Abstract
Microemulsions represent a distinct category of dispersions that can appear either transparent or translucent. Their initial discovery was made by Hoar and Schulman in 1943 during their experimental investigations involving the titration of long-chain fatty acids with medium- and short-chain alcohols, resulting in the formation of translucent or transparent emulsions. These microemulsions are characterized as clear, stable, isotropic mixtures composed of oil, water, and surfactants, often accompanied by a cosurfactant. They exhibit optical isotropy and thermodynamic stability, functioning as liquid solutions of oil, water, and amphiphiles. Research has demonstrated that microemulsions can effectively protect sensitive drugs, regulate drug release, enhance drug solubility, improve bioavailability, and minimize variability among patients. Additionally, formulations have been developed that are suitable for various routes of administration. Since their inception, oral formulations of microemulsions have been recognized for their ability to enhance the bioavailability of poorly soluble drugs. The formation of microemulsions occurs through the simple mixing of their components, eliminating the need for the high shear conditions typically required for conventional emulsions. Moreover, the droplet size within these microemulsions remains consistent, ranging from 10 to 100 nanometers (100-1000 Å), and features a very low oil/water interfacial tension. Due to the droplet size being less than 25% of the wavelength of visible light, microemulsions appear transparent.
Keywords
Microemulsion, Dispersion, Bioavailability, Cosurfactant.
Introduction
A microemulsion is a dispersion made up of water, oil, and surfactant(s) that creates a thermodynamically stable and anisotropic system. The size of the dispersed domains usually falls between about 1 and 100 nm, with a typical range of 10 to 50 nm. [1] Microemulsions are transparent, thermodynamically stable, isotropic liquid mixtures composed of oil, water, and surfactant. The size of the micelles in microemulsions typically ranges from 5 to 100 nm, influenced by various factors such as the type and concentration of surfactant, as well as the degree of dispersion. Consequently, the term "microemulsion" can be somewhat misleading, as it does not accurately represent the size of the co-surfactant. The aqueous phase may contain salts and/or additional components, whereas the "oil" phase can comprise a diverse mixture of hydrocarbons and olefins. In some cases, the addition of a co-surfactant, which serves as a fourth component, is crucial. The ratios of these components lead to variations in the microstructure of microemulsions, which can manifest as small water droplets suspended within an oil phase (water-in-oil microemulsion) or as oil droplets dispersed in a water phase (oil-in-water microemulsion). The microstructure transitions seamlessly between these two extremes, evolving from spherical to cylindrical, tubular, and interconnected phases of oil and water, all separated by a thin layer of surfactant molecules, referred to as a discontinuous microemulsion. Each microemulsion type is characterized by its thermodynamic stability and clarity. There are significant distinctions between emulsions and microemulsions regarding their structure and stability. Unlike microemulsions, emulsions are inherently unstable systems that will separate into distinct phases without agitation. Additionally, droplet sizes in emulsions generally range within the micrometer scale, while in microemulsions, micelle sizes vary from 5 to 100 nanometers, influenced by factors such as the type and concentration of surfactant and the degree of dispersion.[2]
Definition
A micro-emulsion is defined as a thermodynamically stable liquid solution that consists of water, oil, and an amphiphilic agent, characterized by its single optically isotropic nature. [3]
History
The concept of microemulsions was firstly invented by Hoar and Schulman. These systems are characterized as transparent solutions that result from the titration of a conventional coarse emulsion with medium-chain alcohols. Microemulsions represent thermodynamically stable isotropic systems where two immiscible liquids, specifically water and oil, are combined into a single phase through the use of suitable surfactants and co-surfactants. Typically, short to medium-chain alcohols are regarded as co-surfactants within the microemulsion framework. The application of surfactants and co-surfactants effectively reduces interfacial tension, facilitating the spontaneous formation of microemulsions, which possess an average droplet size ranging from 10 to 140 nanometers. [4]
Objectives
- To develop and enhance water-in-oil microemulsions through the strategic combination of surfactants along with organic and aqueous phases, facilitating the characterization of the resultant microemulsions along two dilution lines within the monophasic region of ternary phase diagrams.
