Abstract
A serious consequence linked to group A streptococci invasive infections is streptococcal toxic shock syndrome. Notwithstanding advancements in medical treatment over the past few decades, septic shock continues to be linked to a high death rate. In order to provide patients with streptococcal toxic shock syndrome with prompt diagnosis of the infectious source or sources, surgical therapy, and appropriate and intense support of failing organs, early detection and multidisciplinary management are essential. More research is needed to fully understand the epidemiology and risk factors of streptococcal toxic shock syndrome, including the potential causal involvement of nonsteroidal anti-inflammatory medication exposure. The authors of this review article examine what is now known about streptococcal toxic shock syndrome, go over its pathogenesis, and as well as it’s supportive and target treatment. A severe side effect of group A Streptococcus infection that has a high fatality rate is streptococcal toxic shock syndrome. Treating these patients requires early intensive care assistance, rapid identification, and surgical management.
Keywords
Streptococcal toxic shock syndrome, multiple organ failure, group A streptococcus, septic shock, superantigens
Introduction
A severe consequence of a streptococcus infection, streptococcal toxic shock syndrome (STSS) typically develops quickly and leads to multi-organ failure, Even in healthy people, its death rate is high, and Lancefield group A Streptococcus infection is commonly linked to it [1]. High fever, hypotension, generalized erythematous rash, and numerous organ dysfunction are among the clinical signs, which can quickly escalate to severe and uncontrollable shock. Although the exact cause of this condition is unknown, it is thought to be a mix of enterotoxins with superantigen activity and the host's reaction to streptococcal infection [2]. Although severe invasive GAS infections have long been recognized, it wasn't until the early 1990s that GAS infections linked to shock and multiple organ failure were first documented, after the 1978 discovery of staphylococcal toxic shock syndrome [3]. A "toxic shock-like syndrome" was documented by Steven et al.in 1989 in patients with scarlet fever linked to GAS. Similar to the staphylococcal toxic shock syndrome, the name streptococcal toxic shock syndrome was also created. There have also been reports of nongroup A streptococci causing a comparable but different entity of STSS [4].
Fig-1
The precise mechanism of STSS is not fully understood, but it involves a complex interaction between pathogen virulence and host immunity, as well as the effects of other streptococcal enzymes and toxins, streptococcal toxins, and enterotoxins with superantigen activity [5].
Epidemiology of Streptococcal Toxic Shock Syndrome:
Streptococcus group A (S. pyogenes) is a gram -positive aerobic bacteria that is distinguished by its beta-hemolytic activity, which is the complete hemolysis of blood agar culture plates. Exotoxins with superantigen activity are not always released by GAS strains that possess [6]. Patients with invasive illness have strains of GAS that secrete the pyrogenic exotoxin (superantigen) A, B, or both, and primarily contain types 1 and 3 M protein. In asymptomatic GAS colonization, the skin and nasopharyngeal mucosa are the main sites. It is thought that GAS enter deeper tissues and the bloodstream via rupturing an epithelial barrier, but they can also pass through intact membranes. Although it has been documented in both adults and children worldwide, streptococcal toxic shock syndrome is still a very uncommon illness. Clusters and outbreaks of STSS have been documented in closed settings, including hospitals, nursing homes, and even families, but isolated cases are the norm [7]. It has also been reported that family members can transmit GAS that causes STSS. According to prospective population-based surveillance data, the annual incidence of invasive GAS infections was approximately 3 cases per 100,000 people in Europe and Australia. STSS develops in 13–15% of patients who arrive with invasive GAS infections, with a mortality incidence of 23–44% [8]. According to CDC data, there were 309 instances of STSS infection, an incidence of 0.2 cases per 100,000 inhabitants/year, with a case fatality rate of 36%.
