Abstract
Buccal films represent an innovative drug delivery technology designed to meet essential requirements for effective administration via the buccal route. These films are compact, low in dose, and easy to administer, making them more user-friendly and preferable compared to other buccal drug delivery formats such as wafers, lozenges, microparticles, gels, or tablets. One of the primary benefits of oral films is their ability to bypass first-pass metabolism, leading to improved drug bioavailability. They also effectively mask unpleasant drug flavors and eliminate the need for water during administration. The buccal mucosa, with its rich blood supply and high permeability, serves as an excellent site for both local and systemic drug delivery. When placed on the tongue, the buccal film rapidly absorbs moisture from saliva, hydrating and adhering to the application site for effective drug release. This review explores the advantages, manufacturing techniques, evaluation criteria, and formulation approaches associated with buccal films. Given their benefits, buccal film delivery systems have significant potential as a prominent dosage form in future pharmaceutical and healthcare applications.
Keywords
Microspheres, efficacy, novel, potency, compliance
Introduction
Recent advancements in drug delivery technology have provided effective alternatives to traditional oral routes, particularly for pediatric, geriatric, bedridden, nauseous, or noncompliant patients. Challenges associated with oral administration such as significant first-pass metabolism by the liver, drug degradation in the harsh gastrointestinal environment, and the invasiveness of parenteral routes can be addressed by utilizing the buccal route. [1] In recent years, buccal drug delivery has gained prominence as an essential method of administration. Buccal films, a cutting-edge technology, have been developed to meet these needs, drawing inspiration from the design principles of transdermal patches. These films are compact, low-dose, and easy to administer, making them a more palatable and acceptable dosage form compared to alternatives like tablets, lozenges, wafers, gels, or capsules.[2]
This delivery system is particularly suitable for drugs subject to extensive first-pass metabolism, as it enhances bioavailability while reducing the dosing frequency needed to maintain steady plasma levels. This, in turn, minimizes adverse side effects. The system involves an ultra-thin oral film applied to the tongue or other oral mucosal surfaces. Upon contact with saliva, the film quickly hydrates, adheres to the application site, and begins to disintegrate, releasing the medication for oromucosal absorption. With specific formulation adjustments, the film can also dissolve for subsequent gastrointestinal absorption. [4]
Buccal films can be tailored for systemic or localized therapeutic effects. However, developing high-quality buccal films remains a significant challenge, requiring thorough evaluation and a deep understanding of their performance characteristics.
Special features of mouth dissolving films
- Slim and sophisticated design
- Offered in multiple sizes and shapes
- Discreet and non-intrusive
- Superior mucoadhesive properties
- Quick to disintegrate
- Ensures rapid drug release [6]
Advantages
- Simple and convenient to administer.
- Therapy can be easily discontinued if needed.
- Provides a fast onset of action.
- Eliminates the need for water for swallowing or chewing.
- Delivers the drug directly into systemic circulation, minimizing the hepatic first-pass effect.
- Eliminates the risk of choking.
- Allows for localized and site-specific drug action.
- Compact size enhances patient compliance.
- Increases bioavailability for specific therapeutic agents.
- Effectively masks unpleasant tastes.
- Compact size further improves patient acceptance. [6]
Disadvantages
- Exhibits a delicate, granular characteristic.
- Cannot accommodate larger drug doses in oral film format.
- Requires specialized equipment for packaging.
- Achieving uniformity in the dosage form poses challenges.
- Being hygroscopic, it needs to be stored in a dry environment. [6]
Buccal Mucosa
The oral mucosal drug delivery system can be categorized into two main types: buccal and sublingual. The buccal cavity is widely utilized for administering drugs through the mucosa, whereas the sublingual route is often preferred for achieving a rapid onset of action, as seen in the treatment of angina pectoris. The buccal mucosa refers to the lining inside the cheek. Within the oral cavity, drug delivery methods are further classified into three distinct categories.