- To integrate a model hydrophilic guest molecule, specifically sodium chloride, into the aqueous domains of oil-continuous microemulsions.
- To evaluate the efficacy of chosen salt-containing microemulsion formulations for salt release by employing conductivity measurements and to elucidate the underlying release mechanism.[5]
Theories of Micro Emulsion Formation
- The formation and stability of microemulsions can be explained through three primary theoretical frameworks.
- These frameworks include interfacial or mixed film theories, solubilization theories, and thermodynamic treatments.
- The free energy associated with microemulsion formation is influenced by the degree to which surfactants reduce the surface tension at the oil-water interface, as well as the entropy changes within the system.
Thermodynamic Theory
The equation representing this relationship is
Gf = ?A - TS,
Where Gf denotes the free energy of formation, A represents the change in interfacial area, S indicates the change in entropy, T is the temperature, any ? is the surface tension at the interface of oil-water.
- During microemulsion formation, the change in interfacial area (A) is substantial due to the generation of numerous tiny droplets.
- For a transient microemulsion to form, a negative free energy value is necessary; although A remains positive, its magnitude is minimal and is counterbalanced by the entropic component.
- The significant entropic contribution arises from the high dispersion entropy resulting from the mixing of one phase into another, characterized by the formation of many small droplets.
- Additional favourable entropic contributions are anticipated from dynamic processes, including surfactant diffusion within the interfacial layer and the exchange of surfactant monomers and micelles.
- A negative free energy of formation is achieved when substantial reductions in surface tension coincide with considerable favourable changes in entropy.
- In such scenarios, the microemulsion forms spontaneously, leading to a dispersion that is thermodynamically stable.[6]
Solubilization Theory
The theory of solubilization posits that the creation of a microemulsion involves the interaction between an oil-soluble phase and a water phase, facilitated by micelles or reverse micelles. As these micelles gradually increase in size and swell, they reach a specific range that contributes to the formation of the microemulsion. [7]
The Interface Mixed-Film Theory:
The mixed-film theory of interfacial tension, specifically the negative interfacial tension theory, posits that micro-emulsions can instantaneously and spontaneously generate a negative interfacial tension when surfactants and co-surfactants interact. This film, made up of surfactant and co-surfactant molecules, is considered a liquid "two-dimensional" third phase that maintains equilibrium with both oil and water.This monolayer may function as a duplex film, exhibiting distinct properties on the water side compared to the oil side. According to this theory [ duplex film theory] , the interfacial tension ?T can be expressed by the following equation. ?T = ?(O/W) --- ? Where, ? (O/W) a = Interfacial Tension (reduced by the presence of the alcohol).
? (O/W) a is lower than that ?(O/W) in the absence of the alcohol
Types of Micro Emulsions
Microemulsions are characterized by their thermodynamic stability, which is achieved under specific conditions. Winsor's classification identifies four distinct types of microemulsion phases that exist in equilibrium, commonly known as Winsor phases.
• The first type is oil painting- in- water microemulsion, as Winsor I.
• The alternate type is the water- in- oil painting microemulsion, appertained to as Winsor II.
• The third type is the bi continuous microemulsion, known as Winsor III.
• The fourth type is the single- phase homogeneous admixture, linked as Winsor IV.
Each type of microemulsion plays a unique role in various applications due to its specific properties.
- The stability of microemulsions can be influenced by many factors such as temperature, concentration, and the presence of surfactants.
- Understanding these phases is crucial for optimizing formulations in fields like pharmaceuticals and cosmetics.
- The classification of microemulsions aids researchers in predicting behaviour and interactions in complex systems.
1.Oil-in-water microemulsions, also referred to as Winsor I, consist of oil droplets that are enveloped by a surfactant film, which may include a co-surfactant. This configuration allows the oil to be dispersed within a continuous aqueous phase. Typically, oil-in-water microemulsions exhibit a greater interaction volume compared to their water-in-oil counterparts.