Even when invasive, GAS infections are known to have a low attributable death rate, unless they are invasive and fit the criteria for toxic shock syndrome. The primary factors influencing the fatality rate from streptococcal toxic shock syndrome include the patient's medical history, the infection site, comorbidities, age extremes, and the delay in diagnosis. Additionally, the morbidity associated with STSS can be very significant, especially when extensive surgical debridement is required in cases of necrotizing fasciitis. Organ failure in shocked patients can result in some permanent complications, such as renal and respiratory insufficiency [9].
Current and Historical Perspectives on the Prevalence and Severity of Streptococcal Infections:
Recently, the British tabloids have referred to invasive necrotizing illnesses brought on by GAS as "flesh-eating bacteria" and have predicted the imminence of streptococcal infection epidemics. Despite being false, this kind of exaggeration has helped raise public awareness of this rare but dangerous viral disease. The precise definition of an epidemic is a rise in disease prevalence over the baseline endemic rate. We are indeed facing a major invasive GAS infection outbreak in this environment, but there aren't enough hard, prospective population-based data to back this up. Ten to twenty instances per 100,000 people is the estimated incidence of these illnesses. Therefore, rather than the prevalence of the disease prompting such public interest, the dramatic nature of these infections.
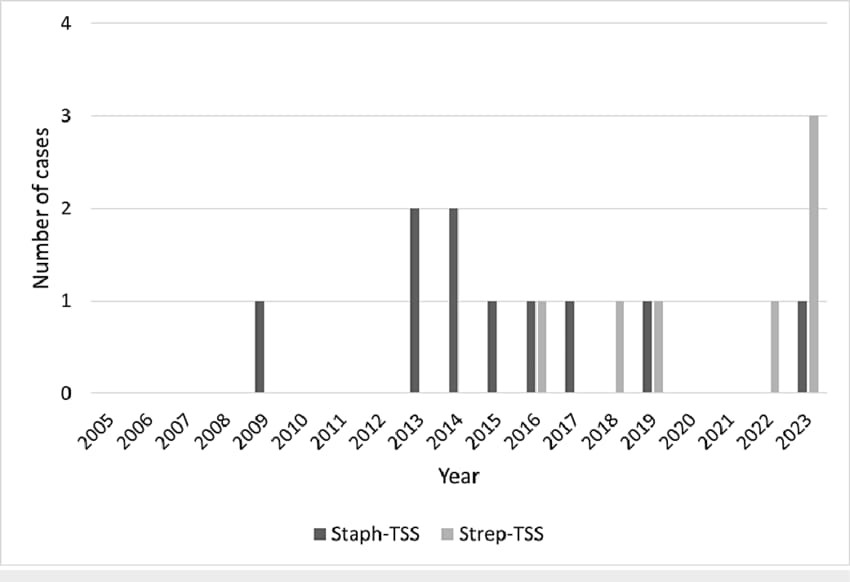
It is unknown if the prevalence of these group A streptococcal infections will rise, fall, or remain the same. There are numerous historical accounts of GAS infection epidemics and associated nonsuppurative aftereffects. Scarlet fever epidemics began to spread from Italy and Spain to Northern Europe in the 1600s, and in 1736, an outbreak in the American colonies claimed 4,000 lives (10). During World War II, there were significant rheumatic fever outbreaks in the American military. Subsequently, post-streptococcal glomerulonephritis occurred in a number of US locales (11). Before the development of antibiotics, many of these outbreaks fluctuated, indicating that either shifts in socioeconomic circumstances or differences in the pathogen's expression of virulence factors were to blame. The remarkable mortality rate of scarlet fever that was recorded in the late 1880s in New York, Chicago, and Norway—between 25% and 30% of children who contracted the disease died at that time—is the best example of this idea (12). In all three places, the mortality rate had decreased to less than 2% by 1900. The decline in mortality rates must have been brought about by either the sluggish uptake of antibiotics or the decreased expression of a streptococcal virulence factor, given socioeconomic conditions probably did not change significantly during that time.