- Sublingual Delivery
- Buccal Delivery
- Local Delivery
The oral cavity includes various structures such as the lips, cheeks, hard palate, soft palate, and the floor of the mouth. It is anatomically divided into two main regions: the outer oral vestibule, which is bordered by the lips, cheeks, teeth, and gingiva (gums), and the oral cavity proper, which stretches from the teeth and gums to the fauces, leading to the pharynx. The hard and soft palates form the roof, while the tongue arises from the floor of the cavity. Specific areas within the oral cavity can be further identified, including: [8]
-
- Gingiva
- Hard palate
- Soft palate
- Tonsil
- Tongue
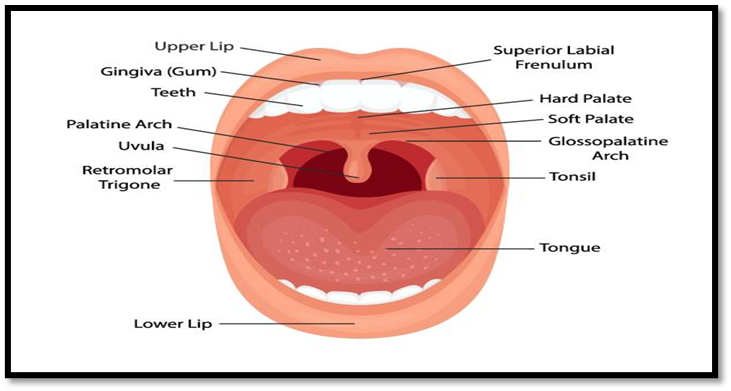
Figure No.1 Anatomy and Physiology of Buccal cavity [8]
The oral mucosa consists of several layers, with the outermost being the stratified squamous epithelium. Beneath this lies the basement membrane, followed by the lamina propria and the submucosa, which forms the deepest layer. The epithelium resembles other stratified squamous epithelia in the body, featuring a basal cell layer that undergoes active mitosis. This layer transitions through multiple intermediate stages of differentiation before forming the superficial layers. [5]
Tongue
The tongue is a voluntary muscular organ located on the floor of the mouth. It is anchored at its base to the hyoid bone and connected to the floor of the mouth by a mucous membrane fold known as the frenulum. The upper surface of the tongue is covered with stratified squamous epithelium and is adorned with numerous papillae, which are small projections housing the sensory nerve endings responsible for taste, commonly referred to as taste buds. [9]
Formulation Consideration For Buccal Film:
The development of orodispersible films (ODFs) requires careful consideration of various attributes, including taste masking, rapid dissolution, visual appeal, and mouthfeel, among others. The excipients used in ODF formulations are categorized based on their specific functions. From a regulatory standpoint, all excipients included in the formulation must be recognized as safe and approved for use in oral pharmaceutical dosage forms. The key components of the formulation include: [9]
- Drug
- Water soluble film forming polymers
- Plasticizers
- Saliva stimulating agent
- Sweetening agent
- Flavouring agent
- Surfactant
- Colours, Filler
Table 1. Concentration of Component [9]
|
Sr. No.
|
Ingredients
|
Amounts
|
|
1
|
Drug
|
1-30%
|
|
2.
|
Film forming polymer
|
40-50%
|
|
3.
|
Plasticizer
|
0-20%
|
|
4.
|
Saliva stimulating agent
|
2-6%
|
|
5.
|
Sweetening agent
|
3-6%
|
|
6.
|
Flavouring agent
|
Q. S.
|
|
7.
|
Surfactant
|
Q. S.
|
|
8.