2. Water-in-oil microemulsions, known as Winsor II, are characterized by water droplets that are surrounded by a continuous oil phase. These systems are often described as "reverse micelles," where the polar headgroups of the surfactant orient towards the water droplets, while the hydrophobic tails extend into the oil phase. When administered orally or parenterally, these water-in-oil microemulsions may face destabilization due to the presence of aqueous biological environments.
3. Bi-continuous microemulsions, or Winsor III, are distinguished by the comparable amounts of water and oil, resulting in both components forming a continuous phase. This unique structure resembles a "sponge phase," where irregular channels of oil and water are intermingled. Transitions between oil-in-water and water-in-oil microemulsions can occur through this bi-continuous state. Such microemulsions may exhibit non-Newtonian flow characteristics and plasticity, making them particularly advantageous for drug delivery applications, whether topically or intravenously.
4.In the case of single-phase homogeneous mixtures, or Winsor IV, the oil, water, and surfactants are uniformly blended together. [8,9,10]
ADVANTAGES
- Microemulsions are prepared easily and it require no energy contribution during the preparation this is due to better thermodynamic stability.
- The formation of microemulsion is reversible.
- They may become unstable at low or high temperature but when the temperature returns to the stability range, the microemulsion reforms.
- Microemulsions are thermodynamically stable systems and allows it self-emulsification of the system.
- Microemulsions have low viscosity compared to the emulsions.
- Microemulsions acts as the super solvents for the drugs, that can solubilize both hydrophilic and lipophilic drugs including the drugs that are insoluble in both aqueous and hydrophobic solvents.
- Having the ability to carry both lipophilic and hydrophilic drugs.
- The dispersed phase, lipophilic or hydrophilic (O/W, or W/O microemulsions) can act as a potential [11,12,13 ,14].
DISADVANTAGES:
- Microemulsion systems exhibit a restricted ability to solubilize substances with high melting points.
- A significant quantity of surfactants is necessary to maintain the stability of the droplets.
- The stability of microemulsions is affected by environmental factors, including temperature and pH levels. [11,12]
Formulation
Microemulsions are colloidal mixtures that consist of an oil phase, a water phase, surfactants, and co-surfactants in appropriate ratios.
Surfactants
Surfactants play a crucial role in stabilizing the microemulsion system. In the formulation of microemulsions, surfactants are employed to reduce interfacial tension, thereby facilitating the dispersion process and forming a microemulsion around the droplets. These surfactants can be classified as non-ionic, zwitterionic, cationic, or anionic. [15]
Co-surfactants
Co-surfactants are chemicals introduced to enhance the efficacy of surfactants. They are utilized to augment the oil-solubilizing capacity of the microemulsion surfactant system. Typically, co-surfactants consist of short to medium-chain alcohols (C3-C8), which help to lower interfacial tension and improve the fluidity of the interface. Common examples include alcohols, amines, and cholesterol. [16-21]
Oils
The oil component plays a crucial role in influencing the curvature of the microemulsion due to its capacity to infiltrate the tail group region of surfactants. In contrast to long-chain alkenes, short-chain oils are more likely to enhance negative curvature and reduce the hydrophilic-lipophilic balance (HLB) [22]. A variety of oil types are employed in the formulation of microemulsions, including.:
• Saturated fatty acids such as lauric acid, myristic acid, capric acid.
• Unsaturated fatty acids such as oleic acid, linoleic acid, linolenic acid.
• Fatty acid esters: ethyl or methyl esters of lauric acid, myristic acid, oleic acid.
The primary criterion for selecting an oil is its high solubility for the drug, while also aiming to minimize any adverse effects.
The formulation volume is critical. The oil must provide the therapeutic dosage of the drug in an encapsulated format. The microemulsion formulation should exhibit low allergenic potential, favorable physiological compatibility, and high biocompatibility.
The essential components of microemulsion formulations typically include:
(a) an oil phase,
(b) An aqueous phase that includes hydrophilic active ingredients, with the potential inclusion of preservatives and buffers.
(c) a primary surfactant [which can be anionic, non-ionic, or amphoteric], and
(d) secondary surfactants or co-surfactants.
