of "side effect", as this last expression might also imply that the effects can be beneficial. The study of ADRs is the concern of the field known as pharmacovigilance. An adverse drug event (abbreviated ADE) refers to any injury caused by the drug (at normal dosage and/or due to overdose) and any harm associated with the use of the drug (e.g. discontinuation of drug therapy) [3]. ADRs are a special type of ADEs. In everyday clinical practice, almost all physicians come across many instances of suspected adverse cutaneous drug reactions (ACDR) in different forms. Although such cutaneous reactions are common, comprehensive information regarding their incidence, severity and ultimate health effects are often not available as many cases go unreported. In the present world, almost everyday a new drug enters market; therefore, a chance of a new drug reaction manifesting somewhere on some form in any corner of world is unknown on unreported. Although many a times, presentation is too trivial and benign, the early identification of the condition and identifying the culprit drug and omit it at earliest holds the keystone in management and prevention of a more severe drug rash. Therefore, not only the dermatologists, but all practicing physicians should be familiar with these conditions to diagnose them early and to be prepared to handle them adequately. Combined use of multiple drugs may cause adverse events. Drug interactions can lead to an increase or a decrease of the drug effects or cause other serious reactions
Classification of Adverse Drug Reaction
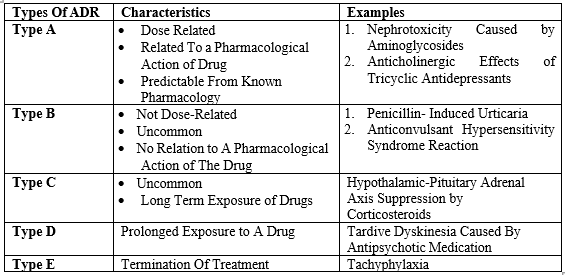
Adverse drug reactions are frequently classified as ‘type A’ and ‘type B’ reactions. An extended version of this classification system is shown here:
Type A Reactions:
Type A (augmented) reactions result from an exaggeration of a drug’s normal pharmacological actions when given at the usual therapeutic dose and are normally dose- dependent. Examples include respiratory depression with opioids or bleeding with warfarin. Type A reactions also include those that are not directly related to the desired pharmacological action of the drug, for example dry mouth that is associated with tricyclic antidepressants. Commonest (up to 70%) - Dose dependent, severity increases with dose. Preventable in most part by slow introduction of low dosages. Predictable by the pharmacological mechanisms, e.g., hypotension by beta-blockers, hypoglycaemia caused by insulin’s or oral hypoglycemic, or NSAID induc ed gastric ulcers.
Type B Reactions:
Type B (bizarre) reactions are novel responses that are not expected from the known pharmacological actions of the drug. These are less common, and so may only be discovered for the first time after a drug has already been made available for general use. Examples include anaphylaxis with penicillin or skin rashes with antibiotics. Rare, idiosyncratic, genetically determined, unpredictable, mechanisms are unknown, serious, can be fatal; unrelated to the dose, e.g., hepatitis caused by halothane, aplastic anemia caused by chloramphenicol, neuroleptic malignant syndrome caused by some anesthetics’ and antipsychotics.
Type C Reactions (continuous drug use):
Type C, or ‘continuing’ reactions, persist for a relatively long time. An example is osteonecrosis of the jaw with bisphosphonates. Occurs as a result of continuous drug use. May be irreversible, unexpected, unpredictable, e.g. tardivedyskinesias by antipsychotics, dementia by anticholinergic medications
Type D Reactions (Delayed):
Type D, or ‘delayed’ reactions, become apparent sometime after the use of a medicine. The timing of these may make them more difficult to detect. An example is leucopoenia, which can occur up to six weeks after a dose of lomustine. Delayed occurrence of ADRs, even after the cessation of treatment, e.g., corneal opacities after thioridazine, ophthalmopathy after chloroquine, or pulmonary/peritoneal fibrosis by methyserzide.
Type E Reactions (End of dose):
Type E, or ‘end-of-use’
reactions, are associated with the withdrawal of a medicine. An example is insomnia, anxiety and perceptual disturbances following the withdrawal of benzodiazepines. Withdrawal reactions. Occurs typically with the depressant drugs, e.g., hypertension and restlessness in opiate abstainer, seizures on alcohol or benzodiazepines withdrawal; first dose hypotension caused by alpha-blockers (Prazosin) or ACE inhibitors.
Type-F (Failure of therapy):
Results from the ineffective treatment (previously excluded from analysis according to WHO definition), e.g., accelerated hypertension because of inefficient control [8][9][10][11][12].
