Abstract
A Review on Lidocaine
Keywords
Lidocaine, skin permeability, local analgesic, Topical anaesthesia
Introduction
Clinically, lidocaine, a local anaesthetic of the amide class, is used to block pain perception. [1,2]. Lidocaine possesses antinociceptive, antiarrhythmic, anti-inflammatory, and antithrombotic properties when administered systemically. Lidocaine uses mechanisms that are separate from its main mechanism of action, sodium channel inhibition, to produce these effects in both acute and chronic pain situations as well as acute respiratory distress syndrome.
Structure of lidocaine:
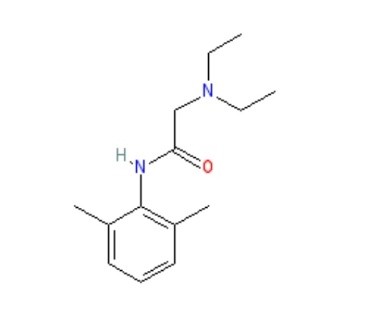
According to the Vaughan-Williams classification, lidocaine is a class Ib antiarrhythmic agent, and its use is indicated in the management of acute ventricular tachyarrhythmia. Lidocaine has long served as the foundation for local anaesthesia, providing efficient pain management for a variety of operations, from minor surgery to dental procedures. With a molecular weight of 234.3 g/mol and log p equal to 2.84, lidocaine is a low soluble and good penetrating medication (Biopharmaceutical Classification System class II), making it a good choice for cutaneous delivery. The most popular, dependable, and efficient amide derivative is this one. Because of its advantageous qualities, including its minimal systemic toxicity, intermediate duration of action, and low risk of allergic responses, lidocaine is prepared as a topical medication. Nils Lofgren initially synthesised lidocaine in 1935 in Professor Hans von Euler’s laboratory in Stockholm. Lofgren then started testing the compounds he and his colleagues had created [3]. When Lofgren tried the 57th compound in 1943, he discovered that it quickly numbed his tongue. According to Goldberg’s (toxicology) and Gordh’s (clinical data) publications, which suggested that lidocaine had a potent and surprising anaesthetic effect, the patent for Xylocaine® was granted in Sweden on May 11, 1948. In November 1948, the Food and Drug Administration authorised the use of Xylocaine® in the United States [4]. Clinical trials conducted by Torsten Gordh showed that lidocaine was a notable advancement above procaine, which at the time was the gold standard for treating surgical pain [5,6]. For the treatment of ventricular arrhythmias, neuropathic pain alleviation, and surgical discomfort, this amide-class anaesthetic is still often employed [7,8]. Along with its anti-arrhythmic and local anaesthetic uses, lidocaine also offers analgesic qualities for several pain problems. The ability of intravenous lidocaine (IVL) to alleviate complicated regional pain syndrome, hyperalgesia, and peripheral neuropathic pain was well acknowledged in the 1980s. Late-onset and insufficient local anaesthetic action are the main issues with using commercial lidocaine preparations. According to reports, commercial creams and gels cannot effectively transport lidocaine base (or its HCl salt) through undamaged skin. To overcome these issues, it is necessary to increase the skin’s permeability to lidocaine given topically. Topical anaesthetics such as lidocaine are frequently utilised for localised pain management during medical and cosmetic treatments. Conventional formulations often suffer from systemic absorption hazards, poor penetration, and short duration of action.
Table 1:Physicochemical Properties:
|
IUPAC Name
|
2-(Diethylamino)-N-(2,6Dimethylphenyl)Acetamide.
|
|
Formula
|
C14H22N2O
|
|
Molecular Weight
|
234.34
|
|
Melting Ranges
|
68-69.0°C.
|
|
Colour, Odour
|
White Odourless Substances.
|
|
Crystal Forms
|
The Base Crystallizes From N-hexane as Fine Needles While the Hydrochloride Is Obtained as A Microcrystalline Powder from Aqueous Acetone
|
|
Solubility
|
Insoluble In Water. Soluble In Ethanol, Methanol, Acetic Acid, Diethyl ether, Dimethyl Sulfoxide.
|
|
Rheological Behaviour
|
Ensures Ease Of Application And Stability During Storage
|
|
pH Compatibility
|
Maintains Skin Compatibility And Prevents Irritation
|
|
Drug Release Profiles
|
Controlled-Release Systems Improve Therapeutic Outcomes and Reduce Dosing Frequency.
