Abstract
The emergence of innovative drug delivery systems has transformed pharmacology, facilitating the precise and efficient administration of therapeutics to designated locations within the body. This extensive review offers a thorough analysis of the latest developments in drug delivery technologies, with a particular emphasis on nanoparticle systems, liposomes, hydrogels, and materials responsive to stimuli. Traditional drug delivery techniques frequently encounter challenges such as limited bioavailability, systemic toxicity, and insufficient targeting capabilities. The objective of novel drug delivery systems is to address these issues by enabling controlled release, targeted administration, and improved therapeutic effectiveness. The role of nanoparticle systems in cancer treatment and management of infectious diseases. The application of liposomes in gene therapy and vaccine formulation. The use of hydrogels for prolonged release and applications in tissue engineering. The potential of stimulus-responsive materials for accurate targeting. Cancer treatment: focused chemotherapy and immunotherapy strategies. Infectious disease management: vaccine administration and antimicrobial treatments. Neurological conditions: targeted delivery to the brain and neurodegenerative approaches. Personalized medicine: customized drug delivery solutions .Combination therapies: enhancing efficacy through synergistic approaches. Scalability and translation: moving towards industrial production and clinical testing. This review serves as a significant resource for researchers, healthcare professionals, and industry stakeholders aiming to create innovative strategies for enhanced therapeutic results. By emphasizing recent advancements and prospective developments, this detailed overview seeks to expedite the progress of novel drug delivery systems
Keywords
Therapeutic efficacy, Liposomes, Nanoparticle based system, novel drug delivery system..
Introduction
The manner in which a drug is administered can greatly influence its effectiveness. Certain medications possess an optimal concentration range that maximizes their therapeutic benefits, while levels that fall outside this range may lead to toxicity or lack any beneficial effects. Conversely, the slow advancements in treating severe illnesses have highlighted the increasing necessity for a multidisciplinary strategy in delivering therapeutics to specific tissue targets. This has led to the development of innovative concepts aimed at managing pharmacokinetics, pharmacodynamics, non-specific toxicity, immunogenicity, bio recognition, and overall drug efficacy. These novel strategies, commonly referred to as drug delivery systems (DDS), integrate various disciplines, including polymer science, pharmaceutics, bio conjugate chemistry, and molecular biology. To reduce drug degradation and loss, mitigate adverse side effects, and enhance drug bioavailability as well as the accumulation of the drug in targeted areas, numerous drug delivery and targeting systems are currently being researched and developed. The concept of Controlled and Novel Drug Delivery, once merely aspirational, has now become a tangible reality. Over the past fifteen years, pharmaceutical researchers and scientists have engaged in extensive and rigorous studies within this domain of drug research. Among the various drug carriers, one can identify soluble polymers, micro particles composed of either insoluble or biodegradable natural and synthetic polymers, microcapsules, cells, cell ghosts, lipoproteins, liposomes, and micelles. These carriers can be designed to be slowly degradable, responsive to stimuli (such as pH or temperature), and even targeted by linking them to specific antibodies that recognize particular components of the area of interest. Targeting refers to the capability of directing the drug-laden system to the desired site. Numerous drug delivery systems have been developed, with several others currently in the pipeline, all aimed at reducing drug loss, mitigating harmful side effects, enhancing drug bioavailability, and promoting the accumulation of the drug at the desired site of action. A variety of novel carriers have been established and documented as effective for controlled and sustained drug delivery. It is essential to assess the various terminologies employed within the broad categories of novel drug delivery systems. [1] Sustained or controlled drug delivery systems administer drug action at a predetermined rate, ensuring prolonged or constant (zero-order) release at therapeutically effective levels within the circulation. Localized drug delivery devices facilitate drug action through rate-limiting drug release in proximity to the target area. Pre-determined rates of drug delivery enable drug action by altering the release of drug molecules through system design, which regulates the molecular diffusion of these molecules in systemic circulation. Targeted drug delivery achieves drug action by utilizing carriers for either passive or active diffusion, or through a self-programmed approach, often in conjunction with appropriate sensory devices that identify their receptors at the targeted site. Novel drug delivery systems encounter a variety