|
Colours, Filler
|
Q. S.
|
1. Active Pharmaceutical Ingredient:
Active pharmaceutical substance can be from any class of pharmaceutically active substances that can be administered orally or through the buccal mucosa. Like antiulcers, antiasthmatics, antitussive, antihistaminic, antiepileptic, expectorants, antianginal etc. For the effective formulation, dose of drug should be in mgs (less than 20 mg/day). Usually 5%w/w to 30%w/w of active pharmaceutical ingredients can be incorporated in buccal film. High dosage of molecules is difficult to incorporate into film. [5]
2. Film Forming Polymers:
Polymers play a crucial role as the primary ingredient in oral fast-dissolving films. The film's strength and durability are influenced by the quantity of polymer used in the formulation. These polymers have garnered significant interest in both the medical and nutraceutical industries. Typically, about 45% w/w of polymer, calculated based on the dry film's total weight, is utilized. Hydrophilic polymers are predominantly employed in buccal films due to their ability to disintegrate quickly when exposed to saliva in the oral cavity. Their unique feature lies in their mucoadhesive properties, enabling them to recognize and bind to specific sugar residues on the mucosal surface without disrupting the ligand’s structure. [23]
Ideal Property of Film Forming Polymer:
- The polymer should be non-toxic and non-irritating.
- It must possess hydrophilic properties.
- It should demonstrate excellent film-forming capability.
- The polymer should have good wetting and spreading characteristics.
- It must be readily accessible and cost-effective.
- A sufficient shelf life is essential.
- The polymer should be tasteless and colorless.
- It should not lead to secondary infections in the oral mucosa.
- Adequate peel, shear, and tensile strengths are necessary.
Currently, both natural & synthetic polymers are used for the preparation of fast dissolving film. Now a day’s various natural & synthetic polymers are available in preparation of fast dissolving film. [23] Polymers used in preparation of fast dissolving film are.
Table 2. Polymers used in preparation of dissolving film [5]
|
Sr.No
|
Natural Polymer
|
Synthetic polymer
|
|
1
|
Pullulan
|
Hydroxypropylmethyl cellulose
|
|
2
|
Starch gelatin
|
Polyvinyl pyrrolidone
|
|
3
|
Pectin
|
Polyvinyl alcohol
|
|
4
|
Sodium alginate
|
Carboxy methyl cellulose
|
|
5
|
Maltodextrin
|
Poly ethylene oxide
|
|
6
|
Polymerized rosin
|
Kollicoat
|
|
7
|
Lycoat NG 73
|
Hydroxypropyl cellulose
|
|
8
|
Xanthan
|
Hydroxyl ethyl cellulose
|
Plasticizers
Plasticizers are vital components in oral film formulations. Their selection is determined by their compatibility with the chosen polymer and the type of solvent used during film casting. These agents enhance the film's flexibility, impart a glossy appearance to the final product, and reduce brittleness. Typically, they are used at concentrations of 1–20% w/w based on the dry polymer weight. Examples of commonly used plasticizers include glycerol, propylene glycol, low molecular weight polyethylene glycols, citrate derivatives such as triacetin and acetylcitrate, phthalate derivatives like dimethyl, diethyl, and dibutyl phthalates, as well as castor oil. [12]
4. Sweetening Agents
Sweetening agents, both natural and artificial, are included to enhance the palatability of fast-dissolving oral thin films. These agents are particularly important in food and pharmaceutical products designed to dissolve or disintegrate in the oral cavity. Common natural sweeteners include sucrose, dextrose, fructose, glucose, liquid glucose, and maltose. Fructose, which is sweeter than both sucrose and dextrose, is often preferred for its rapid perception in the mouth. However, natural sweeteners pose challenges for diabetic patients, making artificial sweeteners more popular in food and pharmaceutical applications. First-generation artificial sweeteners include saccharin, cyclamate, and aspartame, while second-generation options include acesulfame-K, sucralose, alitame, and neotame. [13]
5. Saliva Stimulating Agents