Pathophysiology:
Colonization or infection with bacteria
Production of toxins
Toxins absorbed systemically
Production of cell mediator chemicals (cytokines,
Interleukin [IL1] and tumour necrosis factor (TNF)
Capable of mediating shock and tissue injury and
systemic manifestations of TSS
Materials and Methods:
For the past 20 years, records of all significant hemolytic pyogenic streptococci infections (invasive, bacteremia, or toxic) that have been found in the 150 000-person Harrogate district of North Yorkshire have been preserved. The laboratory and clinical records made at the time, as well as the hospital case notes, were examined for patients who produced pyogenic streptococci and had characteristics meeting the recognized clinical criteria for streptococcal TSS. The review includes one instance from the Northallerton district and two recent additional examples from the York area. Standard laboratory techniques, such as disk diffusion antibiotic susceptibility testing in the Yorkshire laboratories and Lancefield grouping by latex agglutination techniques, were used to identify clinical isolates of streptococci [13]. One of us examined them for T-antigen serotype and M- protein and for genes that produce streptococcal pyrogenic exotoxins A–C the Central Public Health Laboratory, Colindale's Respiratory and Systemic Infection Laboratory [14].
Diagnosis:
- No specific test can diagnose TSS.
- History, difficult to diagnose until characteristic symptoms evolves & source of infection is identified.
- Physical examination.
- Blood culture.
- Culture or throat secretion, vaginal culture.
- Blood test.
- RFT (raised urea & creatinine).
- LFT (decreased liver function).
Fig-2
Treatment:
Antibiotics:
- Penicillin and clindamycin are the first-line antibiotics for STSS.
- Antibiotics should be started as soon as possible and reassessed within 24-48 hours.
- Sometimes it consider as rifampicin or vancomycin for severe cases.
Surgical interventions:
- Debridement of infected tissue.
- Amputation.
- Drainage of abscesses.
Immediate supportive care:
- Fluid resuscitation.
- Vasopressors (e.g., dopamine, norepinephrine) for blood pressure support.
- Oxygen therapy.
- Mechanical ventilation.
Adjunctive therapies:
- Intravenous immunoglobulins (IVIG) to neutralize toxins.
- Steroids (controversial, use with caution).
- Activated protein C (controversial, use with caution).
Other treatments include:
- Renal replacement therapy for renal failure.
- Intubation and ventilatory support.
- Liver functions.
- Coagulation factors.
Duration of treatment:
Typically 7-14 days, depending on severity and response.
Mortality rate:
30-60%, despite aggressive treatment.
Risk Factors:
Most toxic shock syndrome instances are linked to the usage of tampons that are extremely absorbent. Those who use tampons during their periods and are of childbearing age are more likely to get it. Nevertheless, non-menstruating individuals can also experience toxic shock syndrome. TSS can occur in surgical patients as a result of wound infections. Birth control methods like diaphragms or contraceptive sponges, uterine surgery, and childbirth are additional risk factors for those who were assigned female at birth [15].
1. Using tampons, especially if you use menstrual cups or super-absorbent tampons, or if you leave them in for longer than is advised.
2. use barrier contraceptives, like a sponge, cup, or diaphragm.
3. a skin condition, like a cut, burn, boil, insect bite, or surgical wound.
4. recently undergoing surgery, experiencing a miscarriage, or giving birth.
5. treatment of a nosebleed with nasal packing.
6. Having an impetigo, cellulitis, or throat infection caused by a streptococcal or staphylococcal infection.
Symptoms and Causes:
Symptoms:
The following are some potential toxic shock syndrome symptoms:
- A sudden high fever.
- Low blood pressure.
- Vomiting or diarrhea.
- Confusion
- Muscle ache.
- A rash resembling a sunburn, particularly on your palms and soles.
- Seizures.
- Redness of your eyes, mouth and throat.
- Headaches.
Causes:
The most frequent cause of toxic shock syndrome is Staphylococcus aureus, also known as staph. Group A streptococcus (strep) bacteria can also cause the condition [16,17].
