Abstract
Chronic kidney disease is a progressive disease with no cure and high morbidity and mortality occurs in general adult population, especially in people with diabetes and Hypertension. CKD is characterized by the presence of kidney damage or an estimated glomerular filtration rate of less than 60/ml/min persisting for 3months or more .Kidney function can be improved by pharmacological and Non pharmacological therapies, FDA has approved the drugs namely Empagliflozin, Nedosiran, Tenapanor, Budesonide, Vadadustat which act by improving therapeutic action. recently some advanced treatments are introduced to treat CKD by replacing or repairing of renal tissues,replace the function of natural kidney in patients with having end stage renal disease,and by modifying genes to treat CKD or by replicating the kidney structure in CKD .Non pharmacological therapies like Dietary and life style adjustments like low protein, low salt diet might help to mitigate glomerular filtrate in CKD.
Keywords
chronic kidney disease, glomerular filtration rate, Empagliflozin Nedosiran, Tenapanor, replacing renal tissues, modifying genes, Diet control.
Introduction
Kidney damage or an estimated glomerular filtration rate (eGFR) of less than 60 mL/min/1.73 m?2; that lasts for three months or more are indicators of chronic kidney disease (CKD). • Chronic kidney disease is characterized by a gradual, irreversible decline in renal function that leads to an end-stage condition, such as: • Reduced renal nephron activity Reduced glomerular filtration rate and diminished kidney endocrine functions Globally, chronic kidney disease (CKD) ranks as the 16th most common cause of years of life lost.To avoid negative outcomes, primary care physicians must perform appropriate screening, diagnosis, and therapy of chronic kidney disease (CKD).

Figure No 1: Schymatic Representation Of Normal Kidney And Diseased Kidney
Stages of chronic kidney disease
*Stage 1: Normal kidney function with kidney damage The glomerular filtration rate (GFR) is greater than 90 mL/min/1.73 m?2;. Imaging or biopsy shows kidney damage. There are either no symptoms or only minor ones
.Stage 2: Minimal Renal Function Decline GFR: 1.73 m?2;/min 60-89 mL Mild hematuria or proteinuria Blood pressure could be high; electrolyte imbalance or mild anemia
Stage 3: Moderate Renal Function Decline GFR: 1.73 m?2;/min (30–59 mL) Hematuria or proteinuria that is moderate Blood pressure is frequently raised; anemia, electrolyte imbalance, or bone disease may be present.
Stage 3A: Mild symptoms; GFR: 45–59 mL/min/1.73 m?2; Stage 3B: 30-44 mL/min/1.73 m?2; is the GFR. Mild symptoms
Stage 4: Severe Kidney Function Decline* 15–29 mL/min/1.73 m?2; is the GFR. Severe hematuria or proteinuria
Stage 5: Kidney Failure (End-Stage Renal Disease, ESRD)* - Blood pressure is frequently significantly raised - Anemia, electrolyte imbalance, or bone disease are frequently present GFR: less than 15 mL/min/1.73 m?2; Kidney transplantation or dialysis is necessary.