of challenges that impede their development and practical application. A primary concern is the toxicity and biocompatibility of these systems, which must be meticulously designed to ensure safe interactions with the human body. Furthermore, issues related to scalability and manufacturing present significant obstacles, necessitating substantial investment and robust infrastructure. The regulatory landscape and approval processes also complicate matters, as these innovative systems must comply with intricate and rigorous guidelines. The high costs associated with development and the inherent investment risks can be daunting, while the complexity of targeted delivery and release mechanisms demands advanced technical expertise. In addition, concerns regarding stability and shelf-life must be thoroughly addressed to maintain the efficacy of these systems. A limited understanding of biological barriers and transport mechanisms further hinders progress. The increasing demand for personalized medicine and customized therapies introduces an additional layer of complexity. The integration of emerging technologies, such as artificial intelligence and nanotechnology, is vital for the advancement of novel drug delivery systems. Ensuring patient compliance and adherence is also critical for achieving optimal therapeutic outcomes. Despite these challenges, novel drug delivery systems are of great significance in enhancing therapeutic efficacy and outcomes. They provide improved patient compliance and adherence, lower toxicity and side effects, and greater targeting efficiency and specificity. These systems facilitate the development of personalized medicine and tailored therapies, leading to improved quality of life and better disease management. They also hold promise for potential combination therapies and synergistic effects, addressing unmet medical needs and orphan diseases. In particular, these systems have demonstrated potential in cancer treatment through targeted chemotherapy, which minimizes toxicity. Additionally, they offer benefits in the treatment of infectious diseases. [2]
Classfication Of Novel Drug Delivery System:
Advantages Of Novel Drug Delivery System:
1. Drugs are safeguarded against physical and chemical degradation.
2. It facilitates sustained release of medications.
3. The system enhances the distribution of tissue macrophages.
4. There is an improvement in drug stability.
5. The pharmacological activity of drugs is enhanced.
6. It offers protection against various forms of toxicity.
7. Bioavailability is improved.
8. The solubility of drugs is enhanced.[3]
Disadvantages Of Novel Drug Delivery System:
1. Immune responses may arise against carrier systems administered intravenously.
2. The formulation of NDDS drugs necessitates advanced technological capabilities.
3. Skilled personnel are essential for the manufacturing, storage, and administration processes.
4. Maintaining the stability of dosage forms presents significant challenges.
5. The process of drug loading can be time-consuming.
6. There is a potential risk of dose dumping occurring.[4]
Importance Of Novel Drug Delivery System:
1. Improved therapeutic effectiveness: Innovative drug delivery systems enhance the efficacy of medications by ensuring their targeted and sustained release at the desired site.
- Decreased adverse effects: By focusing on targeted delivery, these systems limit the exposure of healthy tissues to the medication, thereby reducing side effects.
- Increased patient adherence: Delivery systems that minimize dosing frequency or streamline administration can enhance patient compliance with treatment protocols.
- Enhanced drug preservation: Certain delivery mechanisms can shield drugs from degradation, thereby improving their stability and extending shelf life.
- Specific drug targeting: These systems are capable of directing drugs precisely to the site of action, thereby elevating their concentration at the target while decreasing systemic exposure.
- Regulated drug release: Advanced delivery systems can facilitate controlled drug release, sustaining therapeutic levels over prolonged periods and lessening the necessity for frequent dosing.
- Improved absorption: Some delivery systems can enhance the bioavailability of poorly soluble medications, thereby increasing their absorption and overall effectiveness.
- Opportunities for combination therapies: Innovative delivery systems can support the simultaneous or sequential administration of multiple drugs, promoting synergistic effects and better treatment outcomes.
- Customized therapies: These systems can be tailored to meet the specific needs of individual patients, enabling personalized medicine strategies.
- Advancement of drug development: Novel drug delivery systems can streamline the creation of new medications by enhancing their delivery and effectiveness, potentially leading to new therapeutic options for various conditions.[5]
Mechanism Of Action:
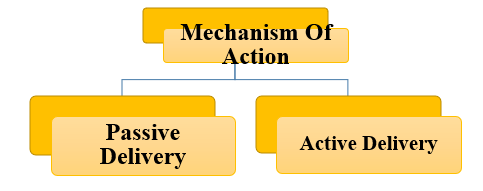
Passive Delivery System:
- Definition: A passive drug delivery system is characterized by its dependence on inherent physiological mechanisms, such as diffusion, osmosis, or dissolution, to facilitate the controlled release of medication.