Prevention:
Chemoprophylaxis
It has been reported that those who have close contact with the index patient can develop secondary invasive GAS illness. In Australia, the incidence rate of contracting an invasive GAS infection is 2011 times greater than the general population (95% CI: 413–5929), according to Carapetis et al. [18]. Uncertainty surrounds the effectiveness of antibiotic prophylaxis in reducing the incidence of secondary invasive GAS infections. In addition to advising contacts to seek medical attention right once when exhibiting symptoms consistent with a GAS infection, the CDC advises prophylaxis for contacts who have risk factors for the illness [19].
Vaccination
GAS causes a wide range of clinical symptoms as well as a high rate of morbidity and mortality. A safe vaccine that covers many strains of GAS and does not cause autoimmune disease would highly desirable. Such a vaccination has not yet been created despite numerous attempts [20].
DISCUSSION:
A severe and quickly developing illness, streptococcal toxic shock syndrome (STSS) is usually brought on by an invasive infection and is caused by the Streptococcus pyogenes bacteria. It is characterized by a fast onset of fever, hypotension, organ failure, and extensive tissue destruction, frequently with soft tissue necrosis and a characteristic rash. Despite prompt treatment with supportive care and antibiotics, STSS's high death rate and quick development provide a clinical challenge. Superantigen release is the main cause of the underlying pathogenicity, which results in a severe immune response that causes shock, cytokine storm, and systemic inflammation. Although the precise pathways are still being investigated, contemporary research aims to comprehend how host factors and bacterial virulence factors contribute to the development of disease.
Future Perspectives:
The advancement of research into therapy and prevention strategies is critical to the management and understanding of streptococcal toxic shock syndrome (STSS) in the future. Certain Streptococcus pyogenes strains can cause STSS, a serious and potentially fatal illness, by releasing strong toxins that cause shock, organ failure, and widespread systemic inflammation. Despite intensive supportive care and antibiotic therapy, STSS death rates are still high today, highlighting the need for novel therapeutic approaches and preventative measures. The development of quick diagnostic instruments for the early identification of STSS and the organisms that cause it may also be essential to bettering results. Faster intervention would be possible with earlier detection of high-risk cases, which could lessen symptom intensity and increase survival rates. Long-term changes in the treatment and management of STSS may result from these developments in early diagnosis, as well as the creation of toxin-neutralizing medications and prophylactic vaccinations. In general, a multidisciplinary strategy integrating clinical care, microbiology, and immunology is necessary to lessen the future burden of STSS.
CONCLUSION:
Streptococcal toxic shock syndrome, is still a dangerous and sometimes fatal illness with high rates of morbidity and death. Notwithstanding improvements in healthcare, medical professionals still face difficulties in treating STSS because of its quick progression brought on by the release of streptococcal toxins. While strong antibiotic medication and supportive care are two examples of current therapeutic approaches that have improved outcomes, they are not always enough to prevent death. Future improvements in treatment and prevention may be possible to research into targeted therapeutics, such as vaccinations and toxin-neutralizing drugs. In order to guarantee early discovery and timely treatment, the development of quick diagnostic technologies will also be essential. To lessen the burden of STSS and increase patient survival rates, a comprehensive strategy combining prevention, early intervention, and cutting-edge therapies is essential.
REFERENCES
- A case report of streptococcal toxic shock syndrome caused by Streptococcus mitis in a healthy adult. Chen X, Gong YY, Zhang L, et al. BMC Infect Dis. 2021;21:154.doi: 10.1186/s12879-021-05852-y.
- Ferretti JJ, Stevens DL, et al. Fischetti VA. Oklahoma City: University of Oklahoma Health Sciences Center; 2016. Streptococcus pyogenes: basic biology to clinical manifestations.
- Weaver GH, et al. The vitality of bacteria from the throats of scarlet fever patients, with special study of streptococci. J Med Res. 1903;9(3):246–256.
- Sims KD, et al. Barton TD. Group B streptococcal toxic shock syndrome in an asplenic patient: case report and literature review. Eur J Clin Microbiol Infect Dis. 2006;25(3):208–210. doi: 10.1007/s10096-006-0106-2.