|
Mechanism of action of lidocaine:
When used as a local anaesthetic, lidocaine works by blocking voltage-gated sodium channels (VGSCs), which reversibly stops action potentials from propagating. Lidocaine inhibits the priming of human peripheral PMNCs and neutrophils, for instance, which has an impact on inflammatory cells in vitro [9]. Additionally, lidocaine can decrease the release of pro-inflammatory mediators such as TNF-?, IL-4, and IL-6. Lidocaine reduces inflammation by preventing the release of histamine, leukocyte metabolism, and the expression of pro-inflammatory cytokines [10]. Lidocaine works by inhibiting NF-k? activation and the cytokine storm that follows [11]. Lidocaine dramatically lowers TNF-? levels in comparison to vehicle-treated controls. By controlling cellular metabolic activity, migration, exocytosis, and phagocytosis through reversible interactions with membrane proteins and lipids, it also has anti-inflammatory effects by reducing the accumulation of PMN (polymorphonuclear) granulocytes in the lung[12]. By preventing human peripheral polymorphonuclear cells or neutrophils from priming, lidocaine has an impact on inflammatory cells in vitro [13,14]. Lidocaine also could decrease mediator release. The antiarrhythmic effect's underlying mechanism seems to be like procaine's. As dosage increases, conduction time slows, refractory period lengthens, and ventricular excitability decreases. Therapeutic dosages do not reduce cardiac contractility[15]. Doses of lidocaine that result in similar increases in the diastolic stimulation threshold do not lower myocardial contractility or lower blood pressure, in contrast to procaine and procaine amide. Before the approval of amiodarone, the Advanced Cardiac Life Support (ACLS) algorithm included lidocaine as the main medication of choice for treating ventricular fibrillation (VF) or ventricular tachycardia (VT). Although lidocaine had been used for years to treat VF/VT, there was no proof that it was more effective than other medications. Lidocaine is still regarded as a good substitute for amiodarone in cases of cardiac arrest from VT/VF, even if it was later taken out of the ACLS Algorithm[16]. Other mechanisms allow lidocaine to produce extra analgesic effects. Numerous studies demonstrate that systemic lidocaine can be used as an analgesic during surgical procedures, particularly laparoscopic abdominal surgery. Lidocaine is often given at first dosages of 1.5–2 mg/kg BW and thereafter at 1.5–3 mg/BW/hr or 2-3 mg/min. As a result, serum lidocaine levels vary from 0.5 to 5?g/ml (~2–21 ?M), like what happens following epidural treatment. Lidocaine has several advantages, such as a speedy recovery, shorter hospital stays, unaffected bowel motions, and less pain following surgery.[17]. The effects of lidocaine on the central nervous system include blocking nicotinic and acetylcholine receptors, blocking opioid receptors, blocking presynaptic calcium channels in the dorsal root ganglion, blocking neurite growth, blocking muscarinic cholinergic receptors, and blocking substance P from attaching to receptors on natural killer (NK) cells[18,19]. Lidocaine is also effective in treating COVID-19. By lowering the cytokine storms linked to COVID-19, nebulised lidocaine seems to be a novel treatment for alleviating lung harm caused by the virus, potentially reversing ARDS. Therefore, additional pre-clinical studies and clinical trials are needed to better characterise the safety and effectiveness of lidocaine in treating individuals with severe acute respiratory distress syndrome (ARDS) brought on by COVID-19.
Pharmacokinetics of lidocaine:
The pharmacokinetics of lidocaine, which encompasses the processes of absorption, distribution, metabolism, and excretion of the drug, is crucial for ensuring its safe and effective application in clinical settings.
Absorption:
- Local Anaesthetic: When administered as a local anaesthetic, lidocaine is swiftly absorbed at the injection site, achieving peak plasma levels generally within 30 to 60 minutes. The absorption rate is influenced by various factors, including the dosage, concentration, and specific location of the injection.
- Topical Anaesthetic: In the case of topical application, lidocaine penetrates the skin or mucous membranes, with the absorption rate being contingent upon the formulation used, the area of application, and the condition of the skin or mucous membranes involved.
Distribution:
Lidocaine exhibits extensive distribution throughout the body, with significant concentrations observed in the heart, lungs, liver, and kidneys. It demonstrates a high degree of protein binding, predominantly to albumin. The volume of distribution for lidocaine is approximately 1-2 liters per kilogram of body weight.