Table 1. Types of Adverse Drug Reaction
REPORTING OF ADVERSE DRUG REACTIONS
All healthcare workers e.g. Doctors, Dentists, Pharmacists, Midwives, Nurses and Allied Health Professionals in Uganda should, as part of their professional responsibility report any suspected adverse drug reactions directly to the National Pharmacovigilance Centre offices or regional Pharmacovigilance Centres located in the regional referral hospitals as soon as possible within 15 calendar days after notification. Patients, caretakers, and the public should also report suspected adverse drug reactions.
What to report ?
ADRs resulting from prescription medicines, (OTC) medicines, complementary medicines and vaccines should be reported. Ideally serious, undocumented and unexpected ADRs are to be reported. If there is any doubt about whether or not an ADR has occurred and should be reported, it is always best practice to submit a report as causality does not need to have been established.
- ADRs in children All suspected ADRs occurring in children under the age of 18, should be reported regardless of whether the medicine is registered for use in children.
- ADRs in the elderly Healthcare professionals should be particularly aware that the elderly may be more susceptible to adverse drug reactions and it is therefore important to monitor drug safety in this age group. Many elderly patients are more likely to be taking multiple medicines and may also metabolise them less effectively or be more sensitive to their effects.
- ADR reports on lack of efficacy Lack of efficacy with medicines used for the treatment of life-threatening diseases (e.g. antimicrobial agents), vaccines or contraceptives or other classes of medicines where lack of efficacy could result in serious consequences, require reporting. Normal progression of disease does not imply lack of efficacy. The batch/lot number of the suspected medicine for a report of lack of efficacy must be included in the report.
- Complementary medicines The ‘natural’ content of complementary medicines means they are often considered to be ‘safe’ by the public who, along with many professionals, fail to recognise the potential potency of many such products. Patients and healthcare professionals should also be aware that interactions between complementary and other, prescribed or OTC, medicines can also cause unexpected reactions. It is therefore important that any suspected ADR which occurs from a complementary medicine is reported and that as much information about the ingredients and the source of the remedy are included.
- Delayed drug effects Some reactions may become manifest months or years after exposure. Any suspicion of such an association should always be reported. Examples of delayed reactions that might need to be reported include:
- kidney disease from long term usage of analgesics or non-steroidal anti-inflammatory drugs (NSAIDs);
- Interactions If an adverse effect is suspected to be related to an interaction between two or more medicines, it should be reported as an adverse reaction.
- Medication errors Medication errors, whether resulting in an adverse drug reaction or not, must be reported.
- Overdose Suspected ADRs, associated with an overdose, should be reported, as well as other reactions that may have occurred due to the overdose.
- Reports relating to pregnancy and breastfeeding The healthcare professional must report suspected ADRs related to pregnancy or breastfeeding regardless of whether the medicine is contra-indicated in pregnancy and/or lactation
- Serious adverse drug reactions All serious suspected reactions must be reported. The side effects of an established medicine may be well known but if a serious reaction occurs it should always be reported regardless of whether it is expected or not.
- Product quality problem Healthcare professionals are encouraged to report product quality problems, whether resulting in an adverse drug reaction or not. The batch/lot number of the suspected medicines must be included in the report.
- Teratogenicity and congenital anomalies The following information should be provided for reports on congenital anomalies or teratogenicity:
- age and sex of the infant;
- the birth date or the date on which the pregnancy was ended; (duration of pregnancy/gestational age of foetus/baby);
- date and/or duration of exposure to teratogen/substance/medicine in preconception period, and/or any or all trimesters of pregnancy;
- the teratogen/substance(s) or medicine(s) exposed to and the dose in case of a medicine and reason(s) for exposure or treatment with the medicine(s)
Suspected ADRs should also be reported in cases where a baby is born with a congenital abnormality or where a pregnancy results in a malformed or aborted foetus and the report should include information of all medicines taken during pregnancy [13].
Detection Method of ADR
- Hypothesis generating methods include
Spontaneous ADR Reporting
which is a system whereby any suspected ADRs are voluntarily notified by health professionals, pharmaceutical companies and other stakeholders to a central authority (Central Drugs Standard Control Organization -CDSCO in India).
Prescription Event Monitoring
represents a method which is hybrid of spontaneous reporting with aspects of formal epidemiological studies.
Systematic methods
public health surveillance data such as death registries are used to identify patterns of reactions that might be associated with drug use [14][15] .
- Hypothesis testing methods include :
Case-Control Studies
In case control studies the research compares the exposure rate in the cases with the exposure rate in the control.
Cohort Studies
These studies involve a group of patients (cohort) followed up for a time duration long enough to detect the outcome of interest.
Randomized Controlled Trials
These studies involve patients divided into two groups randomly into exposed and the other not exposed, so that the outcomes can be compare [14][15] .