- Principles:1. Diffusion-controlled release: The movement of drug molecules occurs through a membrane or matrix. 2. Osmosis-controlled release: The influx of water initiates the release of the drug.
3. Dissolution-controlled release: The drug gradually dissolves in bodily fluids.
1. Straightforward design and manufacturing process
2. Economical
3. Compatible with biological systems
4. Predictable release profiles
5. Limited ability for targeted delivery
1. Matrix-based systems (e.g., hydrogels, polymers)
2. Membrane-controlled systems (e.g., diffusion cells)
3. Osmotic systems (e.g., osmotic pumps)
4. Dissolution-controlled systems (e.g., erodible implants)
1. Enhanced bioavailability
2. Decreased toxicity
3. Improved patient adherence
4. Cost-efficient
1. Oral delivery (e.g., tablets, capsules)
2. Transdermal delivery (e.g., patches)
3. Ocular delivery (e.g., eye drops)
4. Implantable devices (e.g., drug-eluting stents)
1. Sustained-release tablets
2. Transdermal patches (e.g., nicotine, fentanyl)
3. Osmotic pumps (e.g., insulin)
4. Hydrogel-based wound dressings [6]
- ACTIVE DELIVERY SYSTEM:
- Definition: An active drug delivery system refers to a specialized mechanism that employs external energy sources or stimuli to regulate the release and targeting of therapeutic agents.
- Principles:
1. Targeted delivery: Drug molecules are directed towards specific locations or cells.
2. Controlled release: The release of the drug is initiated by external stimuli.
3. Enhanced permeability: The absorption of the drug is facilitated through increased permeability.
1. Temperature-responsive systems
2. pH-responsive systems
3. Light-activated systems
4. Ultrasound-triggered systems
5. Electro stimulation-based systems
6. Magnetic field-controlled systems
1. Improved targeting efficiency
2. Enhanced bioavailability
3. Real-time monitoring and control
4. Capabilities for personalized medicine
5. Potential for combination therapies
1. Increased therapeutic effectiveness
2. Decreased systemic toxicity
3. Better patient compliance
4. Enhanced diagnostic capabilities
1. Complex design and manufacturing processes
2. High costs
3. Risk of technical failures
4. Regulatory hurdles
1. Cancer treatment
2. Gene therapy
3. Vaccine administration
4. Neurological conditions
5. Cardiovascular disorders
1. Liposomes containing pH-sensitive lipids
2. Thermo responsive hydrogels
3. Photo-sensitive nanoparticles
4. Ultrasound-activated micro bubbles
5. Electro responsive drug-eluting stents.[7]
Differenece Between Conventinal Drug Delivery System Controlled Drug Delivery System And Sustained Release.
|
Conventional Dosage Forms
|
Sustained Release Dosage Forms
|
Controlled Release Dosage Form
|
|
The drug solubilities in the gastric contents. These dosage forms do not sustain either their dissolution or their absorption
|
These dosage forms maintain the drug release rate over a sustained period.
|
These dosage forms lead to predictable and constant plasma concentration, independently of the biological environment of application site.
|
|
These dosage forms release the drug in single action following first-order kinetics.
|
The dose in these dosage forms is of less significance than the release rate form the therapeutic system.
|
These dosage forms release the drug in pre-determined pattern over a fixed time period following zero-order kinetics.
|
|
These dosage forms are related to all types of dosage forms.
|
These dosage forms are related to oral dosage forms.
|
The dosage forms are related to oral, vaginal and transdermal dosage forms
|
|
The time interval v/s drug plasma concentration profile for these dosage forms is short and show peak.
|
The drug concentration for these dosage forms is maintained for a certain time period and then reduced.
|
The uniform drug concen. For these dosage forms is maintained for pre-determined time period.