Metabolism:
The metabolism of lidocaine primarily occurs in the liver, facilitated by the enzymes CYP3A4 and CYP1A2. This process results in the formation of two principal metabolites: monoethylglycinexylidide (MEGX) and glycinexylidide (GX). Notably, MEGX is an active metabolite that may enhance the anesthetic and antiarrhythmic properties of lidocaine.[20.21.22].
Excretion:
Lidocaine and its metabolites are predominantly eliminated through the kidneys via urine, with approximately 90% of the drug being cleared within a 24-hour period.[23]
Factors Influencing Pharmacokinetics:
Various factors can influence the pharmacokinetics of lidocaine, including:
Age: The pharmacokinetics of lidocaine may be modified in infants, children, and older adults.
Liver function: Impaired liver function due to disease can hinder the metabolism of lidocaine, resulting in elevated plasma levels and a risk of toxicity.
Kidney function: Renal impairment can affect the excretion of lidocaine and its metabolites, leading to increased plasma concentrations.
Drug interactions: Certain medications may interact with lidocaine, thereby impacting its pharmacokinetics. The pharmacokinetics of lidocaine are intricate and subject to influence from multiple factors. Recognizing these factors is crucial for the safe and effective administration of lidocaine.
Administration:
- Lidocaine is offered in various formulations, including solutions, aqueous gels, and ointments, each available in different concentrations.
- Different routes of administration necessitate distinct preparations of lidocaine to ensure optimal efficacy.
- Lidocaine is also found in products like medicated plasters, which are specifically designed to alleviate chronic postherpetic neuralgia.
- Subcutaneous injections of 0.05% to 0.1% solutions can be administered in large volumes for tumescent local anaesthesia, resulting in localized swelling and firmness beneficial for certain surgical interventions.
- Solutions ranging from 0.25% to 0.5% are utilized for intravenous regional anaesthesia, known as Bier’s block, or for infiltration into subcutaneous tissues.
- Epidural anaesthesia and regional nerve blocks typically employ solutions of 1% to 2%, which are also available in intravenous forms for antiarrhythmic applications.
- Aqueous gels containing 1% to 2% lidocaine, often combined with antiseptics like chlorhexidine, are used to prepare and lubricate the urethra prior to procedures such as Foley catheterization.
- Topical anaesthesia of mucous membranes in the airway, including the mouth and pharynx, is achieved using 4% solutions, which can be applied through gargling, spraying, or atomization.
- An eutectic mixture of lidocaine and prilocaine (EMLA) is effective for providing local cutaneous anaesthesia, particularly to minimize discomfort during needle punctures.
- It can be combined with epinephrine or other local anaesthetics, such as prilocaine, to enhance its effectiveness.
Adult Dosage:
- The dosages for infiltrative or regional anaesthesia are contingent upon the specific type of block being performed.
- When utilizing lidocaine to suppress airway reflexes, the recommended dosage is between 1 to 2 mg/kg, administered 2 to 5 minutes prior to intubation.
- In cases of cardiac dysrhythmias, the initial intravenous dose is set at 1 to 1.5 mg/kg, which may be followed by an infusion if necessary[24].
- A consensus statement from 2020 proposed that for intravenous treatment of acute pain, a loading dose should not exceed 1.5 mg/kg over a period of 10 minutes, followed by a continuous infusion capped at 1.5 mg/kg/h for a maximum duration of 24 hours, with vigilant monitoring for clinical efficacy and potential toxicity[25].
- The panel from the American Society of Anaesthesiologists acknowledged the lack of conclusive evidence regarding the ideal dosing of lidocaine.
- Nevertheless, drawing from clinical experience, the ASA advises an induction dose of 1.5 mg/kg, succeeded by an intraoperative infusion of 2 mg/kg/h for patients undergoing both open and laparoscopic abdominal procedures[26].
- For awake intubation, it is crucial to ensure effective topical application.
- The total lidocaine dosage should not surpass 9 mg/kg of lean body weight.
- To reduce the likelihood of laryngospasm, clinicians are encouraged to consider the use of nebulized lidocaine and to utilize lower concentrations[27].
Specific Patient Populations:
Hepatic impairment: The guidelines established by the American Association for the Study of Liver Diseases indicate that a lidocaine patch may be utilised for pain management in patients suffering from decompensated cirrhosis; however, it is advised to exercise caution in such cases[28,29,].
Renal impairment: Typically, dosage modifications are not required for individuals with renal impairment, as the absorption of topical lidocaine is minimal.