Management of Adverse Drugs Reaction
Successful management of adverse drug reactions requires early identification and prompt treatment of anaphylaxis, whether due to immunoglobulin (Ig) E- or non-IgE-mediated mechanisms of mast cell mediator release.
Acute therapy is directed toward enhancement of oxygenation and maintenance of normotension. Requisite measures include the use of epinephrine, oxygen, and adequate fluid replacement; in some instances, vasopressors or corticosteroid drug therapy may be warranted. Emergency measures may be needed to maintain the airway. Although the offending drug is usually discontinued, a necessary drug for which there is no satisfactory alternative occasionally may be continued without danger of further anaphylaxis as long as therapy is not interrupted. Good management also requires anticipation of adverse reactions whenever a therapeutic program is instituted. Familiarity with the drug groups most commonly responsible for immunologic reactions is helpful, as is knowledge of satisfactory alternatives for these drugs in the presence of known hypersensitivity.
Naranjo Scale
The Naranjo algorithm, Naranjo Scale, or Naranjo Nomogram is a questionnaire designed by Naranjo et al. for determining the likelihood of whether an ADR (adverse drug reaction) is actually due to the drug rather than the result of other factors. Probability is assigned via a score termed definite, probable, possible or doubtful. Values obtained from this algorithm are often used in peer reviews to verify the validity of author's conclusions regarding adverse drug reactions. It is also called the Naranjo Scale or Naranjo Score. The ADR Probability Scale consists of 10 questions that are answered as either Yes, No, or “Do not know”. Different point values (-1, 0, +1 or +2) are assigned to each answer. A simplified version of the 10 questions is provided below [17] .
-
-
- ? 9 = definite ADR
- 5-8 = probable ADR
- 1-4 = possible ADR
- 0 = doubtful ADR
AIM & OBJECTIVE AIM
Aim of this case report observation study is a study of adverse drug reaction. It focus on the adverse drug reaction of IV Paracetamol drug .
OBJECTIVE
Study of adverse drug reaction occurred due of paracetamol
MATERIAL AND METHOD STUDY TITLE
To study adverse drug reaction due to paracetamol drug.
The Present study was conducted at Manipal Hospital, Baner during the period of 22 April, 2023 to 28 April, 2023.
Study Design
Case report observational study.
Source of Study Population
- OPD patient visited to Manipal Hospital, Baner.
- Inclusion
- Patient’s name, age, gender. Drug Prescribed.
- Dosage of Drugs Prescribed & dosage form. Route of Administration.
Exclusion
Incomplete information regarding patient.
Data Collection
Data on the Reported ADRs will be evaluated to understand the pattern of the ADRs with respect to patient demographic disease, Nature of the reactions, characteristics of the drugs involved, and outcome of the reactions.
Criteria For Identifying ADRs
ADR identified by physicians will be considered and will be included in the study.
Analysis of ADRs
Nature and description of ADDRs reported.
Causality Assessment of ADR Based on Algorithm
The degree of association of an adverse of an adverse reaction with a drug is done with the help of Naranjo’s algorithm
CASE STUDY
Patient Observations
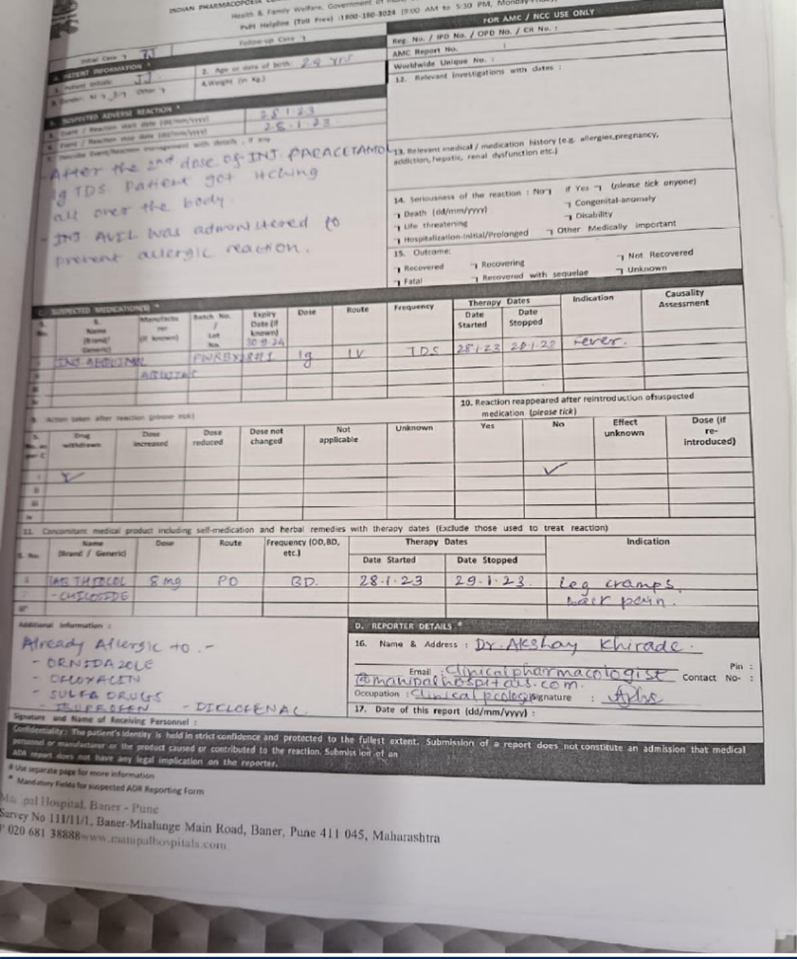
Patient Initials –
JJ
Age –
24 yrs
Sex –
Female
Hospital/Clinic –
Manipal Hospital Baner , Pune
- SUSPECTED MEDICATION DETAILS
Drug - Inj.