Severe symptoms include: Overload of fluids and electrolyte imbalance Uremia Anemia *Extra Categorization: * Proteinuria, or albuminuria: A1: less than 30 mg/g A2: 30–300 mg/g A3:
greater than 300 mg/g GFR classifications: ?90 mL/min/1.73 m?2; is G1. G2: 1.73 m?2;/min 60-89 mL 45–59 mL/min/1.73 m?2; is G3a. 30-44 mL/min/1.73 m?2; for G3b 15–29 mL/min/1.73 m?2; is G4. G5: less than 15 mL/min/1.73 m?2;
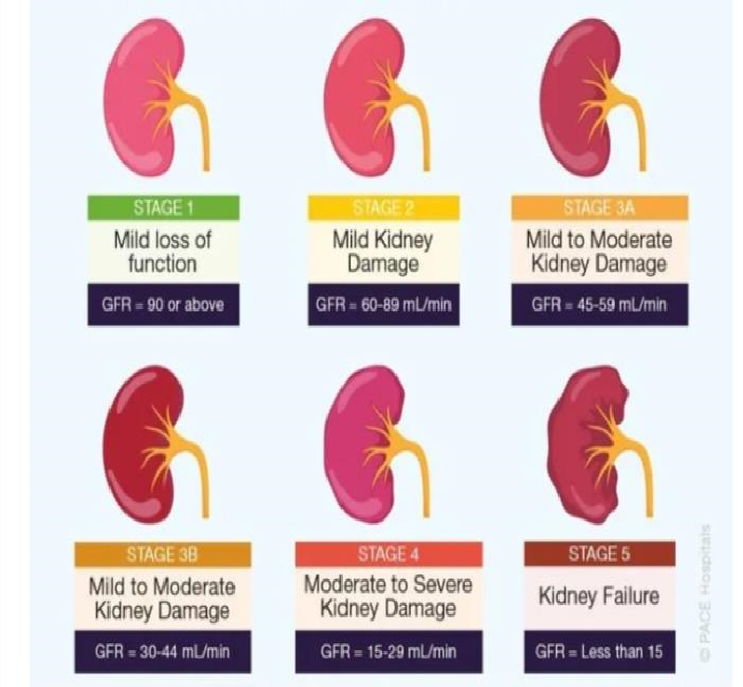
Figure No 2: Stages Of Ckd
Pathophysiology

Figure No 3: Pathophysiology Of Ckd
Kidney injury results in loss of nephrons and increased levels of angiotensin .angiotensin vasoconstrictor that triggers increased levels of blood pressure alongside hypertension ,there is an increase in glomerular permeability ,tubular protein reabsorption ,tubular /interstitial inflamation and eventually renal scarring
Risk Factors of Chronic Kidney Disease (CKD):
- Diabetes mellitus
- High blood pressure
- Being overweight
- Smoking
- Lack of physical exercise
- Excessive consumption of salt
- Consuming a lot of protein
- Specific drugs (NSAIDs, some antibiotics, etc.)
- CKD in the family *
Risk factors that cannot be changed
- Age (over 60)
- Ethnicity (Asian, Hispanic, and African American)
- CKD in the family
- A history of kidney damage or illness
- Genetic susceptibility (polycystic kidney disease, for example)
- Male sex
- Low birth weight
*Healthy Conditions:*
- Heart conditions
- A stroke
- Elevated cholesterol
- Lupus
- Rheumatoid arthritis
- Gout
- Some illnesses (such hepatitis C and HIV) *
Factors related to lifestyle:*
- Poor diet (heavy in sugar, salt, and saturated fat)
- Insufficient exercise
- Drinking too much alcohol
- Using drugs recreationally (such as cocaine and heroin)
Environmental Factors:
- Toxin exposure (heavy metals, pesticides, etc.)
- Pollution of the environment (air, water, etc.)