Pregnancy considerations: Lidocaine is believed to traverse the placenta through passive diffusion. The American College of Obstetricians and Gynecologists (ACOG) considers the use of local anaesthesia, including lidocaine with or without epinephrine, to be safe for treating oral conditions during pregnancy[30]. The maximum recommended dosage for lidocaine is 4.5 mg/kg (300 mg) for plain formulations and 7 mg/kg (500 mg) when combined with epinephrine[31]. It is important that a pregnant woman is not denied necessary surgical procedures or that such procedures are not postponed due to her pregnancy, as delays could adversely affect both her and the foetus. Current research suggests that exposure to anaesthetic or sedative agents in utero does not seem to impact foetal brain development. Furthermore, animal studies have not indicated any negative effects from limited exposures lasting less than three hours.
Breastfeeding considerations: Following continuous intravenous infusion, epidural administration, or high-dose local anaesthesia, the concentrations of lidocaine in breast milk remain low, thereby posing minimal risk to breastfeeding infants. Consequently, it is improbable that lidocaine will result in adverse effects for infants who are breastfed, and no specific precautions are warranted. The potential for lidocaine to interfere with breastfeeding, especially when used alongside other anaesthetics and analgesics, is a topic of discussion, influenced by the variability in research designs and methodologies. With appropriate support for breastfeeding, the use of epidural lidocaine in conjunction with opioids generally has a negligible effect on the success of breastfeeding, although the administration of labor pain medication may postpone the initiation of lactation[32].
Pediatric patients: Neonates exhibit an underdeveloped metabolic clearance system, which heightens their susceptibility to the accumulation of drugs and their metabolites. Furthermore, the levels of ?1-acid glycoprotein in neonates and infants are significantly lower, with the concentration of AAG at birth being approximately 50% of that found in adults. This condition leads to a greater unbound fraction of lidocaine, a prolonged elimination half-life, and an elevated risk of accumulation, especially during continuous infusions.[33] In Pediatric patients, the administration of topical lidocaine must be approached with caution to avoid the risk of overdose. The maximum allowable dose is determined by the patient's weight or age for otherwise healthy children over the age of 3 years. For infants and children under 3 years, the application of no more than 1.2 mL of the 2% solution is recommended, ensuring a minimum interval of 3 hours between doses and a cap of 4 doses within a 12-hour period.
Systemic lidocaine infusions have been widely recognized for their efficacy in managing postoperative acute pain in pediatric patients; however, their validation for other forms of pediatric pain remains limited. The NMDA antagonist characteristics of lidocaine may offer advantages in addressing challenging pain conditions, including mixed nociceptive-neuropathic pain and central sensitization. The typical dosing regimen for these scenarios involves an initial bolus of 1.5 mg/kg, followed by a continuous infusion of 1 mg/kg/hr, with higher dosages often employed in pediatric oncology contexts. Further research is essential to evaluate the safety and efficacy of lidocaine infusions in children and to refine dosing guidelines for various pain types. Additionally, it is crucial to investigate the significance of monitoring plasma levels during continuous infusion[34].
Older Patients: In the case of older patients, local anaesthesia should be the primary choice for surgical interventions. It is advisable to use the minimum effective volume and concentration to mitigate the risk of systemic toxicity. For lidocaine, a concentration of 10 mg/mL is recommended, with a maximum dosage not exceeding 5 mg/kg. This strategy ensures effective local anaesthesia for numerous surgical procedures while minimizing the likelihood of postoperative complications[35].
Monitoring:
- Lidocaine possesses a narrow therapeutic index, necessitating plasma-level monitoring for patients with hepatic impairment who are undergoing extended infusions.
- For dose calculations, it is advisable to utilize the patient’s ideal body weight rather than their actual body weight to prevent excessively elevated plasma concentrations, with a maximum limit of 120 mg/hr for lidocaine infusions.
- Continuous monitoring of vital signs and electrocardiograms (EKG) is essential. Clinicians should employ validated assessment tools such as the McGill Pain Questionnaire (SF-MPQ) and the Visual Analog Scale (VAS).
- Lidocaine formulations that include epinephrine can produce significant cardiovascular effects, even when administered in minimal quantities. Therefore, it is essential to conduct thorough hemodynamic monitoring prior to and during the administration of solutions containing vasopressors, especially if there are specific concerns regarding the patient’s cardiovascular condition[36].
Adverse Effects:
- Many adverse reactions linked to lidocaine occur when plasma concentrations reach toxic levels.
- The drug is most rapidly introduced into the intravascular compartment when administered in the intercostal space, followed by the caudal, epidural, brachial plexus, femoral, and subcutaneous areas.