Aequimol
Batch No.-
PWRBX28H1
Dose –
1g
Route –
Intravenous (IV)
Expiry date – 30/09/2024
Frequency - TDS Therapy Dates :
Date Started –
28/01/2023
Date Stopped –
28/01/2023
Indication
– Fever
- CONCOMITANT MEDICATION DETAILS
Drug –
Tab.Thiocolchicoside
Route –
Oral Frequency – BD Dose – 8 mg Therapy Dates
Date Started –
28/01/2023
Date Stopped –
29/01/2023
Indication –
Leg cramps and back pain
Adverse Drug Reaction
The patient who had fever , Inj. Aequimol 1g for three times a day by intravenous route was prescribed to her. After the 2nd dose of Inj. Aequimol , patient got itching all over the body Action Taken After Reaction
- Inj. Avil was administered to prevent allergic reaction.
- Reaction rappeared after reintroduction of suspected medication - No
Reporter Details
Name –
Dr. Akshay Khirade
Address-
Clinical Pharmacologist, Manipal Hospitals.
Contact –
9604020940
Occupation-
Clinical Pharmacologist
Additional Information –
Patient was already allergic to following drugs -
- Ornidazole
- Ofloxacin
- Sulfa Drugs
- Ibuprofen
- Diclofenac
- Suspected Drug and Its Pharmacology
Class of Drug:-
Analgesic and Antipyretic
Brand name:-
Aequimol
Dose and Strength:
- Injection-150mg
- Tablet-500mg
- Capsule-500mg
- Syrup you swallow (oral solution)-120mg,250mg or 500mg in 5ml
- Powder you add to water and drink
- Suppository (medicine that you push gently into your bottom)-60mg
Mechanism Of Action
Paracetamol has a central analgesic effect that is mediated through activation of descending serotonergic pathways. Debate exists about its primary site of action, which may be inhibition of prostaglandin (PG) synthesis or through an active metabolite influencing cannabinoid receptors.
Pharmacokinetic Properties of IV Paracetamol Maximum plasma concentration :
Single 1g dose ;30µg/mL
Volume of distribution : 1L/kg
Therapeutic doses :
predominantely in the liver by glucuronidation and sulfation
Higher doses :
via the cytochrome P450 2E1 pathway, producing N-acetyl-p-benzoquinone (NAPQI), which rapidly forms conjugates with gluathione to generate nontoxic metabolites Acute overdose : gluthaione stores may be depleted, NAPQI may accumulate and hepatotoxicity may occur (administer N-acetylcysteine as antitode within 10hr of overdose) Systemic clearance :
18L/hr
Mean elimination half-life (Adults):
2.7 hours
Excretion :
Predominantly in the urine as glucuronide (60-80%) and sulfide (20-30%) conjugates
<5>
Adult Indications and Dosage :
Acetaminophen injection is indicated for:
-
-
-
- The management of mild to moderate pain
- The management of moderate to severe pain with adjunctive opioid analgesics
- The reduction of fever.
General Dosing Information :
Acetaminophen infusion may be given as a single or repeated dose for the treatment of acute pain or fever. No dose adjustment is required when converting between oral acetaminophen and acetaminophen infusion dosing in adults and adolescents who weigh 50 kg and above. Calculated maximum daily dose of acetaminophen is based on all routes of administration (i.e., intravenous, oral, and rectal) and all products containing acetaminophen. Exceeding the maximum mg/kg daily dose of acetaminophen as described in tables may result in hepatic injury, including the risk of liver failure and death. To avoid the risk of overdose, ensure that the total amount of acetaminophen from all routes and from all sources does not exceed the maximum recommended dose.