- Exposure at work (e.g., lead, cadmium)
Genetic Factors:
- Kidney illness with polycystic
- The Alport syndrome
- Fabry illness
- Cystinuria
- Additional hereditary conditions that impact kidney function
Additional factors include:
- A history of kidney stones
- Prior kidney surgery or transplantation
- Some medical treatments (such as angiography)
- Nephropathy brought on by contrast
High-Risk Groups:
- People with high blood pressure or diabetes
- Senior citizens 3. Minority groups (Asian, Hispanic, and African American)
- People with a family history of chronic kidney disease
- People who are obese or sedentary
Screening Guidelines:
- For people with diabetes or hypertension, the annual urine protein-to-creatinine ratio (UPCR) and serum creatinine
- Serum creatinine and biannual UPCR for patients with stage 3-5
Signs and Symptoms of Chronic Kidney Disease (CKD):
Early Signs and Symptoms (Stages 1-3):
- Fatigue
- Weakness
- Pale skin
- Shortness of breath
- Swelling in legs, ankles, and feet (edema
6. Frequent urination
- Nocturia (urinating at night)
- Blood in urine (hematuria)
- Proteinuria (excess protein in urine)
- Mild electrolyte imbalance *
Moderate to Advanced Signs and Symptoms (Stages 4-5)
1. Anemia
- Bone pain or fractures (osteodystrophy)
- Itching (pruritus)
- Muscle cramps
- Numbness or tingling in hands and feet
- Restless leg syndrome
- Sleep disturbances
- Decreased appetite
- Nausea and vomiting
- Confusion, cognitive impairment
Severe Signs and Symptoms (End-Stage Renal Disease, ESRD)::
1. Fluid overload (heart failure, pulmonary edema)
- An imbalance in electrolytes (hyperkalemia, hypocalcemia)
- Uremia (waste product accumulation)
- Epilepsy
- Coma
- Death (if treatment is not received)
Results of the physical examination:
1. Edema
- Hypertension
- Pale skin;
- Dry skin;
- Loss of hair
- Inadequate wound care
- Cardiovascular illness, such as coronary artery disease and heart failure
Laboratory Results:
- A higher level of serum creatinine
- A lower GFR (glomerular filtration rate)
- The presence of proteinuria
- Hematuria
- Unbalanced electrolytes
- Low hemoglobin, or anemia
- Lipid profile abnormality
* Imaging Research:
- Ultrasonography
- A CT scan
- MRI
- Kidney biopsy (sometimes)
Additional diagnostic tests: *
- The ratio of urine protein to creatinine (UPCR)
- The ratio of urine albumin to creatinine (UACR)
- Cytostatin serum c
Chronic Kidney Disease (CKD) Diagnosis:
Clinical Assessment: *
- Health background
- Physical assessment
- Tests in the lab
- Imaging research *
Experiments in the lab
1. Creatinine in serum
- The rate of glomerular filtration (GFR)
- BUN, or blood urea nitrogen
- Electrolyte panel (phosphate, calcium, potassium, and sodium)
- The ratio of urine protein to creatinine (UPCR)
- The ratio of urine albumin to creatinine (UACR)
- Cytostatin C in serum 8. CBC, or complete blood count
Imaging Research:
1. Ultrasonography
- A CT scan, or computed tomography
- MRI, or magnetic resonance imaging
- Kidney biopsy (sometimes)
Stages;
Stage 1: Normal GFR but kidney damage
Stage 2: GFR declines somewhat (60-89 mL/min/1.73 m?2;).
Stage 3: GFR declines moderately (30-59 mL/min/1.73 m?2;).
Stage 4: GFR drops significantly (15–29 mL/min/1.73 m?2;).
5. Kidney failure in stage five (GFR <15>
Acute renal damage is the first differential diagnosis
- Syndrome of nephrotic
- The syndrome of nephropathy
- Stones in kidneys
*Genetic Examination:
- Kidney illness caused by polycystic
- The Alport syndrome
- Fabry illness
- Cystinuria
Observation:
- Frequent GFR assessment
- Ratio of urine protein to creatinine
- The electrolyte panel 4. Monitoring blood pressure
Recent FDA approval Drugs to treat chronic kidney disease

FIGURE NO 4 :Symbolization of FDA Approved drugs
For too long,new treatment for people with chronic kidney disease have been few far Recently five new kidney disease related drugs have been approved by FDA(food and drug administration).
It is important for the kidney community to be aware of these recently approved medicines.so I want to take an opportunity to provide an overview of these drugs
Those are:
1. Empaglifozin
2. Nedosiran
3. Tenapanor
4. Budesonide
5. Vadadustat
1. EMPAGLIFOZIN
On September 22,2023 the FDA approved empaglifozin brand name (jardiance)to treat kidney disease in adults.it is a 10 mg pill that is taken every day it reduced the risk of reduced the risk of decline in glomular filtration rate,kidney failure and cardiovascular death
Mechanism of action of Empaglifozin
Empagliflozin, an SGLT2 inhibitor, has a multifaceted mechanism of action in Chronic Kidney Disease
- *SGLT2 Inhibition*: Blocks reabsorption of glucose in the proximal tubule, increasing urinary glucose excretion.