Saliva-stimulating agents are added to increase saliva production, facilitating the rapid disintegration of film formulations. These agents indirectly assist in the quick breakdown and dissolution of the film. Commonly used substances include food-grade acids such as citric acid, lactic acid, maleic acid, and ascorbic acid. [13]
6. Cooling Agents
Cooling agents like monomethyl succinate are used to enhance flavor strength and improve the mouthfeel of the film. Other cooling agents such as WS-3, WS-23, and Utracoll II can be used in combination with flavoring agents for an improved sensory experience. [34]
7. Colouring Agents
Coloring agents, including pigments like titanium dioxide and FD&C-approved colorants, may be added to the buccal film formulation. These are typically used at concentrations of up to 1% w/w, especially when insoluble ingredients or suspended drugs are part of the formulation. [31]
8. Surfactants
Surfactants function as solubilizing or wetting agents, promoting rapid dissolution of the film within seconds and facilitating immediate drug release. They can also improve the solubility of poorly soluble drugs in buccal formulations. Examples of surfactants include Poloxamer 407, sodium lauryl sulfate, benzalkonium chloride, benzethonium chloride, and substances like Tweens and Spans. [8]
9. Stabilizing agent
Stabilizers and thickeners are essential for enhancing the viscosity and consistency of the dispersion or solution before casting the film. Examples include natural gums such as xanthan gum, locust bean gum, carrageenan, and cellulosic derivatives. These agents are generally used at concentrations of up to 5% w/w. [21]
Manufacturing Method Of Buccal Film:
- Solvent Casting Method
- Hot Melt Extrusion Method
- Direct Milling Method
1. Solvent Casting Method
In the solvent casting method, the appropriate amount of polymer is dissolved in distilled water. The active pharmaceutical ingredient (API) is then incorporated into the solution in small quantities. A plasticizer is added to the mixture, and the solution is thoroughly stirred to ensure uniformity. This prepared solution is poured onto a petri dish and placed in a hot air oven set at 40°C for drying. Once dried, the film is carefully removed from the petri dish using a blade and stored in a desiccator for 24 hours. Afterward, it is cut into the desired size and shape. [2]
Step 1: Preparation of casting solution
Step 2: Deaeration of solution
Step 3: Transfer of appropriate volume of solution into the mould
Step 4: Drying the casting solution
Step 5: Cutting the final dosage form to contain desired amount of drug
Advantages
- Provides excellent uniformity in thickness and superior clarity compared to extrusion methods.
- Films exhibit a fine gloss and are free from defects like die lines.
- Enhanced flexibility and improved physical properties.
Disadvantages
- Requires polymers that are soluble in volatile solvents or water.
- A stable solution with an optimal balance of solids content and viscosity must be prepared.
2. Hot Melt Extrusion Method
The hot melt extrusion process involves melting a blend of the drug and excipients, followed by forcing the molten mixture through an orifice to create a uniform material in various forms such as granules, tablets, or films. This technique is widely utilized in transdermal drug delivery systems. [2]
Steps in Hot Melt Extrusion Method:
- Mixing the drug with solid carriers.
- Melting the mixture using a heated extruder.
- Shaping the molten mixture into films using dies.
Advantages:
- Solvents or water are not required in the process.
- Fewer steps, leading to greater efficiency.
- Compressibility properties of the API are less critical.
- Suitable for poorly soluble drugs.
- Provides uniform dispersion due to intense mixing and agitation.
- Consumes less energy compared to high-shear methods.
Disadvantages:
- Potential thermal degradation due to high temperatures.
- The polymer must have suitable flow properties for processing.
- Limited selection of available polymers.
- Excipients must be free of water or volatile solvents.