The benefits of microemulsions compared to other dosage forms are as follows:
• Enhanced absorption rates
• Elimination of variability in the absorption process
• Solubilization of lipophilic drugs
• Provision of aqueous dosage forms for water-insoluble drugs
• Increased bioavailability
• Versatile delivery routes that including topical route, oral route, and intravenous route.
• Rapid and effective penetration of the drug moiety.
• Assistance in taste masking
• Improved patient compliance with liquid dosage forms
• Minimal energy requirements.
Preparation of Micro Emulsions
The preparation of micro-emulsions can be achieved through various methods, notably the phase titration method and the phase inversion method.These techniques are essential for formulating stable micro-emulsions, which are thermodynamically stable mixtures of oil, water, and surfactants.
Phase Titration Method
A pseudo-ternary phase diagram is utilized to identify distinct zones within the system. This diagram serves as a valuable tool for visualizing the relationships between the components involved in the micro-emulsion formulation. Each corner of the pseudo-ternary phase diagram represents a 100% concentration of a specific component—typically one of the three primary ingredients: oil, water, or surfactant. By systematically varying the ratios of these components, researchers can pinpoint the micro-emulsion zone, which is characterized by the formation of stable micro-emulsions. The resulting micro-emulsions can be categorized as either water-in-oil (w/o) or oil-in-water (o/w) based on their composition. In a w/o micro-emulsion, water droplets are dispersed within a continuous oil phase, while in an o/w micro-emulsion, oil droplets are dispersed within a continuous water phase. This classification is crucial for determining the application and behavior of the micro-emulsion in various contexts, such as pharmaceuticals, cosmetics, and food products. Constructing phase diagrams is crucial for understanding the intricate interactions among different components in the micro-emulsion system. These diagrams not only illustrate the stability regions for micro-emulsions but also provide insights into the conditions under which different structures can form. Micro-emulsions can exhibit a variety of structures, including emulsions, micelles, and gels, all of which are influenced by the chemical composition and concentration of the ingredients involved. For instance, the type and concentration of surfactants can significantly affect the size and stability of the droplets, while the presence of co-surfactants can enhance the flexibility of the micro-emulsion system. By analyzing the phase behavior of the components, researchers can optimize formulations to achieve desired properties, such as improved solubilization of active ingredients, enhanced bioavailability, or tailored release profiles. Overall, a thorough understanding of phase diagrams and the factors influencing micro-emulsion formation is essential for the successful development of these versatile systems. [23,24]
Phase Inversion Method
- The phase inversion of microemulsions is induced by either the introduction of an excess of the dispersed phase or variations in temperature. Significant physical transformations occur during this process, including alterations in particle size, which can influence drug release in both in vivo and in vitro settings.
- The methods employed involve modifying the spontaneous curvature of the surfactant; for non-ionic surfactants, this is accomplished by adjusting the system's temperature, leading to a transition from an oil-in-water microemulsion at lower temperatures to a water-in-oil microemulsion at elevated temperatures.
- The generation of finely dispersed oil droplets is achieved through a technique known as the phase inversion temperature (PIT) method. This approach can also take into account other factors, such as salt concentration and pH levels, rather than relying A modification in the spontaneous radius of curvature can be induced by varying the volume fraction of water.
- By gradually introducing water into the oil, water droplets are initially created within a continuous oil phase.
- As the water volume fraction increases, the spontaneous curvature of the surfactant transitions from stabilizing a water-in-oil (w/o) microemulsion to an oil-in-water (o/w) microemulsion at the point of inversion. Short-chain surfactants at the o/w interface form a flexible monolayer, leading to a discontinuous microemulsion at this inversion point.
Characterization of Micro Emulsion
The characterization of microemulsions involves assessing various parameters, including droplet size, viscosity, density, turbidity, refractive index, phase separation, and pH levels.
- To determine the droplet size distribution, techniques such as light scattering or electron microscopy are employed, which are recognized as effective methods for evaluating the stability of microemulsions.
- Dynamic Light Scattering (DLS) measurements are conducted at a 90° angle using a dynamic light-scattering spectrophotometer equipped with a 632 nm neon laser, while polydispersity is analyzed using an Abbe refractometer. [25]
- Microemulsion characterization employs various analytical techniques to reveal their phase type, viscosity, structure, and dynamics.