- *Reduced Sodium Reabsorption*: Inhibits sodium-glucose co-transport, decreasing sodium reabsorption and increasing urinary sodium excretion.
*Renal Effects:*
- *Increased Glucose Excretion*: Reduces glucose reabsorption, increasing urinary glucose excretion.
- *Reduced Glomerular Hyperfiltration*: Decreases glomerular pressure, slowing CKD progression.
- *Increased Tubular Flow*: Enhances tubular flow, reducing fibrosis and inflammation.
Systemic Effects:
- *Blood Pressure Reduction*: Decreases systolic blood pressure, reducing cardiovascular risk.
- *Weight Loss*: Increases urinary glucose and calorie loss, aiding weight reduction.
*CKD-Specific Benefits:*
- *Slowed eGFR Decline*: Reduces CKD progression.
- *Reduced Albuminuria*: Decreases proteinuria.
- *Improved Cardiovascular Outcomes*: Reduces risk of cardiovascular events.
- *Reduced Hospitalization*: Decreases hospitalization for heart failure.
Dosing and Administration:
- *10-25 mg/day*: Oral, once daily.
- *Dose adjustment*: Based on eGFR and renal function.
Contraindications
- *Severe Renal Impairment*: Avoid use in ESRD or dialysis.
- *Hypotension*: Monitor blood pressure.
- *Ketoacidosis*: Rare but serious risk.
Interactions:
- *Diuretics*: Monitor electrolytes.
- *Insulin/Secretagogues*: Adjust dose.
- *Other oral antidiabetics*: Monitor glucose.
2. NEDOSIRAN
For people living with primary hyperoxaluria type 1(PH1),a rare liver genetic disease that can cause kidney stone and kidney damage the FDA approved NEDOSIRAN .brand name (Rivfloza)on October 2 2023.Nedosiran is a RNAi therapeutic designed to treat Chronic Kidney Disease (CKD) by targeting patatin-like phospholipase domain-containing protein 3 (PNPLA3).
Mechanism of Action:
- *RNA Interference (RNAi)*: Nedosiran uses RNAi technology to specifically silence the PNPLA3 gene.
- *PNPLA3 Inhibition*: PNPLA3 is involved in lipid metabolism and liver fat accumulation. Inhibiting PNPLA3 reduces liver fat and decreases lipid accumulation in the kidneys.
- *Reduced Renal Lipotoxicity*: Decreased lipid accumulation in the kidneys mitigates renal lipotoxicity, inflammation, and fibrosis.
- *Improved Renal Function*: Nedosiran slows CKD progression by improving renal function, reducing proteinuria, and decreasing eGFR decline.
Benefits in CKD:
- *Slowed CKD Progression*
- *Reduced Proteinuria*
- *Improved Renal Function*
- *Decreased eGFR Decline*
- *Reduced Cardiovascular Risk*
Dosing and Administration:
- *Subcutaneous Injection*: Administered via subcutaneous injection every 12 weeks.
- *Dose Adjustment*: Based on renal function and response to treatment.
Contraindications
- *Severe Renal Impairment*: Avoid use in ESRD or dialysis.
- *Liver Disease*: Monitor liver function.
- *Hypersensitivity Reactions*: Rare but serious risk.
Interactions:*
- *Lipid-Lowering Agents*: Monitor lipid profiles.
- *Other CKD Therapies*: Monitor renal function.
3. TENAPANOR
It was approved by the FDA on october 17 2023 as the treatment for high phosphorus (hyper phosphatemia)in adults with kidney disease who are receiving dialysis treatment It's brand name is XPHOZAH®
Tenapanor is a sodium-free, minimally absorbed, small-molecule inhibitor of the sodium/hydrogen exchanger 3 (NHE3) in the gastrointestinal tract.
*Mechanism of Action in Chronic Kidney Disease (CKD):*
- *NHE3 Inhibition*: Tenapanor blocks NHE3 in the gut, reducing sodium absorption and increasing sodium excretion.
- *Reduced Sodium Reabsorption*: Decreased sodium reabsorption in the gut reduces sodium levels in the blood.