3. Direct Milling Method
This solvent-free technique involves mixing the drug and excipients directly, either through milling or kneading. The resulting mixture is rolled out on a release liner until the desired thickness is achieved. This method is preferred due to the absence of residual solvents and associated health risks. [2]
Evaluation Parameter Of Buccal Film
- Observation of Physical Characteristics
The oral films were assessed for properties such as homogeneity, color, transparency, flexibility, brittleness, and surface texture through visual inspection. [2]
- Film Weight
The weight of individual buccal films was determined using a calibrated balance. The average weight of the films was calculated to ensure consistency in the manufacturing process. [4]
- Thickness Measurement
A calibrated micrometer screw gauge was used to measure the thickness of the buccal films at five different locations. The average value was calculated to ensure uniform thickness, which is critical for accurate dosing and reproducibility of the formulation process. [8]
- Surface pH Analysis
To measure surface pH, the films were hydrated in contact with 1 ml of distilled water for 2 hours at room temperature. The pH was recorded after equilibrating the electrode with the film's surface for 1 minute. [9]
- Folding Endurance
Folding endurance was assessed by repeatedly folding the film at the same point until it broke. The number of folds the film withstood before breaking was recorded as the folding endurance value. [5]
- Tensile Strength
The tensile strength, which reflects the load required to deform or rupture the film, was evaluated by securing film strips of specific dimensions between clamps at a set distance. Tensile strength was calculated using the formula:
Tensile Strength (N/mm2)=Breaking Force (N)/CrossSectional Area (mm2) [4]
- Percentage Moisture Loss
The films were weighed, placed in a desiccator with anhydrous calcium chloride for 72 hours, and reweighed. The percentage moisture loss was calculated using the formula:
%Moisture Loss=(Initial Weight?Final Weight)×100/Initial Weight [4]
- In Vitro Disintegration Time
The disintegration time was determined by placing the films in a petri dish containing 2 ml of distilled water. The time taken for the films to disintegrate was recorded. [10]
- Drug Content Uniformity
The films were dissolved in 100 ml of pH 6.8 buffer and appropriately diluted. The drug content was analyzed spectrophotometrically at a wavelength of 242 nm, and the average content was calculated. [5]
- In Vitro Dissolution Study
Dissolution studies were performed using a USP Type II (Basket Type) apparatus with 50 ml of pH 6.8 buffer as the dissolution medium, maintained at 37°C and stirred at 50 rpm. At specific intervals, 1 ml samples were collected and replaced with fresh medium. The drug content was determined spectrophotometrically at the active ingredient's ?max. [6]
- Swelling Index
The films' swelling behavior was evaluated by weighing the initial film (W0?), allowing it to swell on a petri dish in an incubator at 37°C, and reweighing (Wt?) at specified intervals.[5] The swelling percentage (%S) was calculated as:
%S= (Wt?W0)×100/W0
- Dissolution Kinetics
It is done by determining the best fit mathematical model for formulations. R and k values for different mathematical models are determined putting the dissolution data in respective mathematical models. The model for which the R value is the highest that model is considered as the best fit model for the concerned formulation. The n value for the best fit model is recorded and it is used to determine the fickian or non-fickian diffusion pattern the formulation follows. [5]
-
- Zero-order kinetic
Qt = Qo + k0t
Where, Qt is amount of drug release at time t K0 is zero order release rate constant.
Q0 is amount of drug present initially at t = 0
-
- First-order kinetic:
ln (100 – Q) = lnQ0 – k1t Where,
Q = amount of drug release at time t Q0 = amount of drug present initially K1 = first order release rate constant
-
- Higuchi equation: Q = kH t1/2 Where,
Q = amount of drug release at time t KH = Higuchi dissolution
- Ex-vivo diffusion study
For in vitro release study, goat buccal mucosa membrane is used as a barrier membrane with Phosphate buffer (pH 6.8) as a medium. Drug release from film is evaluated by Franz diffusion cell. Buccal mucosa membrane is mounted between the donor and receptors compartments. The film is placed on the mucosal membrane. The diffusion cell is placed in simulated saliva maintained at 37±2°C.The receptor compartment is filled with 50 mL phosphate buffer (pH 6.8) and hydrodynamics is maintained by stirring with a magnetic bead at 50 rpm. 1 mL sample is withdrawn and replaced with 1 mL fresh medium to maintain the sink condition. The samples are analyzed by U.V. spectrophotometer at specific wavelength. [9]
- Stability Studies
Stability was evaluated according to ICH guidelines under different storage conditions: 40°C/75% RH for 6 months and 30°C/75% RH for 24–36 months. Films were packaged in materials like aluminum foil and assessed for properties such as DSC, FTIR, folding endurance, disintegration time, drug content, and in vitro drug release.[25]
Future Aspects Of Buccal Film
Muccoadhesive buccal films can be incorporated potent drug which fulfilled criteria for buccal film as drug delivery system. [1,5,8,10]
- We can evaluate the dissolution of buccal film for drug release profile studies.