- Phase Type Identification: Determining whether a microemulsion is oil-in-water (o/w) or water-in-oil (w/o) is crucial for understanding its properties. This is achieved through electrical conductivity measurements using a conductometer. O/w microemulsions, with water as the continuous phase, exhibit higher conductivity due to ionic species, while w/o microemulsions show lower conductivity due to reduced ion mobility in oil. Analyzing conductivity values allows accurate classification of the microemulsion phase type.
- Viscosity Assessment: Viscosity significantly affects microemulsion flow behavior and stability. A Brookfield rotary viscometer measures viscosity under controlled conditions at various shear rates, relevant for applications in pharmaceuticals, cosmetics, and food. Measurements are conducted at a constant temperature of 37 ± 0.2°C to reflect physiological conditions [26]
- Structural and Dynamic Investigation: Advanced Nuclear Magnetic Resonance (NMR) techniques are used to explore the structure and dynamics of microemulsions, providing insights into molecular interactions. Self-diffusion measurements, a specific NMR application, further enhance understanding. [27,28]
Drug Release Studies:
- In vitro drug release studies were conducted utilizing a Franz diffusion cell equipped with cellophane paper. The recipient compartment, which was water-jacketed, had a total volume capacity of 25 ml and featured two arms: one designated for sampling and the other for a thermometer. The donor compartment had an internal diameter of 2 cm and was positioned to ensure contact with the diffusion medium in the receptor compartment. The receptor compartment was filled with phosphate buffer saline (PBS), maintained at a temperature of 37°C ± 1°C. Prior to the application of the micro-emulsion, equivalent to 10 mg of drug, the membrane was equilibrated. Periodic samples were withdrawn from the receptor compartment, with each withdrawal being replaced by an equal volume of fresh PBS solution, and the samples were analyzed using a spectrophotometer at a wavelength of 254 nm. For the physical stability study, selected formulations were subjected to centrifugation at 3000 rpm for 30 minutes. Formulations that exhibited no phase separation were then subjected to a heating and cooling cycle, specifically a freeze-thaw cycle. This involved six cycles between temperatures of 4°C (in a refrigerator) and 45°C (in a hot air oven), with each temperature maintained for a minimum of 48 hours. Formulations that remained stable under these conditions were chosen for further investigation. The optimized micro-emulsion formulation was stored at 4°C, room temperature, and 45°C for a duration of three months, with samples evaluated for physicochemical parameters such as globule size and drug content at one-month intervals.
- Phase Behaviour:
Phase behaviour: Lavender essential oil (LO) is extensively utilized as a bioactive ingredient in cosmetic formulations. This research involved the construction of pseudo ternary phase diagrams for microemulsions consisting of an oil phase (LO: short-chain alcohol in a 1:1 weight ratio), a non-ionic surfactant (Tween 80), and water, aimed at assessing the influence of co-surfactant type on the dilution capacity of microemulsion systems. The solubilization of LO was enhanced by the inclusion of 1,3-butylene glycol. Consequently, the microstructural inversion of a water titration line D82 was examined through dye diffusion, conductivity, viscosity, and differential scanning calorimetry (DSC). The transition of microemulsions from water-in-oil (W/O) to a bi-continuous phase occurred at a water content of 20%, followed by a shift to an oil-in-water (O/W) structure at 50% water content. In the bi-continuous phase, viscosity decreased significantly with an increase in temperature. This structural transition influenced the free radical scavenging activity. The DPPH radical scavenging activity exhibited a continuous increase with water content, ranging from 10% to 90%, suggesting that a higher free water content may enhance the interaction between LO and DPPH radicals. The ABTS radical scavenging activity for both W/O and bi-continuous formulations was concentration-dependent. [29]
Identification Tests
Dilution test: The continuous phase will remain intact and will not undergo splitting or fracturing when incorporated into microemulsions. It will maintain its stability even when water is introduced to the oil-in-water type of microemulsions.