- *Blood Pressure Reduction*: Lower blood sodium levels decrease blood pressure, reducing cardiovascular risk and slowing CKD progression.
- *Reduced Aldosterone Secretion*: Tenapanor decreases aldosterone secretion, which reduces fibrosis and inflammation in the kidneys.
- *Improved Renal Function*: Reduced sodium reabsorption and aldosterone secretion slow CKD progression, improving renal function.
*Benefits in CKD:*
- *Blood Pressure Control*
- *Reduced Sodium Levels*
- *Slowed CKD Progression*
- *Improved Renal Function*
- *Reduced Cardiovascular Risk*
*Dosing and Administration:*
- *21-42 mg/day*: Oral, twice daily.
- *Dose Adjustment*: Based on renal function and response to treatment.
*Contraindications
- *Severe Renal Impairment*: Avoid use in ESRD or dialysis.
- *Hypotension*: Monitor blood pressure.
- *Electrolyte Imbalance*: Monitor electrolyte levels.
*Interactions:*
- *Diuretics*: Monitor electrolytes.
- *ACE Inhibitors*: Monitor blood pressure.
- *Other CKD Therapies*: Monitor renal function.
4. BUDESONIDE
The end of 2023 brought new hope for people with immunoglobulin A nephropathy (IgAN) when FDA fully approved budesonide(brand name TARPEYO®)on December 20 Budesonide, a corticosteroid, has anti-inflammatory and immunosuppressive properties, making it a potential treatment for Chronic Kidney Disease (CKD).
Mechanism of Action in CKD:
- Anti-Inflammation_: Budesonide reduces inflammation in the kidneys, decreasing cytokine production and inflammatory cell infiltration.
- Immunosuppression_: Inhibits immune cell activation, reducing renal damage.
- Antifibrotic Effects_: Decreases fibrosis and extracellular matrix deposition. 4. Reduced Oxidative Stress_: Antioxidant properties mitigate oxidative stress.
5. Improved Renal Function_: Slows CKD progression, improving renal function.
Benefits in CKD:
- Reduced Proteinuria
- Slowed CKD Progression
- Improved Renal Function
- Reduced Inflammation
- Improved Cardiovascular Outcomes Dosing and Administration:
- 8-16 mg/day_: Oral, once daily
- Dose Adjustment: Based on renal function and response to treatment.
Contraindications
- Severe Renal Impairment: Avoid use in ESRD or dialysis.
- Infections: Monitor for infections.
- Hyperglycemia: Monitor blood glucose.
- Osteoporosis: Monitor bone density.
Interactions:
- CYP3A4 Inhibitors: Monitor budesonide levels.
- Diuretics: Monitor electrolytes.
- Other CKD Therapies: Monitor renal function.
5. VADADUSTAT
The most recent FDA approval was announced on March 27,2024. brand name(vafseo®) is approved to treat anemia in adults who have been receiving dialysis treatment for at least three months.Vadadustat is an oral hypoxia-inducible factor prolyl hydroxylase (HIF-PH) inhibitor, designed to treat anemia in Chronic Kidney Disease (CKD).
Mechanism of Action:
- HIF-PH Inhibition:Vadadustat inhibits HIF-PH, increasing hypoxia-inducible factor (HIF) levels.
- Erythropoietin (EPO) Stimulation: Elevated HIF levels stimulate EPO production.
- Red Blood Cell Production:Increased EPO levels stimulate red blood cell production.
- Improved Oxygen Delivery: Increased red blood cells improve oxygen delivery to tissues. Benefits in CKD:
- Corrects Anemia: Increases hemoglobin levels.
- Reduces ESA Requirements: Decreases need for erythropoiesis-stimulating agents (ESAs).
- Improved Quality of Life: Enhances physical function and reduces fatigue.
- Reduced Cardiovascular Risk: May reduce cardiovascular events.
Dosing and Administration:
- 800-1200 mg/day: Oral, once daily.
- Dose Adjustment: Based on hemoglobin levels and response.