- The maximum safe dosage based on body weight can vary from 3 mg/kg to 7 mg/kg when using formulations that include epinephrine, although other dosages have also been reported. Even smaller doses can lead to adverse effects and toxicity if given intravenously.
- Lidocaine is more neurotoxic compared to other local anaesthetics, particularly when high concentrations are applied directly to nerve tissue.
- The use of highly concentrated lidocaine (2.5% to 5%) for spinal anaesthesia is associated with an increased occurrence of transient radicular irritation syndrome, a painful yet self-limiting condition that affects the calves, thighs, and buttocks[37].
Drug-Drug interactions:
- The simultaneous administration of lidocaine and propranolol significantly elevates the serum levels of lidocaine.
- Caution is advised when giving lidocaine hydrochloride to patients with digitalis toxicity or atrioventricular block.
- Lidocaine is metabolized by the enzymes CYP1A2, CYP2B6, and CYP2D6, and it also acts as an inhibitor of CYP1A2. Therefore, care should be taken when lidocaine is used in conjunction with fluvoxamine[38,39].
- Furthermore, there is an increased risk of methemoglobinemia in patients using local anaesthetics like lidocaine alongside other medications such as nitrates, nitric oxide, hydroxyurea, dapsone, sulphonamides, chloroquine, phenobarbital, and phenytoin[40].
- The metabolism of lidocaine can yield O-toluidine, a metabolite linked to methemoglobinemia, especially at high doses, though it can also occur at lower doses in patients taking other drugs that may induce methemoglobinemia or in those with hemoglobinopathies or various types of anaemia[41].
Contraindications:
- Lidocaine is not recommended for individuals who have a documented history of severe adverse reactions. Although rare, anaphylactic reactions to lidocaine can occur.
- Additionally, methemoglobinemia may arise in patients with hemoglobinopathy or other forms of anaemia.
- Lidocaine should be avoided as an antiarrhythmic agent if the dysrhythmia is potentially a result of local anaesthetic toxicity.
Warnings and Precautions:
Lidocaine viscous: Severe adverse events, including seizures, cardiopulmonary arrest, and fatalities, have been documented in children under the age of 3 due to the inappropriate use of lidocaine viscous 2%. This medication is contraindicated for treating teething pain and should only be utilized in this demographic when no safer alternatives are available[42]. It is imperative to strictly follow dosing guidelines and ensure secure storage to minimize associated risks. This boxed warning issued by the FDA specifically addresses these concerns and does not apply to all formulations, such as intravenous lidocaine. Previous research has indicated that lidocaine can suppress premature ventricular complexes (PVCs) and nonsustained ventricular tachycardia (NSVT), which were believed to be precursors to ventricular fibrillation (VF) or pulseless ventricular tachycardia (pVT). However, recent studies have associated the prophylactic use of lidocaine for PVC suppression with increased mortality rates following acute myocardial infarction, likely due to a rise in asystole and bradyarrhythmias, resulting in the discontinuation of routine prophylactic administration. Observational studies suggest that while lidocaine may reduce the recurrence of VF/pVT after the return of spontaneous circulation (ROSC), it does not enhance survival rates and offers no advantage for patients experiencing non-shockable rhythms[43].
Toxicity:
Signs and Symptoms of Overdose:
- Mild toxicity is indicated by plasma levels exceeding 5 ?g/mL, with initial symptoms including slurred speech, tinnitus, circumoral paresthesia, and lightheadedness.
- When levels rise above 10 ?g/mL, patients may experience seizures or loss of consciousness. At 15 ?g/mL, there is further depression of the myocardium and central nervous system, leading to cardiac arrhythmias, respiratory arrest, and cardiac arrest at levels above 20 ?g/mL[44].
- Research involving animals indicates that the dosage of lidocaine necessary to induce cardiovascular collapse is approximately 7.1 ± 1.1 times greater than that required to elicit central nervous system effects[45]. This ratio, referred to as the "(cardiovascular collapse/CNS (CC/CNS) ratio," is considerably higher than that of other local anaesthetics; for instance, bupivacaine has a CC/CNS ratio of about 2.0.
- In conscious patients experiencing toxic doses, lidocaine is less likely than other local anaesthetics to transition swiftly from neurological symptoms to complete cardiovascular failure.
- Conversely, neurological signs may be obscured in patients under sedation or general anaesthesia, resulting in cardiovascular instability or arrhythmias being the initial signs of toxicity[46].