Recommended Dosage: Adults and Adolescents
Adults and adolescents weighing 50 kg and over
The recommended dosage acetaminophen infusion is 1000 mg every 6 hours or 650 mg every 4 hours, with a maximum single dose of acetaminophen infusion of 1000 mg, a minimum dosing interval of 4 hours, and a maximum daily dose of acetaminophen of 4000 mg per day (includes all routes of administration and all acetaminophen- containing products including combination products).
Adults and adolescents weighing under 50 kg -
The recommended dosage of acetaminophen infusion is 15 mg/kg every 6 hours or 12.5 mg/kg every 4 hours, with a maximum single dose of acetaminophen infusion of
15 mg/kg, a minimum dosing interval of 4 hours, and a maximum daily dose of acetaminophen of 75 mg/kg per day (includes all routes of administration and all acetaminophen-containing products including combination products).
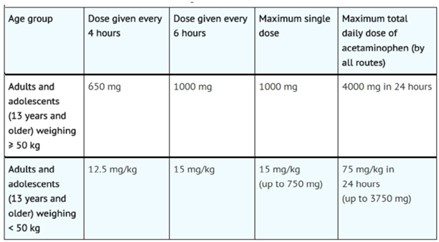
Table.2. Dosing for Adults and Adolescents
Recommended Dosage: Children
Children 2 to 12 years of age:
The recommended dosage of acetaminophen infusion is 15 mg/kg every 6 hours or 12.5 mg/kg every 4 hours, with a maximum single dose of acetaminophen infusion of 15 mg/kg, a minimum dosing interval of 4 hours, and a maximum daily dose of acetaminophen of 75 mg/kg per day [19].
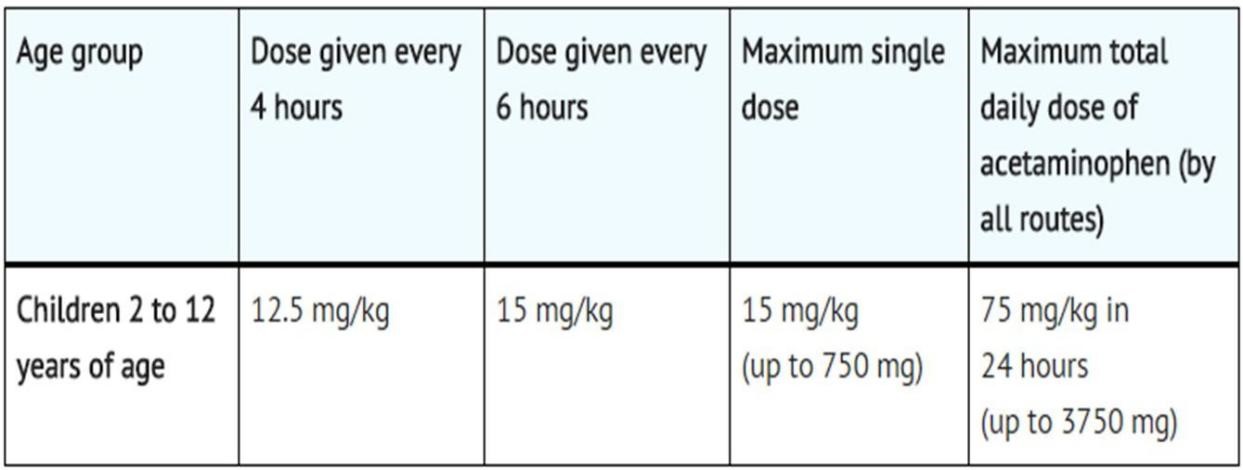
Table.3.Dosing for children
Symptoms of Overdose
- Diarrhea
- Increased sweating
- Loss of appetite
- Nausea or vomiting
- Stomach cramps or pain
- Swelling, pain, or tenderness in the upper abdomen or stomach area.
Side Effects
- An allergic reaction, which can cause a rash and swelling
- Flushing, low blood pressure and a fast heartbeat – this can sometimes happen when paracetamol is given in hospital into a vein in your arm
- Blood disorders, such as thrombocytopenia (low number of platelet cells) and leukopenia (low number of white blood cells)
- Liver and kidney damage, if you take too much (overdose) – this can be fatal in severe cases [20] .
Pregnancy and breastfeeding
- Tell your healthcare provider if you are pregnant or plan to become pregnant. If necessary, paracetamol can be used during pregnancy. Paracetamol can be taken if you are breastfeeding. Small amounts of paracetamol pass into breast milk.
- Use the lowest possible dose that reduces your pain and/or fever and use it for the shorted time possible. Contact your doctor or midwife if the pain and/or fever are not reduced or if you need to take this medicine more often.
Contraindications
- Do not administer to patients with severe hepatic impairment.
- Administer with caution to patients with moderate hepatic impairment, severe renal impairment, chronic alcoholism, malnutrition, dehydration.