- Proft T, Fraser JD. Streptococcal superantigens: biological properties and potential role in disease. In: Ferretti JJ, Stevens DL, Fischetti VA et.al. editors. Streptococcus pyogenes: basic biology to clinical manifestations.
- Streptococcal Toxic Shock Syndrome (STSS) (Streptococcus pyogenes) 2010 Case Defnition.https://wwwn.cdc.gov/nndss/conditions/streptococcal-toxic-shock-syndrome/case-defnition/2010/. Accessed 4 Apr 2018.
- Thigpen MC, Thomas DM, Gloss D, Park SY, Khan AJ, Fogelman VL, et al. Nursing home outbreak of invasive group a streptococcal infections caused by 2 distinct strains. Infect Control Hosp Epidemiol. 2007;28(1):68–74.
- O’Loughlin RE, Roberson A, Cieslak PR, Lynfeld R, Gershman K, Craig A, et al. The epidemiology of invasive group A streptococcal infection and potential vaccine implications: United States, 2000–2004. Clin Infect Dis.
- Factor SH, Levine OS, Schwartz B, Harrison LH, Farley MM, Mc Geer A, et al. Invasive group A streptococcal disease: risk factors for adults. Emerg Infect Dis. 2003;9(8):970–7.
- The Working Group on Severe Streptococcal Infections. Defining the group A streptococcal toxic shock syndrome: rationale and consensus definition. JAMA1993; 269:390-1.
- Dillon HC, et al. Impetigo contagious: suppurative and non- suppurative complication Clinical, bacteriologic and epidemiologic characteristics of impetigo. Am J Dis Child 1968; 115:530-41.
- Wannamaker LW, Rammelkamp CH, Jr., Denny FW, Brink WR, Houser HB, Hahn EO, et al. Prophylaxis of acute rheumatic fever by treatment of the preceding streptococcal infection with various amounts of depot penicillin. Am J Med 1951; 10:673-95.
- Colman G Streptococcus and lactobacillus Parker MT, Duerden BI (Eds.), et al. Topley and Wilson's principles of bacteriology, virology and immunity, 8th edition, 2, Edward Arnold, London (1990), pp. 119-159.
- Nunthapisud P, et al. Sirilertpanrana S, Reinprayoon S, Tanna A Detection of the erythrogenic toxin A, B and C genes in group A streptococci isolated from clinical specimens Adv Exp Med Biol, 418 (1997), pp. 729-731.
- Lin YC, et al. Peterson ML. New insights into the prevention of staphylococcal infections andsyndrome(https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3102526/). Expert Rev Clin Pharmacol. 2010;3(6):753-767. Accessed 8/17/2022.
- Cohen J, et al. Dermatologic manifestations of systemic infections. In: Infectious Diseases. 4th ed. Elsevier; 2017.
- Kellerman RD, et al. Toxic shock syndrome. In: Conn's Current Therapy 2020. Elsevier; 2020. https://www.clinicalkey.com. Accessed Feb. 6, 2020.
- Carapetis JR, et al. Jacoby P, Carville K, Ang S-JJ, Curtis N, Andrews R. Effective-ness of clindamycin and intravenous immunoglobulin, and risk of disease in contacts, in invasive group a streptococcal infections. Clin Infect Dis. 2014;59(3):358–65.
- Robinson KA, et al. Rothrock G, Phan Q, Sayler B, Stefonek K, Van Beneden C, et al. Risk for severe group A Streptococcal disease among patients’ household contacts. Emerg Infect Dis. 2003;9(4):443–7.
- Excler J-L, Kim JH.et.al. Accelerating the development of a group A Streptococcus vaccine: an urgent public health need. Clin Exp Vaccine Res. 2016;5(2):101–7.


 Rudraraju Greeshma *
Rudraraju Greeshma *
 Dr. C. Bhuvaneswara Rao
Dr. C. Bhuvaneswara Rao



 10.5281/zenodo.14247092
10.5281/zenodo.14247092