|
[8]Novel Drug Delivery System Includes:
- Mucosal drug delivery system:
- Implantable drug delivery system:
- Transdermal drug delivery system:
- Gastro retentive drug delivery system:
- Nasopulmonary drug delivery system:
- Targeted drug delivery system:
- Mucosal drug delivery system:
Bio adhesion can be described as the condition in which two materials, with at least one being of biological origin, are maintained in contact for an extended duration through interfacial forces. In the realm of pharmaceutical sciences, when the adhesive bond occurs with mucus or a mucous membrane, this process is termed mucoadhesion. The efficacy of mucoadhesive polymers has been demonstrated in drug delivery systems for ocular, nasal, vaginal, and buccal applications, resulting in a notably extended residence time for sustained release formulations on these mucosal surfaces. Furthermore, the advancement of oral mucoadhesive delivery systems has consistently garnered significant interest, as systems that can adhere to specific segments of the gastrointestinal (GI) tract present numerous advantages.[9]
- Implantable drug delivery system:
Implantable drug delivery systems are sophisticated medical devices engineered to administer therapeutic agents at a regulated rate directly into the body over an extended duration. These systems facilitate targeted delivery, enhance bioavailability, minimize systemic side effects, improve patient adherence, and support long-term treatment. A variety of implantable systems are available, such as pumps, reservoirs, matrices, and microchips. These systems find applications in managing chronic pain, diabetes, cancer, cardiovascular diseases, and neurological disorders. For example, insulin pumps have significantly transformed diabetes care, while drug-eluting stents have advanced the treatment of cardiovascular conditions. The design of these implantable systems necessitates meticulous attention to factors such as biocompatibility, biostability, sterilization, the implantation process, and patient safety. Innovative materials, including nanomaterials and biomaterials, are being investigated to improve the performance of these systems. Future advancements may include wireless communication and control, personalized medicine, combination therapies, and minimally invasive implantation techniques. Nonetheless, several challenges remain, including concerns regarding biocompatibility and toxicity, complications related to implantation and explantation, risks of infection and inflammation, potential device malfunctions, and the need for regulatory approvals. Despite these obstacles, implantable drug delivery systems possess significant potential to enhance therapeutic outcomes.[10]
- Transdermal drug delivery system:
Transdermal drug delivery systems represent non-invasive techniques for administering medications through the skin, allowing for a controlled release of therapeutic agents into the bloodstream. These systems present numerous benefits, such as extended drug action, minimized side effects, enhanced patient adherence, and targeted delivery. A variety of transdermal systems are available, including patches, gels, creams, ointments, and sprays. These systems operate through mechanisms like diffusion, osmosis, iontophoresis, and electroporation to promote drug delivery. Factors influencing permeability encompass skin thickness, molecular weight of the drug, solubility, and pH levels. Transdermal systems are frequently employed in pain management, hormone replacement therapy, treatment of cardiovascular diseases, neurological disorders, and local anesthesia. Notable examples of transdermal systems include nicotine patches for aiding smoking cessation, fentanyl patches for pain relief, estradiol patches for hormone therapy, nitroglycerin patches for angina treatment, and rivastigmine patches for managing Alzheimer's disease. Future advancements in transdermal systems may involve nanotechnology, enhanced delivery through electroporation, ultrasound-mediated techniques, and applications in personalized medicine. Despite their advantages, transdermal systems encounter challenges such as skin irritation, inconsistent drug absorption, the need for dose adjustments, and issues with adhesion. Continued research is essential to overcome these obstacles and enhance the efficacy of transdermal drug delivery.[11]
- Gastro retentive drug delivery system:
Gastroretentive drug delivery systems (GRDDS) are engineered to maintain the presence of drug formulations in the stomach for prolonged durations, thereby enhancing bioavailability and therapeutic effectiveness. These systems are particularly advantageous for medications that exhibit narrow absorption windows, limited solubility, or instability within the intestinal milieu. GRDDS can be classified into four primary categories: floating systems, mucoadhesive systems, expandable systems, and superporous hydrogel systems.Floating systems, which include hollow microspheres and gas-generating mechanisms, leverage buoyancy to remain suspended in the stomach. Mucoadhesive systems, such as bioadhesive polymers and microspheres, attach to the gastric mucosa. Expandable systems, exemplified by swellable tablets and balloons, increase in size upon interaction with gastric fluids. Superporous hydrogel systems absorb liquids, swelling to retain the drug. GRDDS have demonstrated enhanced therapeutic results for a range of conditions, including gastroesophageal reflux disease (GERD), peptic ulcers, and diabetes. Medications such as proton pump inhibitors, H2 receptor antagonists, and antacids are particularly benefited by GRDDS. Crucial factors that affect the design of GRDDS encompass gastric residence time, drug release kinetics, and gastric acidity. Ongoing research is focused on discovering innovative materials and technologies to further improve the performance of GRDDS.[12]
- Nasopulmonary drug delivery system:
Nasopulmonary drug delivery systems (NPDDS) are designed to direct medications to the lungs and nasal passages, thereby enhancing therapeutic effectiveness while reducing systemic side effects. These systems are particularly beneficial for treating respiratory conditions such as asthma, chronic obstructive pulmonary disease (COPD), and cystic fibrosis. NPDDS can utilize a variety of formulations, including solutions, suspensions, dry powders, and liposomes. Intranasal administration enables swift absorption and provides direct access to the brain, making NPDDS advantageous for central nervous system (CNS) disorders, including Alzheimer's disease, Parkinson's disease, and migraines. Conversely, pulmonary delivery focuses on the lungs, taking advantage of their extensive surface area and thin epithelial barrier to promote efficient drug absorption.To facilitate drug delivery, NPDDS utilize devices such as nebulizers, metered-dose inhalers (MDIs), and dry powder inhalers (DPIs). Key factors that influence the design of NPDDS include particle size, aerosolization techniques, and mucociliary clearance. Recent advancements in NPDDS have significantly enhanced disease management and improved patient outcomes. Current research efforts are concentrated on refining formulations, optimizing device designs, and targeting specific regions within the lungs. [13]
- Targeted drug delivery system:
Targeted drug delivery systems (TDDS) are designed to administer therapeutic agents directly to the affected area of a disease, thereby reducing systemic side effects and improving treatment efficacy. These systems employ a range of strategies, such as passive and active targeting, to achieve accurate drug delivery. Passive targeting is based on characteristics like particle size, utilizing liposomes and nanoparticles to concentrate in diseased tissues, whereas active targeting involves the use of ligands, antibodies, and aptamers that specifically attach to target cells.TDDS have significantly transformed the management of various medical conditions, including cancer, cardiovascular diseases, neurological disorders, and infections. For example, antibody-drug conjugates (ADCs) and immunoliposomes have advanced cancer treatment, while targeted nanocarriers have improved the administration of cardiovascular medications.Crucial elements that affect the design of TDDS include particle size, surface modifications, and the choice of targeting moiety. Ongoing research is focused on discovering innovative materials and technologies to enhance the effectiveness of TDDS.[14]
Recent Advances In Novel Drug Delivery System:
- Liposomes:
- Nanoparticles:
- Niosomes:
- Micelles:
Dendrimers:
- LIPOSOMES:
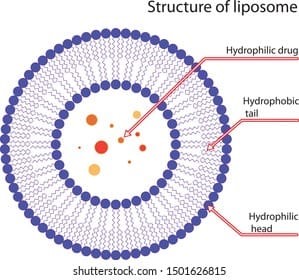