- May cause (very rarely): malaise, hypotension and rash.
- Do not exceed indicated doses, especially in children and elderly patients. Paracetamol intoxications are severe (hepatic cytolysis) [21].
Drug Interaction
- Aceclofenac : may decrease the excretion rate of Abacavir which could result in a higher serum level.
- Acyclovir: Acyclovir may decrease the excretion rate of Abacavir which could result in a higher serum level.
- Baclofen: Baclofen may decrease the excretion rate of Abacavir which could result in a higher serum level.
- BCG vaccine: The therapeutic efficacy of BCG vaccine can be decreased when used in combination with Abacavir.
- Bendroflumethiazide: Bendroflumethiazide may increase the excretion rate of Abacavir which could result in a lower serum level and potentially a reduction in efficacy.
Uses
-
-
-
- Acetaminophen injection is used to relieve mild to moderate pain and to reduce fever.
- Acetaminophen injection is also used in combination with opioid (narcotic) medications to relieve moderate to severe pain.
- Ear Pain
- Cold
- Fever
- Muscle Pain
- Headaches, Migraine
- Arthritis
Suspected Medication - IV Paracetamol
Fig.1. Aequimol IV 100 ml, 1000 mg
CONCOMITANT MEDICATION
Drug –
Tab.Thiocolchicoside
Route –
Oral Frequency – BD
Dose –
8 mg Therapy Dates :
Date Started –
28/01/2023
Date Stopped –
29/01/2023
Drug Class:
Muscle relaxant
Tablet Thiocolchicoside
Brand Name:
Muscoril,Myoril,Neoflax
Dosages Of Thiocolchicoside:
Oral :Adult: Initially,16mg daily.
Intramuscular; Muscular spasms Adult: upto 8mg daily.
Mechanism Of Action
Thiocolchicoside binds to GABA-A andstrychnine sensitive glycine receptors. Thiocolchicoside acting as a GABA-A receptor antagonist, its myorelaxant effects could beexerted at the supra-spinal level, via complex regulatory mechanisms, although a glycinergic mechanism of action cannot be excluded. The characteristics of the interaction of Thiocolchicoside with GABA-A receptors are qualitatively and quantitatively shared by its main circulating metabolite, the glucuronidated Derivative. Thiocolchicoside is rapidly absorbed after oral administration, and metabolized into 3 main metabolites. The two main circulating forms were the Thiocolchicoside aglycon and the glucuronidated derivative of Thiocolchicoside, which is active. Thiocolchicoside is well tolerated oral administration for periods of up to 6 months.
Side Effect
- Sedation, drowsiness, blurred or double vision
- Constipation or diarrhea, dizziness
- Drowsiness, nervousness and confusion
- Dyspepsia (chronic or recurrent pain in the upper abdomen, upper abdominal fullness,
- And feeling full earlier than expected when eating),
- Fatigue, headache, heartburn, hiccups
- Nausea, insomnia, stomach cramps
- Trembling, vomiting, and weakness
- Long-term use photosensitivity reactions.
Therapeutic Uses
- Thiocolchicoside is a muscle-relaxant(skeletal)agent used for the treatment of orthopedic, traumatic and rheumatologic disorders
- Anti-inflammatory & Analgesic properties.
- Used in combination with glafenine and meprobamate to tranquilize patients undergoing hysterosalpingography.
- In the treatment of painful muscle spasms.
- Spastic sequelae of hemiparesis, Parkinson's disease and latrogenic Parkinson symptoms, particularly the neurodyslectic syndrome.
- Acute and chronilumbar and sciatic pain, cervico-brachial neuralgia, persistent torticollis, post-traumatic and post-operative pain.
Contraindications
It is contraindicated to pregnant women, lactating mother and also peoples about to undergo surgery and having ulcer to stomachs. Should not be used during pregnancy and lactation. Should not be given to children. Avoid in people who develop hypersensitivity [22][23].
Scoring for Naranjo algorithm
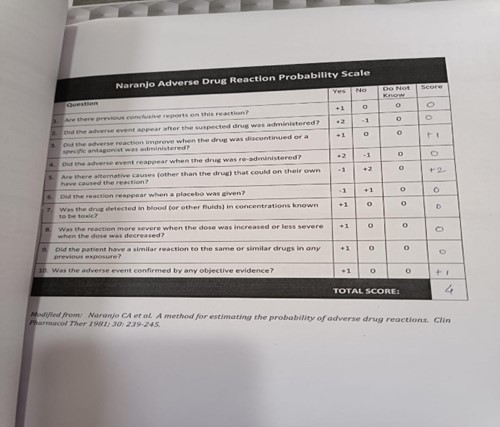
>9 = definite ADR 5–8 = probable ADR 1–4 = possible ADR 0 = doubtful ADR. A 24 year patient prescribed IV PARACETAMOL 1g of dose thrice a day, after 2nd dose of injection patient got itching all over the body which means patient suffering from hypersensitivity reaction (Anaphylaxis). The patient reported and measure naranjo scale and the total probability score of naranjo scale was 4, that show patient reported with possible adverse drug reaction type.