Test for Strains:
Water-soluble dyes such as methylene blue or amaranth are incorporated, resulting in the formation of an oil-and-surfactant microemulsion. When observed under a microscope, a droplet of the microemulsion reveals a background that appears either blue or red, while the globule itself appears colorless.[30]
Conductivity:
Measurements of conductivity are essential for assessing whether the micro-emulsion system is oil-continuous or water-continuous. The solubilization of the water phase within the chosen oily mixture was quantitatively evaluated through electrical conductivity measurements. The conductivity of the optimized micro-emulsion (B-9), as measured by the conductivity meter, was determined to be 0.283 ?. The findings from the electroconductivity study indicate that the system is of the oil-in-water (o/w) type.
Zeta Potential:
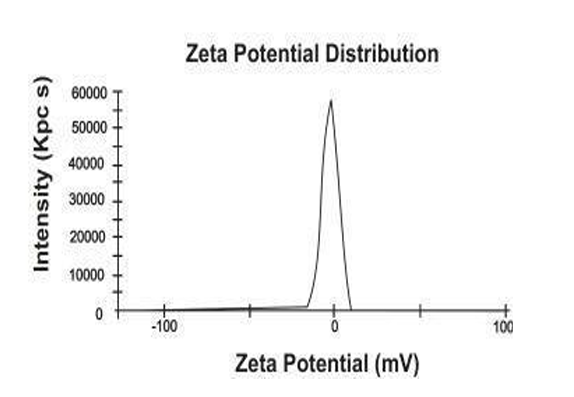
The zeta potential measurements for the optimized micro-emulsion and its diluted variant (diluted 100 times with 0.1N HCl) are illustrated in Figure , revealing values of -6.34 mV and -3.02 mV, respectively. The slightly negative charge of the droplets suggests that aggregation is unlikely to occur. pH: The pH of a 10% aqueous solution of the base was assessed. For the SA ME systems, the measurement was conducted by directly immersing the electrode of the pH meter (Hanna-213, Portugal) into the system. The pH values of the optimized formulation were determined by inserting the electrode directly into the dispersion using a calibrated pH meter (Digital Potentiometer Model EQ-601 Equip-tonics).
Applications of Microemulsion
1) Pharmaceutical Applications
In recent decades, microemulsions have emerged as a promising drug delivery system, attributed to their thermodynamic stability, optical clarity, and ability to facilitate penetration. [31]
2) Oral Delivery
The creation of effective oral delivery systems has consistently posed challenges for researchers, as the efficacy of drugs can be limited by instability or inadequate solubility in gastrointestinal fluids. Microemulsions possess the capability to enhance the solubilization of poorly soluble drugs, particularly those classified as BCS class II or class IV, thereby addressing issues related to dissolution and bioavailability. The presence of polar, non-polar, and interfacial domains allows for the encapsulation of hydrophilic drugs, including macromolecules, with varying solubility. These systems provide protection for the incorporated drugs against oxidation and enzymatic degradation while improving membrane permeability. At present, the microemulsion formulations that are available for commercial use comprise Sandimmune Neoral (Cyclosporine A), Fortovase (Saquinavir), and Norvir(Ritonavir). Microemulsion formulations hold significant potential for enhancing the oral bioavailability of poorly water-soluble drugs by improving their solubility in gastrointestinal fluids.
3)Topical Delivery
Topical administration of medications offers several advantages compared to alternative methods. One significant benefit is the circumvention of hepatic first-pass metabolism, as well as the prevention of salivary degradation and potential toxicity associated with gastric processing. Additionally, this method allows for direct delivery of the drug to the specific areas of the skin or eyes that require treatment.
4) Ophthalmic Delivery
In traditional ophthalmic dosage forms, water-soluble medications are administered in aqueous solutions, whereas water-insoluble drugs are prepared as suspensions or ointments. These systems face significant challenges, including low corneal bioavailability and insufficient efficacy in the posterior segment of ocular tissues.