- We can examine in-vivo studies for the prepared buccal film.
- We can perform the stability study for buccal film.
CONCLUSION
The review concluded that buccal films are among the most acceptable and palatable dosage forms available. This formulation presents a promising avenue for continued research, particularly for the systematic delivery of drugs that are inefficient when taken orally. By bypassing first-pass metabolism, buccal films enhance the bioavailability of active pharmaceutical ingredients. Their unique characteristics make them superior to other innovative buccal drug delivery systems.Buccal films are particularly advantageous for geriatric and pediatric patients, as well as individuals with difficulty swallowing. This innovative dosage form offers a cost-effective, non-irritating solution for drug delivery within the oral cavity. Additionally, it provides a non-invasive alternative for delivering potent peptides and protein-based drugs. With strong mucoadhesive properties, buccal films enable rapid onset of action, improving the safety, efficacy, and stability of active ingredients. The development of oral thin film technology also supports brand extension and serves as a tool for product lifecycle management by extending the patent life of existing drugs. This novel technology optimizes therapeutic efficacy and is extensively studied for its potential applications. Buccal films thus offer significant advantages over traditional dosage forms, and there remains a broad scope for future research in this area
REFERENCES
- Muthadi Radhika Reddy, An Introduction to Fast Dissolving Oral Thin Film Drug Delivery Systems: A Review. J. Pharm. Sci. & Res, 2020; 12(7): 925- 940.
- Dnyaneshwar H. R, Wale K.K., Sayyed S.F., Dr. Chaudhari S.R, Orodispersible Film Dosage Form: A Review. World Journal Of Pharmaceutical Research, 2014; Volume 39(5): 1093-1111.
- Dhobale Avinash V, Nikose Karishma2 , Mrunal Pharate.R3, Mahendra Datir4, Recent Advances In Mucoadhesive Buccal Drug Delivery System And Its Marketed Scope And Opportunities. International Journal Of Advanced Pharmaceutical Sciences, 2018; 1(08): Nishi Thakur, Mayank Bansal, Neha Sharma, Ghanshyam Yadav and Pragati Khare, Overview “A Novel Approach of Fast Dissolving Films and Their Patients”. Advances in Biological Research, 2013; 7 (2): 50-58.
- Bibhu Prasad Panda, Development of Innovative Orally Fast Disintegrating Film Dosage Forms: A Review. International Journal of Pharmaceutical Sciences and Nanotechnology, 2012; 5(2): 1666-1674.
- Vishakha Dhananjay Jagtap, Buccal Film - A Review on Novel Drug Delivery System. International Journal of Research and Review, 2020; 7(6): 17-28.
- Shruthi B K , Dr V. Chandrakala, S. Srinivasan, Role of Superdisintegrants in Rapid Dissolving Oral Films. Int. J. Pharm. Sci. Rev. Res., 2022; 75(2): 110- 116.
- Yogyata S. Pathare*, Vishakha S. Hastak, Amruta N. Bajaj, Polymers used for Fast Disintegrating Oral Films: A Review. Int. J. Pharm. Sci. Rev. Res., 2013; 21(1):169-178.
- V. M. Vaidya, Dr. n. s. Bhajipale, S.P. Deshmukh, A.G. Sinhe, BUCCAL BIOADHESIVE DRUG DELIVERY- A PROMISING OPTION FOR CONVENTIONAL THERAPY. European Journal of Biomedical and Pharmaceutical Sciences, 2019; 6(12):138-147.
- Patel, J.; Patel, K.R.; Patel, N.M.Review on fast dissolving film.Int. J.Univers. Pharm. Bio. Sci., 2013, 2(1): 149-162.