Management of Overdose :
- If toxicity or overdose is suspected, the administration of lidocaine should be halted immediately.
- In cases of cardiorespiratory collapse, priority should be given to airway management and respiratory support to avert respiratory acidosis, which can worsen toxicity and enhance lidocaine's detrimental chronotropic and inotropic effects[47].
- Support for vital functions, including oxygen supplementation, intravenous fluids, and inotropic agents, should be provided as necessary.
- The use of intravenous lipid emulsion is recommended as a rescue treatment, particularly in instances of refractory cardiovascular collapse[48].
CONCLUSION:
In conclusion, Lidocaine is an important choice for pain relief and antiarrhythmic effects with a favourable safety profile. This review examines its history, pharmacokinetics, mechanism of action, administration, drug interactions, contraindications, adverse effects, toxicity and clinical applications. It also assesses lidocaine use in pain relief, anaesthesia and cardiology. It also discusses different administration forms i.e., injectable, topical and oral with special benefits. This review provides a bit of knowledge regarding lidocaine’s characteristics for healthcare experts and analysts pointing to optimize its clinical applications.
REFERENCES
- Garutti, L. Rancan, C. Simon, G. Cusati, G. Sanchez-Pedrosa, F. Moraga,et al. Intravenous lidocaine decreases tumor necrosis factor Alpha expression both locally and systemically in pigs undergoing lung resection surgery, Anesth. Analg. 119 (2014) 815–828,https://doi.org/ 10.1213/ANE.0000000000000360.
- F. Wen, Y. Liu, H. Wang, W. Tang, Y.D. Hou, H.L. Wang, Lidocaine inhibits the production of IL-1? from macrophages RAW264.7 induced with lipopolysaccharide, Int. J. Clin. Exp. Pathol. 10 (2017) 6582–6588.
- T. Gordh, T.E. Gordh, K. Lindqvist, D.S. Warner, Lidocaine, The origin of a modern localanesthetic,Anesthesiology113(2010)14331437,https://doi.org/10.1097/ALN.0b013e3181fcef48.
- H.Hermanns, M.W. Hollmann,M.F. Stevens, P. Lirk, T. Brandenburger, T. Piegeler,et.al. Molecular mechanisms of action of systemic lidocaine in acute and chronic pain: a narrativereview,Br.J.Anaesth.123(2019)335349,https://doi.org/10.1016/j.bja.2019.06.014.
- M.H. Holmdahl, Xylocain (lidocaine, lignocaine), its discovery and Gordh’s contribution to its clinical use, Acta Anaesthesiol. Scand. Suppl. 113 (1998) 8–12, https://doi.org/10.1111/j.1399-6576.1998.tb04979.x.
- A.J. Johansson, Inga fischer-hjalmars (1918–2008): Swedish pharmacist, humanist, and pioneer quantum chemist, J. Chem. Educ. 89 (2012) 1274–1279, https://doi. org/10.1021/ed300024g.
- C. Berger, J. Rossaint, H. Van Aken, M. Westphal, K. Hahnenkamp, A. Zarbock, Lidocaine reduces neutrophil recruitment by abolishing chemokine-induced arrest and transendothelial migration in septic patients, J. Immunol. 192 (2014) 367–376, https://doi.org/10.4049/jimmunol.1301363.
- Y. Zhang, G.-J. Tao, L. Hu, J. Qu, Y. Han, G. Zhang, et al. Lidocaine alleviates morphine tolerance via AMPK-SOCS3-dependent neuroinflammation suppression in the spinal cord, J. Neuroinflammation 14 (2017) 211, https://doi.org/10.1186/s12974-017-0983-6.
- A. Ploppa, R.T. Kiefer, D.M. Haverstick, D.S. Groves, K.E. Unertl, M.E. Durieux, Local anesthetic effects on human neutrophil priming and activation, Reg. Anesth. Pain Med. 35 (2010) 45–50, https://doi.org/10.1097/AAP.0b013e3181c75199.
- F.F. Cruz, P.R.M. Rocco, P. Pelosi, Anti-inflammatory properties of anesthetic agents, Crit. Care 21 (2017) 67, https://doi.org/10.1186/s13054-017-1645-x.
- H.-L.Wang, Y.Q. Xing, Y.-X. Xu, F. Rong, W.-F. Lei, W.-H. Zhang, The protective effect of lidocaine on septic rats via the inhibition of high mobility group box 1 expression and NF- ?B activation, Mediat. Inflamm. (2013) 1–9, https://doi.org/ 10.1155/2013/570370, 2013.