5) Nasal Delivery Recently
Recent research has focused on microemulsions as a potential delivery system to improve drug absorption through the nasal mucosa. The use of mucoadhesive polymers contributes to an extended residence time on the mucosal surface. Lianly et al. examined the impact of diazepam in the emergency management of status epilepticus. Their findings indicated that the nasal absorption of diazepam occurs relatively quickly at a dosage of 2 mg/kg-1, with peak plasma concentration achieved within 2 to 3 minutes.[32]
6)Parenteral Delivery
The development of parenteral dosage forms for both lipophilic and hydrophilic drugs present significant challenges. The formulation of water-in-oil (w/o) microemulsions offers advantages for the parenteral administration of poorly soluble drugs, eliminating the need for suspensions. Frequent drug administration necessitates high concentrations, and these microemulsions exhibit greater physical stability in plasma compared to liposomes and other delivery vehicles, with the internal oil phase demonstrating enhanced resistance to drug leaching. Numerous poorly soluble drugs have been successfully formulated into oil-in-water (o/w) microemulsions for parenteral use. [33]
CONCLUSION
Microemulsion serve as an efficient and user-friendly medium for the administration of pharmaceuticals, capable of improving drug absorption while minimizing systemic side effects. They offer the potential to refine drug targeting without a corresponding rise in systemic absorption. The careful selection of suitable excipients and a thorough safety assessment, particularly concerning cosurfactants, are essential in the formulation of microemulsions. Furthermore, they hold promise as drug delivery systems that can facilitate the simultaneous administration of multiple medications. This review articles explains the main aspects regarding to the micro emulsions.
REFERENCES
- Prince B, Leon M. Micro emulsions in Theory and Practice, Academic Press, New York, 1197.
- Henri L, Clausse, Marc, Marcel Dekker. Micro emulsion Systems, 1987, 6.
- Sagar R. Wagh, Mayur B. Patil, Aparna S. Musale, Harshal D. Mahajan, Rajendra D. Wagh . A Review on Microemulsion for Drug Delivery System, Vol 9, Issue 7, 2023.
- Hoar TP, Schulman JH. Transparent Water in oil dispersions: the oleopathic hydro micelle. Nature 1943; 152:102-103, Vol 9, Issue 7, 2023.
- Sujatha B, Himabindu E, Bttu S, Abbulu K. Microemulsions-A review. Journal of Pharmaceutical Sciences and Research. 2020 Jun 1;12(6):750-3.
- Amit A. Kale and Vandana B. Patra vale. Development and Evaluation of Lorazepam, Microemulsions for Parenteral Delivery. AAPS Pharm SciTech 2008; 9: 966-971.
- Vandana Patel et al. Development of Microemulsion for Solubility Enhancement of Clopidogrel. Iranian Journal of Pharmaceutical Research 2010; 9(4): 327-334.
- Tenjarla S. Micro emulsions: an overview and pharmaceutical applications. Crit Rev Ther Drug Carrier Syst. 16(5), 1999, 461-521.
- Lawrence MJ, Rees GD. Micro emulsion-based media as novel drug delivery systems. Adv Drug Deliv Rev. 45(1), 2000, 89-121.
- Vandamme TF. Micro emulsions as ocular drug delivery systems: recent developments and future challenges. Prog Retina Eye Res. 21(1), 2002, 15-34.
- Kumar. K. Senthil et al. Microemulsions as Carrier for Novel Drug Delivery: A Review. International Journal of Pharmaceutical Sciences Review and Research, 2011; 10: 37-45.
- Patel R. Mrunali. Microemulsions: As Novel Drug Delivery Vehicle, 2007; 5. 8. Madhav. S and Gupta. D. A review on microemulsion based system. International Journal of Pharmaceutical Sciences and Research, 2011; 2(8): 1888.
- Ghosh, P.K. and Murthy R.S.R. Microemulsions: A Potential Drug Delivery System. Current Drug Delivery, 2006; 3: 167-180.
- Chandra A. and Sharma P.K. Microemulsions: An Overview. Pharmainfonet, 2008; 6(2).
- Bhargava HN, Narurkar A, Lieb LM. “Using microemulsions for drug delivery”, PharmaTech, 11, 1987.
- Kreuter J. “Microemulsions; In: Colloidal drug delivery systems”, Marcel Dekker, New York, 1994, 31-71.