The structure of liposomes is characterized by distinct internal and external zones that exhibit varying affinities for drug molecules, organized in a vesicular configuration. The core of the vesicle demonstrates a greater affinity for hydrophilic drugs, while hydrophobic drugs are situated within the peripheral zone, nestled between the lipid and phospholipid layers. Liposomes can be categorized into several types, including noisomes, phytosomes, ethosomes, and transfersomes. The formation of niosomes occurs when non-ionic surfactants are present in low or negligible concentrations of phospholipids, resulting in enhanced aqueous dispersibility and stability. Transfersomes are recognized as flexible liposomes, exhibiting increased elasticity due to the incorporation of single-chain surfactants that function as edge activators. Ethosomes are produced when ethanol serves as a primary component in liposome preparation. When phospholipids encapsulate active ingredients derived from herbal sources or plants, they are referred to as phytosomes. The vesicular architecture of liposomes has emerged as a compelling option for encapsulating a diverse range of biotherapeutic agents, which possess various physicochemical properties and three-dimensional structures. Their capacity to incorporate multiple peptides and proteins renders liposomes particularly advantageous for vaccine development and cancer therapy delivery. Recently, immunoliposomes have gained traction, as the liposomal surface can be directly linked to antibodies or covalently bonded to polyethylene glycol (PEG) chains in PEGylated liposomes. The inclusion of PEG chains on the liposomal surface represents a novel approach to shield liposomes from the reticuloendothelial system (RES), a topic that will be elaborated upon later. Presently, numerous liposome-based pharmaceutical products are available in the market, and ongoing development continues in the field of liposome formulations.[15]
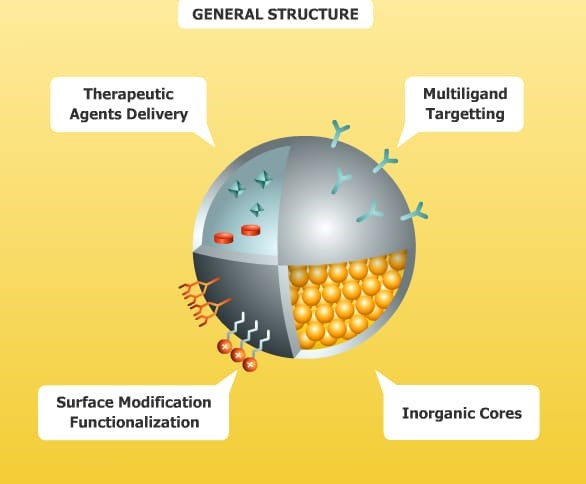
Nanoparticles (NPs) are categorized into various types of nanomaterials, including nanocapsules, nanospheres, nanopores, and nanoshells, with particle sizes ranging from 20 to 250 nm. Polymeric nanoparticles can encapsulate drugs within their internal structure, or alternatively, free drug molecules may be adsorbed onto their surfaces, allowing for an initial burst release shortly after administration. Commonly used polymeric materials, such as polylactic-co-glycolic acid (PLGA) and its copolymers (PLGA-PEG-PLGA or PEG-PLGA-PEG), are favored in NP preparation due to their biocompatibility and biodegradabili ty. Additionally, the surfaces of NPs can be functionalized with specific groups that bind to receptors on cell membranes. This results in SMART NPs, which are designed for active transport to targeted cells, enhancing cellular uptake and selectivity while minimizing adverse effects on surrounding healthy tissues. The development of SMART NPs has progressed through several phases aimed at improving site selectivity, specificity, and cellular uptake. Initially, NPs are transported via passive diffusion without selectivity, such as those containing peptides or anticancer drugs. Subsequently, an active targeting strategy is implemented, involving the binding of targeting ligands to receptors that are overexpressed on the surfaces of targeted cells. A contemporary approach in the design of SMART NPs involves integrating multiple drug release mechanisms to enhance internalization into the target cells. For effective cellular targeting, the initial step involves activating the surfaces of SMART NPs with functionalized groups for cell targeting. This is followed by the formation of a linkage between the targeting ligand and the receptors on the cell surface. Additionally, a second mechanism utilizing pH-responsive polymers for encapsulating anticancer drugs can be applied, where the polymeric matrix begins to disintegrate in the acidic environment of cancerous tissues, facilitating the release of drug molecules.[16]
C .Micelle:
The structure of polymeric micelles consists of a hydrophilic outer shell and a hydrophobic core at the center, which is ideal for encapsulating water-insoluble pharmaceuticals. The fundamental concept behind this delivery mechanism is that the hydrophilic shell conceals the nanosystem, thereby shielding it from immune system attacks. This phenomenon is referred to as the Stealth effect. The Stealth effect allows the nanosystem to traverse blood vessels with reduced immunogenic responses and diminished uptake by macrophages of the reticulo endothelial system (RES). Consequently, this leads to an extended circulation time and improved kinetics. Niosomes are vesicular structures that feature an aqueous core surrounded by a bilayer composed of cholesterol and one or more nonionic surfactants.