- Z.A. Ali, R.S. El-Mallakh, Nebulized lidocaine in COVID-19, an hypothesis, Med. Hypotheses 144 (2020) 109947, https://doi.org/10.1016/j.mehy.2020.109947.
- Kanbara T, Tomoda MK, Sato EF, Ueda W, Manabe M. Lidocaine inhibits priming and protein tyrosine phosphorylation of human peripheral neutrophils. Biochem Pharmacol 1993; 45(8): 1593-8.doi:10.1016/0006-2952(93)90299-c.
- Hollmann MW, Gross A, Jelacin N, Durieux ME. Local anesthetic effects on priming and activation of human neutrophils. Anesthesiology 2001; 95: 113-2.
- J. Frieden, Antiarrhythmic drugs. Part VII. Lidocaine as an antiarrhythmic agent, Am. Heart J. 70 (1965) 713–715, https://doi.org/10.1016/0002-8703(65)90399-6.
- A. Mizzi, T. Tran, D. Mangar, E.M. Camporesi, Amiodarone supplants lidocaine in ACLS and CPR protocols, Anesthesiol. Clin. 29 (2011) 535–545,https://doi.org/10.1016/j.anclin.2011.05.001.
- G.C. McCarthy, S.A. Megalla, A.S. Habib, Impact of intravenous lidocaine infusion on postoperative analgesia and recovery from surgery, Drugs 70 (2010) 1149–1163, https://doi.org/10.2165/10898560-000000000-00000.
- K.S.P. Abelson, A.U. Hoglund, ¨ Intravenously administered lidocaine in therapeutic doses increases the intraspinal release of acetylcholine in rats, Neurosci. Lett. 317 (2002) 93–96, https://doi.org/10.1016/S0304-3940(01)02440-5.
- H. Hiruma, K. Shimizu, T. Takenami, H. Sugie, T. Kawakami, Effects of clonidine on lidocaine-induced inhibition of axonal transport in cultured mouse dorsal root ganglion neurones, Br. J. Anaesth. 101 (2008) 659–665, https://doi.org/10.1093/ bja/aen265.
- Lewin NA, Nelson LH (2006). “Chapter 61: Antidysrhythmics”.
- In Flomenbaum N, Goldfrank LR, Hoffman RL, Howland MD, Lewin NA, Nelson LH (eds.). Goldfrank’s Toxicologic Emergencies (8th ed.). New York: McGraw-Hill. Pp. 963–4. ISBN 978-0-07-143763-9.
- Kim JH, Kang DW, Choi GW, Lee SB, Lee S, Cho HY. Evaluation of Lidocaine and Metabolite Pharmacokinetics in Hyaluronic Acid Injection. Pharmaceutics. 2021 Feb 02;13(2).
- Collinsworth KA, Kalman SM, Harrison DC (1974). “The clinical pharmacology of lidocaine as an antiarrhythymic drug”. Circulation. 50 (6): 1217–30. Doi:10.1161/01.CIR.50.6.1217.
- Al-Khatib SM, Stevenson WG, Ackerman MJ, Bryant WJ, Callans DJ, Curtis AB, et al. 2017 AHA/ACC/HRS Guideline for Management of Patients With Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. Circulation. 2018 Sep 25;138(13):e272-e391.
- Foo I, Macfarlane AJR, Srivastava D, Bhaskar A, Barker H, Knaggs R,et al. The use of intravenous lidocaine for postoperative pain and recovery: international consensus statement on efficacy and safety. Anaesthesia. 2021 Feb;76(2):238-250.
- Chou R, Gordon DB, de Leon-Casasola OA, Rosenberg JM, Bickler S, Brennan T, et al. Management of Postoperative Pain: A Clinical Practice Guideline from the American Pain Society, the American Society of Regional Anesthesia and Pain Medicine, and the American Society of Anesthesiologists' Committee on Regional Anesthesia, Executive Committee, and Administrative Council. J Pain. 2016 Feb;17(2):131-57.
- Ahmad I, El-Boghdadly K, Bhagrath R, Hodzovic I, McNarry AF, Mir F,et al. Difficult Airway Society guidelines for awake tracheal intubation (ATI) in adults. Anaesthesia. 2020 Apr;75(4):509-528.
- Rogal SS, Hansen L, Patel A, Ufere NN, Verma M, Woodrell CD,et al. AASLD Practice Guidance: Palliative care and symptom-based management in decompensated cirrhosis. Hepatology. 2022 Sep;76(3):819-853.