RESULT
The Naranjo Adverse Drug Reaction Probability Scale is a tool used to assess the likelihood that a given adverse drug reaction (ADR) is caused by a particular medication. The scale consists of ten questions, and each question is assigned a score of -1, 0, or +1, depending on the response. The total score can range from -4 to +13, with higher scores indicating a higher probability of the ADR being drug-related. A score of 4 on the Naranjo scale indicates a "Possible" likelihood of the ADR being drug- related. The scale ranges from 1-4. The patient developed anaphylaxis reaction after 2nd dose of inj paracetamol,the adverse drug reaction (ADR) in the patient with a Naranjo scale score of 4 suggests a possible causal relationship between the medications (Inj. Paracetamol) and the observed reaction. Due to which it states the reaction occurred after the administration of drug
Recognized pattern associated with the suspected drugs followed a known or recognized pattern. This could mean that the adverse event is consistent with the expected side effects or known adverse reactions of Inj. Paracetamol. The reaction could be explained by features or characteristics of the patient's underlying disease. Certain conditions or patient factors can make individuals more susceptible to experiencing adverse reactions to medications.
DISCUSSION
A female patient of 24 years came to the hospital having chief complaint of fever. She was adviced Injection Aequimol. Inj. Aequimol 1g for three times a day by intravenous route was prescribed to her. After the 2nd dose of Inj. Aequimol, patient got itching all over the body. Concomitant medications in which advised tablet thiocolchicoside indicated for leg cramps and back pain. Patient has history of allergy of Ornidazole, Ofloxacin, Sulfa Drugs, Ibuprofen, Diclofenac. In given study the total Naranjo scale scored was 4 that described that given adverse reaction type was reported as possible adverse reaction . The study described that in present case study is dechallenge type and in replacement of this the treatment prescribed to the patient i.e Inj. Avil was administered to prevent allergic reaction. Further investigation is needed. Clearly, there remains considerable uncertainty regarding the chronic adverse effects of paracetamol use. The evidence base in each of the above sections relies mostly on observational and cohort studies, and so is prone to inherent biases. The positive associations found in these studies are generally weak, and often contradictory. Few RCTs have been performed but, when undertaken, usually give reassuring results. Further studies are required in many areas, but RCTs may be difficult to perform, either because they would need to be very large to detect the modest increases in risk seen in the observational studies, or because of the significant ethical issues of using placebo in patients in pain, as well as of conducting trials in children and pregnant women. The two areas in which the evidence is most convincing are hypertension and GI bleeding. A small BP rise of 4 mmHg would be clinically important at the population level, and the outcome of ongoing RCTs should clarify the reliability of this estimate. This may be particularly important in patients with angina or pre?existing hypertension. The fairly consistent evidence for GI bleeding associated with paracetamol use, along with its additive effect when combined with NSAIDs, may be less well known but similarly important. When considering prescribing paracetamol in the chronic setting it would seem wise to consider these adverse effects, based on current data, and discuss them with the patient. Whether paracetamol use in the chronic setting should be restricted is doubtful, given that the alternatives are NSAIDs and opioids. Indeed, in patients intolerant of NSAIDs, their next option would be opioid medication, which comes with risks of addiction, drowsiness and fatal accidental overdose. In summary, the average therapeutic effect for chronic pain syndromes is small, but there is accumulating evidence of clinically significant adverse effects in chronic use. Despite this, for patients who derive clear symptomatic benefit, or only take occasional therapeutic doses, the risks are probably very small. For this reason, paracetamol can be seen as the ‘least?worst’ option – which probably means that it will remain, for now at least, the first?line analgesic of choice.
CONCLUSION
In present study concluded that the patient prescribed with paracetamol in which after the second dose of Inj. paracetamol 1gm TDS patient get itching all over the body , so in this case the type of case study is dechallenge and to overcome this patient prescribed with antihistamine drug that is Inj. Avil . The Naranjo scale scored 4 which shows that the type of ADR is possible ADR type & it states that the following reported ADR reaction is a temporal sequence after a drug was prescribed . The reported ADR followed a recognized pattern to the suspected drug as well as it could be explained by characteristics of the patient’s disease. It concluded that there is causality assessment between adverse drug reaction and suspected medication. So,further investigation is needed.
REFERENCES
-
-
-
- WHO. International monitoring of adverse reactions to drugs: adverse reaction terminology. WHO Collaborating Centre for International Drug Monitoring, Uppsala, Sweden, 1992.