- CENTER FOR DRUG EVALUATION AND RESEARCH. CMC review.http://www.accessdata.fda.gov/drugsatfda_docs/nda/2010/0 22524Orig1s000ChemR.pdf. (Accessed March 4, 2013).
- F. Cilurzo, I. E. Cupone, P. Minghetti, F. Selmin, L. Montanari, Fast dissolving films made of maltodextrins, Eur. J. Pharm. Biopharm. 70 ;(2008) 895–900.
- P. Lakshmi, J. Sreekanth, A. Sridharan, Formulation development of fast releasing oral thin films of levocetrizinedihydrochloride with Eudragit® Epo and optimization through Taguchi orthogonal experimental design, Asian J Pharm ; 2011;5 (2): 84-92.
- Khanna R., Agrawal S.P. and Ahuja A., et al “Mucoadhesive Buccal drug delivery a potential alternative to conventional therapy.” Indian Journal of pharmaceutical sciences: 1998; 60(1): 1-11.
- Gawas S.M., Dev A, Rathod S., et al, “current approaches in buccal drug delivery system” Pharmaceutical and Biological evaluations,: 2016;(3): 165- 177.
- Shinde P, Salunkhe V, Magdum C.Buccal film:an innovative dosage form designed to improve patient compliance. Int. J of Pharmaceutical and Chemical science, 2012; 1(4):1262-1278.
- Mundhe B, Kadam V, Jadhv S. A short review on fast dissolving oral film.Wprld J of Pharmacy and pharmaceutical sciences. 2014;3(3):463-475.
- Bhyan B, Jangra S. Formulation and Evaluation of fast dissolving sublingual film of Rizatriptan Benzoate. Int J of Drug Development and Research. (2012); 4(1):133-144.
- Anjudip Yadav, Vandana Sharma, Shailendra Tripathi, Shankar Lal Soni, Oral Fast Dissolving Film: A Novel Formulation, Asian Journal of Pharmaceutical Research and Development. 2020; 8(4): 77-82.
- Muthadi Radhika Reddy, An Introduction to Fast Dissolving Oral Thin Film Drug Delivery Systems: A Review, Journal of pharmaceutical Science and Research. 2020; 12(7): 925-940.
- Arya A, Chandra A, Fast Dissolving Oral Films: An Innovative Drug Delivery System and Dosage Form, International Journal of Chemtech Research,2010;(2): 576-583.
- Indian Pharmacopoeia 2010, vol- ,Published By The Indian Pharmacopoeia Commission, Central Indian Pharmacopoeia Laboratory, Govt. of India, Ministry of Health & Family Welfare, Sector-23, Raj Nagar, Ghaziabad, 2010, 1815-1820.
- M. G. Ahmed, R. N. Charyulu, N. M. Harish, P. Prabhu, Formulation and In- vitro evaluation of Chitosan films containing tetracycline for the treatment of periodontitis, Asian J Pharm, 3 (2009); 113-119.
- Kennedy, S.W. Tributyl citrate, in Rowe, R.C.; Sheskey, P.J.; Owen (Eds.), Handbook of Pharmaceutical Excipients, Pharmaceutical press, London, 2006, pp. 792-793.
- Shridhar I, Joshi A. Formulation and characterization of buccal patch of Ondanserton hydrochloride. Int. J of Pharmaceutical Research and development. 2013:5(8):84-94.
- Radha MB, Murthy VS. Buccal film drug delivery system-an innovative and emerging Technology. Molecular Pharmaceutics and Organic Process Research. 2013; 1(3):1-6.
- Kulkarni A.S., H.A. Deokule, M.S. Mane and D.M. Ghadge (2010). ‘Exploration of different polymers for use in the formulation of oral fast disintegrating strips. J Current Pharmaceutical Res 2(1): 33-35.
- “A Novel Approach in Oral Fast Disintegrating Drug Delivery Systems and their patents”. Adv Biol Res 5(6): 291-303.
- Priyanka N, Chauhan I, Yasir M, Insights into Polymers: Film Formers in Mouth Dissolving Films, Drug Invention Today, 2011; (1):280-289.