- Lawrence MJ . “Surfactant systems: microemulsions and vesicles as vehicles for drug delivery”, European Journal of Drug Metabolism and Pharmacokinetics, 3, 1994, 257-269.
- Tenjarla S. “Microemulsions: an overview and pharmaceutical applications”, Critical Reviews in Therapeutic Drug Carrier Systems, 16, 1999, 461- 521. 10. Lawrence MJ, Rees GD, “Microemulsion based media as novel drug delivery systems” Advanced Drug Delivery Reviews, 47, 2000, 89121.
- Aboofazeli R, Lawrence CB, Wicks SR, Lawrence MJ. “Investigations into the formation and characterization of phospholipid microemulsions III. Pseudo-ternary phase diagrams of systems containing water-lecithin-isopropyl myristate and either an alkanoic acid, amine, alkanediol, poly ethylene glycol alkyl ether or alcohol as co-surfactant”, International Journal of Pharmaceutics, 111, 1994, 63-72.
- Stilbs P, Lindman B, Rapacki K .“Effect of alcohol cosurfactant length on microemulsion structure”, Journal of Colloid Interface Science, 95, 1983, 583-585.
- Ghosh PK, Murthy RS. “Microemulsions: A potential drug delivery system”, Current Drug Delivery, 3(2), 2006, 167-180.
- Martin, A., Coarse Dispersions in Physical Pharmacy, Fourth Edition; B.I. Waverly Pvt. Ltd., New Delhi, 1994; 495 – 496. 16.
- Shafiq un Nabi S, Shakeel F, Talegaonkar S; Formulation development and optimization using nanoemulsion technique: A technical note; AAPS Pharm Sci Tech., 8, 2007, 1-6
- Jha Sajal Kumar, Dey Sanjay, Karki Roopa, Micro emulsions Potential Carrier for Improved Drug Delivery, International Pharmaceutical Science., 1(2), 2011, 25-31.
- Regev, O., Ezrahi, S., Aserin, A., Garti, N., Wachtel, E., Kaler, E.W., et al., A study of the microstructure of a four-component nonionic microemulsion by cryo-TEM, NMR, SAXS and SANS, Langmuir, 1996;12: 668–674. 17.
- Bellare, J.R., Haridas, M.M., Li, X.J; Characterization of microemulsions using Fast Freeze – Fracture and Cryo-Electron Microscopy in Handbook of Microemulsion, Science and Technology; Ed: Kumar, P., Mittal, K.L.; Marcel Dekker, Inc., New York, 1999; 411-523.
- Shinoda, K., Araki M., Sadaghiani, A., Khan, A., Lindman, B.; Lecithin-based microemulsions: phase behaviour and microstructure, J. Phys. Chem., 1991; 95: 989–993. 18.
- Corswant, C.V., Engstrom, S., Soderman, O.; Microemulsions based on soybean phosphatidylcholine and triglycerides. Phase behaviour and microstructure, Langmuir, 1997; 13: 5061–5070. 19
- Bhavani, Jeeva , Karthick,Raghul, Pravin Kumar, Vijay A Review on Formulation and Evaluation of Microemulsion (1)Professor ,Psv College Of Pharmaceutical Science And Research Krishnagiri , india
- Nilesh.S.Shinde, Sandesh.R.Nikam, Pritam.S.Deore A Brief Review On Method Preparation And Importance Of Microemulsion In Drug Delivery System.
- Sagar R. Wagh, Mayur B. Patil, Aparna S. Musale, et al., A Review on Microemulsion for Drug Delivery System
- Syamasri Gupta, S.P. Moulik, Biocompatible microemulsions and their prospective uses in drug delivery. Journal of Pharmaceutical Sciences. 2008; 97:22-45.
- Corswant C, Thoren P, Engstrom S, Triglyceride-based microemulsion for intravenous administration of sparingly soluble substances. J. pharm. Sci. 1998; 200-208.


 Nagaveni Pommala*
Nagaveni Pommala*
 Mounika Gandham
Mounika Gandham

 10.5281/zenodo.14670480
10.5281/zenodo.14670480