Future Scope For Novel Drug Delivery System:
The future of innovative drug delivery systems presents significant opportunities for transforming healthcare. Emerging technologies, including nanotechnology, gene editing, and artificial intelligence, are anticipated to be crucial in improving the efficacy and precision of drug delivery. The rise of personalized medicine, targeted therapies, and combination treatments is expected to gain greater prominence. Advanced materials such as biomaterials, hydrogels, and graphene will support the creation of sophisticated drug delivery platforms. The incorporation of the Internet of Medical Things (IoMT) and wearable technology will facilitate real-time monitoring and customized drug release. Additionally, 3D printing and bioprinting will allow for the development of tailored drug delivery systems. Moreover, progress in stem cell therapy and regenerative medicine will require the implementation of novel drug delivery approaches. Future investigations will aim to address biological barriers, enhance drug solubility, and improve patient adherence. Collaborative efforts among academic institutions, industry stakeholders, and regulatory bodies will foster innovation and accelerate the transition to clinical applications.[18]
REFERENCES
-
-
-
- Reddy PD, Swarnalatha D. Recent advances in Novel Drug Delivery Systems. IJPTR 2010; 2(3): 2025-2027.
- Santini JT, Richards AC, Scheidt R, Cima MJ, Langer R. Microchips as controlled drug delivery devices. Angew Chem Int Ed 2000; 39(23): 2396-2407.
- Reddy PD, Swarnalatha D. Recent advances in Novel Drug Delivery Systems. IJPTR 2010; 2(3): 2025-2027.
- Müller RH. Physicochemical characterization of colloidal drug delivery systems such as reverse micelles, vesicles, liquid crystals and nanoparticles for topical administration. J Pharm Pharmacol 2004; 58(2): 343-356.
- Zhao CX. Multiphase flow microfluidics for the production of single or multiple emulsions for drug delivery. Adv Drug Deliv Rev 2013; 65: 1420-1446.
- Labhasetwar V, Leslie-Pelecky D. Drug Delivery Systems. Boca Raton (FL): CRC Press; 2010
- Bergeson SM. Active Drug Delivery Systems. Philadelphia (PA): Lippincott Williams & Wilkins; 2012.
- Prajapati ST, Manivannam R, Katedeshmukh RG. Novel Drug Delivery System. Lucknow: Thakur Publication Pvt. Ltd; 2022.
- Vinod KR, Reddy R, Banji D, Reddy V, Sandhya S. Critical review on mucoadhesive drug delivery systems. Hygeia J Drugs Med 2012; 6(1): 7-28.
- Anonymous. Implantable Drug Delivery Systems: A Review. J Control Release 2020;.321: 1-15.
- Anonymous. Transdermal Drug Delivery Systems: A Review. J Pharm Sci 2020; 109(5): 1531-1544.
- National Institutes of Health (NIH). Gastro retentive Drug Delivery Systems [Internet]. Bethesda (MD): NIH; [cited 2022)
- Anonymous. Nasopulmonary Drug Delivery Systems: A Review. J Pharm Sci 2020; 109(3): 931-943.
- Anonymous. Targeted Drug Delivery Systems: A Review. J Control Release 2020; 319: 1-14.
- Nekkanti V, Kalepu S. Recent advances in liposomal drug delivery: a review. Pharm Nanotechnol 2015; 3: 35-55.
- Colson YL, Grinstaff MW. Biologically responsive polymeric nanoparticles for drug delivery. Adv Mater 2012; 24: 3878-3886.
- Hunter CA. Vesicular System (Niosomes and Liposomes) for Delivery of Sodium Stibogluconate in Experimental Murine Visceral Leishmaniasis. J Pharm Pharmacol 1988; 40: 161-164.
- Anonymous. Novel Drug Delivery Systems: A Review of Current Status and Future Directions. Biomaterials 2022; 280: 121234


 Prajwal Game *
Prajwal Game *
 Bhagyashri Vikhe
Bhagyashri Vikhe




 10.5281/zenodo.14234513
10.5281/zenodo.14234513