- Mahajan A. Formulation and Evaluation, 2012. Fast dissolving Buccal films of Sertraline. International Journal of Drug Development Research, 4(1): 220- 226.
- Dhobale, A. V. Recent Advances in Mucoadhesive Buccal Drug Delivery System and Its Marketed Scope and Opportunities; LSDP College of Pharmacy Mandavgan Pharata, 2018. [Online] Available: https://www.researchgate.net/publication/327111123.
- Jani, R.; Patel, D. Hot Melt Extrusion: An Industrially Feasible Approach for Casting Orodispersible Film. Asian J. Pharm. Sci. 2015, 10 (4), 292–305. https://doi.org/10.1016/j.ajps.2015.03.002.
- Development and Evaluation of Buccal Film Containing Antihypertensive Agent. Pharma Innov. J. 2015, 4 (1), 53–60. Available online: www.thepharmajournal.com
- Siddiqui, N.; Garg, G.; Sharma, P. K. et al; A short review on “a novel approach in oral fast dissolving drug delivery system and their patents.” Adv. Biol. Res. 2011, 5 (6), 291–303.
- Sudhakar, Y.; Kuotsu, K.; Bandyopadhyay, A. K. et al; Buccal bioadhesive drug delivery—a promising option for orally less efficient drugs. J. Control. Release 2006, 114 (1), 15–40.
- Thakur, R.; Narwal, S.; Narang, A. et al; Formulation and in vitro characterization of ondansetron HCl-loaded buccal patches. Drug Dev. Ind. Pharm. 2018, 44 (8), 1262–1269.
- Lakshmi, P. K.; Rajesh, A. P. et al; Buccal patches for ondansetron hydrochloride using gellan gum and sodium alginate: Formulation and in vitro evaluation. Int. J. PharmTech Res. 2012, 4 (3), 1321– 1330.
- Patel, M.; Patel, N. et al; Design and development of ondansetron HCl buccal films: in vitro and in vivo evaluation. J. Pharm. Sci. Res. 2015, 7 (8), 514–518.
- Madhav, N. S.; Kulkarni, V. S. et al; Development and evaluation of natural polymer-based buccal films of ondansetron HCl. Int. J. Pharm. Bio Sci. 2014, 5 (2), 321–326.
- Ahuja, A.; Khar, R. K.; Ali, J. et al; Mucoadhesive drug delivery systems. Drug Dev. Ind. Pharm. 1997, 23 (5), 489–515.
- Tejashri, M.; Sharma, B. et al; Buccal films for enhanced drug delivery: formulation and characterization. Indian J. Pharm. Biol. Res. 2014, 3 (2), 23–31.
- Palem, C. R.; Gannu, R.; Yamsani, V. V. et al; Development of bilayered mucoadhesive buccal films for buccal drug delivery of ondansetron hydrochloride. Int. J. Pharm. Sci. Nanotechnol. 2011, 4 (3), 1461–1469.
- Shohreh Alipour1, Sahar Akbari1, Fatemeh Ahmadi2, et al; Development and in vitro evaluation of fast-dissolving oral films of ondansetron hydrochlorid, Trends in Pharmaceutical Sciences 2015; 1(1): 25-30
- Verma, N.; Wahi, A. K.; Verma, A.; Chattopadhayay, P. Evaluation of a Mucoadhesive Buccal Patch for Delivery of Atenolol: In Vitro Screening of Bioadhesion. J. Pure Appl. Microbiol. 2007, 1, 115–118.
- Rachna Kumria, Vishant Gupta1, Sanjay Bansal2, Jyoti Wadhwa3, Anroop B Nair.et al; Oral buccoadhesive films of ondansetron: Development and evaluation, International Journal of Pharmaceutical Investigation | April 2013 | Vol 3 | Issue 2


 Chandrika Khanolkar*
Chandrika Khanolkar*
 Tushar GRukari
Tushar GRukari

 10.5281/zenodo.14249511
10.5281/zenodo.14249511