- Zopf Y. Risk factors associated with adverse drug reactions following hospital admission: a prospective analysis of 907 patients in two German university hospitals. Drug Safety 2008; 31: 789-798.
- Word Health Organization. International drug monitoring: the role of the hospital. Geneva, Switzerland: word health organization;1966. technical report series no.425.
- Khraenbuhl-Melcher A, Schlienger R, Lampert M, et al. Drug – related problems in hospitals. A review of the recent literature. Drug Safety 2007; 30: 379-407.
- Stephens MDB. Definitions and classifications of adverse reaction terms in: Stephens MDB, Talbot JCC, Routledge PA, eds. The detection of new adverse reactions, 4th edn. London: Macmillan reference, 1998: 32-44.
- Edwards IR, Aronson JK. Adverse drug reactions: definitions, diagnosis, and management. Lancet 2000; 356: 1255-259.
- Nebeker JR, Barach P, Samore MH. Clarifying adverse drug events: a clinican’s guide to terminology, documentation, and reporting. An Intern. Med 2004; 140: 795-801.
- G Paithasarathi, Karin N, Nahata M. Essential concepts and skills.lst Universities Press Impression. 2008: 84-97.
- Tripathi K. Text book of Pharmacology. Jaypee Brothers Medical publishers Pvt. Ltd.2013: 218-222.
- W Roger, Whittle C. Clinical pharmacy and Therapeutics. Churchill Livingstone Elsevier; 2007: 51-60.
- BotharaK. Fundamentals of Pharmacology. NiraliPrakashan; 1, 2012: 10.1-10.8.
- Tipnis H, Bajaj A. Clinical Pharmacy. career publication. 2013: 330-342.
- https://www.sahpra.org.za/wp-content/uploads/2020/04/ADR-Reporting- Guideline_HCPs_v1_for-commenting_March-2020.pdf
- Mann R and Andrews E. Pharmacovigilance. hichester England: John Wiley and Sons Ltd; 2002.
- Belhekar MN, Taur SR, Munshi RP.A study of agreement between the Naranjo algorithm and WHO-UMC criteria for causality assessment of adverse drug reactions. Indian J Pharmacol 2014;Vol 46:117-20.
- Aronson J.K., Ferner R.E. Clarification of terminology in drug safety. Drug Saf. 2005;28:851–870. doi: 10.2165/00002018-200528100-00003.
- Naranjo CA, Busto U, Sellers EM, Sandor P, Ruiz I, Roberts EA, Janecek E, et al. A method for estimating the probability of adverse drug reactions. Clin Pharacol Ther. 1981;30:239–45.
- https://www.semanticscholar.org/paper/The-hemodynamic-effects-of-intravenous- paracetamol-Chiam-Bellomo/71d4bd99032556e97559a2904e6508f20dff3b6f
- "Tylenol, Tylenol [[infants]]' Drops (acetaminophen) dosing, indications, interactions, adverse effects, and more". Medscape Reference. WebMD. Retrieved 10 May 2014.
- https://www.drugs.com/sfx/paracetamol-side-effects.html
- Allanore L., Roujeau J.-C. Clinic and Pathogenesis of Severe Bullous Skin Reactions: Stevens-Johnson Syndrome, Toxic Epidermal Necrolysis. In: Pichler W.J., editor. Drug Hypersensitivity. Karger; Basel, Switzerlands: 2007. pp. 267–277.
- Carta M, Murru L, Botta P, Talani G, Sechi G, De Riu P, Sanna E, Biggio G The muscle relaxant thiocolchicoside is an antagonist of GABAA receptor function in the central nervous system.Neuropharmacology. 2006 Sep;51(4):805-15. Epub 2006 Jun 30.
- Mascia MP, Bachis E, Obili N, Maciocco E, Cocco GA, Sechi GP, Biggio G Thiocolchicoside inhibits the activity of various subtypes of recombinant GABA(A) receptors expressed in Xenopus laevis oocytes. Eur J Pharmacol. 2007 Mar 8;558(1-3)


 Sushma Shivaji Dhas*
Sushma Shivaji Dhas*
 Sushma Shivaji Dhas
Sushma Shivaji Dhas
 Kiran H. Bibave
Kiran H. Bibave
 Rupali G. Chaudhari
Rupali G. Chaudhari
 Sailee S. Girigosavi
Sailee S. Girigosavi
 Sejal M. Chavan
Sejal M. Chavan
 Sakshi V. Gilbile
Sakshi V. Gilbile
 Kiran R. Dadas
Kiran R. Dadas
 Akash S. Domate
Akash S. Domate






 10.5281/zenodo.11203574
10.5281/zenodo.11203574