- Daraz YM, Abdelghffar OH. Lidocaine Infusion: An Antiarrhythmic With Neurologic Toxicities. Cureus. 2022 Mar;14(3):e23310.
- Committee Opinion No. 569: oral health care during pregnancy and through the lifespan. Obstet Gynecol. 2013 Aug;122(2 Pt 1):417-422.
- Toledano RD, Kodali BS, Camann WR. Anesthesia drugs in the obstetric and gynecologic practice. Rev Obstet Gynecol. 2009 Spring;2(2):93-100.
- Drugs and Lactation Database (LactMed®) .National Institute of Child Health and Human Development; Bethesda (MD): Nov 16, 2020. Lidocaine.
- Heath C, Hii J, Thalayasingam P, von Ungern-Sternberg BS, Sommerfield D. Perioperative intravenous lidocaine use in children. Paediatr Anaesth. 2023 May;33(5):336-346.
- Hall EA, Sauer HE, Davis MS, Anghelescu DL. Lidocaine Infusions for Pain Management in Pediatrics. Paediatr Drugs. 2021 Jul;23(4):349-359.
- Cuvillon P, Lefrant JY, Gricourt Y. Considerations for the Use of Local Anesthesia in the Frail Elderly: Current Perspectives. Local Reg Anesth. 2022;15:71-75.
- Carroll IR, Younger JW, Mackey SC. Pain quality predicts lidocaine analgesia among patients with suspected neuropathic pain. Pain Med. 2010 Apr;11(4):617-21.
- Zaric D, Christiansen C, Pace NL, Punjasawadwong Y. Transient neurologic symptoms (TNS) following spinal anaesthesia with lidocaine versus other local anaesthetics. Cochrane Database Syst Rev. 2003;(2):CD003006.
- Konieczny KM, Dorian P. Clinically Important Drug-Drug Interactions Between Antiarrhythmic Drugs and Anticoagulants. J Innov Card Rhythm Manag. 2019 Mar;10(3):3552-3559.
- Isohanni MH, Neuvonen PJ, Olkkola KT. Effect of fluvoxamine and erythromycin on the pharmacokinetics of oral lidocaine. Basic Clin Pharmacol Toxicol. 2006 Aug;99(2):168-72.
- Iolascon A, Bianchi P, Andolfo I, Russo R, Barcellini W, Fermo E, et al., SWG of red cell and iron of EHA and EuroBloodNet. Recommendations for diagnosis and treatment of methemoglobinemia. Am J Hematol. 2021 Dec 01;96(12):1666-1678.
- Barash M, Reich KA, Rademaker D. Lidocaine-induced methemoglobinemia: a clinical reminder. J Am Osteopath Assoc. 2015 Feb;115(2):94-8.
- Meyers RS, Thackray J, Matson KL, McPherson C, Lubsch L, Hellinga RC, et al. Key Potentially Inappropriate Drugs in Pediatrics: The KIDs List. J Pediatr Pharmacol Ther. 2020;25(3):175-191.
- Panchal AR, Berg KM, Kudenchuk PJ, Del Rios M, Hirsch KG, Link MS, et al. 2018 American Heart Association Focused Update on Advanced Cardiovascular Life Support Use of Antiarrhythmic Drugs During and Immediately After Cardiac Arrest: An Update to the American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation. 2018 Dec 04;138(23):e740-e749.
- Becker DE, Reed KL. Local anesthetics: review of pharmacological considerations. Anesth Prog. 2012 Summer;59(2):90-101; quiz 102-3.
- Morishima HO, Pedersen H, Finster M, Hiraoka H, Tsuji A, Feldman HS, et al. Bupivacaine toxicity in pregnant and nonpregnant ewes. Anesthesiology. 1985 Aug;63(2):134-9.
- Yukioka H, Hayashi M, Fujimori M. Lidocaine intoxication during general anesthesia. Anesth Analg. 1990 Aug;71(2):207-8.
- Covino BG. Recent advances in local anaesthesia. Can Anaesth Soc J. 1986 May;33(3 Pt 2):S5-8.
- Sekimoto K, Tobe M, Saito S. Local anesthetic toxicity: acute and chronic management. Acute Med Surg. 2017 Apr;4(2):152-160.


 Nagaveni Pommala *
Nagaveni Pommala *

 10.5281/zenodo.14636911
10.5281/zenodo.14636911