Contraindications
- Severe Renal Impairment: Avoid use in ESRD or dialysis.
- Hypersensitivity Reactions: Rare but serious risk.
- Thrombosis: Monitor for thrombotic events.
Interactions:
- CYP3A4 Inhibitors: Monitor vadadustat levels.
- ESAs: Monitor hemoglobin levels.
- Other CKD Therapies*: Monitor renal function.
Formulations:
1. Tablets: Oral, once daily.
SOME ADVANCED TREATMENT FOR CHRONIC KIDNEY DISEASE
1.Stem cell therapy is an experimental treatment for Chronic Kidney Disease (CKD),
aiming to repair or replace damaged renal tissue.
Types of Stem Cells Used:
- Mesenchymal Stem Cells (MSCs): Derived from bone marrow, adipose tissue, or umbilical cord.
- Hematopoietic Stem Cells (HSCs): Derived from bone marrow.
- Embryonic Stem Cells (ESCs): Derived from embryos.
Mechanisms of Action:
- Differentiation: Stem cells differentiate into renal cells, replacing damaged tissue.
- Paracrine Effects: Stem cells secrete factors promoting renal repair.
- Immunomodulation: Stem cells modulate immune responses, reducing inflammation.
- Angiogenesis: Stem cells promote new blood vessel formation.
Benefits in CKD:
- Improved Renal Function
- Reduced Proteinuria
- Slowed CKD Progression
- Enhanced Regenerative Capacity
- Reduced Inflammation
Stem Cell Sources:
- Autologous: Patient's own stem cells.
- Allogenic: Donor-derived stem cells.
Delivery Methods:
- Intravenous Infusion
- Intra-arterial Injection
- Intrarenal Injection
2. The artificial kidney, also known as the implantable bioartificial kidney, is a medical device designed to replace the function of a natural kidney in patients with End-Stage Renal Disease (ESRD).
Mechanism:
- Blood Filtration: The device filters waste products, excess fluids, and electrolytes from the blood.
- Ultrafiltration: Removes excess fluids and toxins through a semipermeable membrane.
- Dialysis: Uses a dialysate solution to remove waste products.
- Electrolyte Management: Regulates electrolyte levels.
- Hormone Regulation: Mimics kidney function by regulating hormones.
Types:
- Implantable Bioartificial Kidney: Permanent implant.
- Wearable Artificial Kidney: Portable device.
Benefits:
- Improved Quality of Life
- Reduced Dialysis Need
- Increased Mobility
- Enhanced Nutrient Absorption
- Potential for Regenerative Therapy
3. Genetic Engineering in Chronic Kidney Disease (CKD) involves modifying genes to prevent or treat kidney damage.
Mechanisms:
- RNA Interference (RNAi): Silencing genes involved in CKD progression.
- MicroRNA (miRNA) Regulation:Modulating miRNA expression to reduce fibrosis and inflammation.
- Gene Silencing:Using small interfering RNA (siRNA) or short hairpin RNA (shRNA) to reduce harmful gene expression.
Targeted Genes:
- TGF-?1: Involved in fibrosis and CKD progression.
- TNF-?: Contributes to inflammation and kidney damage.
- VEGF: Regulates angiogenesis and kidney function.
Delivery Methods:
- Viral Vectors: Adenovirus, lentivirus, or adeno-associated virus.
- Non-Viral Methods: Electroporation, liposomes, or nanoparticles.
- Gene-Edited Stem Cells: Transplanting gene-corrected stem cells.
Benefits:
- Slowed CKD Progression
- Improved Renal Function
- Reduced Fibrosis and Inflammation
- Enhanced Regenerative Capacity
4. Kidney Regeneration Technology aims to restore or replace damaged kidney tissue in Chronic Kidney Disease (CKD) patients.
Mechanisms of Action:
- Stem Cell Therapy: Utilizes stem cells to differentiate into renal cells, replacing damaged tissue.
- Cellular Reprogramming: Converts non-renal cells into functional renal cells.
- Tissue Engineering:Creates artificial kidney tissue using biomaterials and cells.
- Bioactive Molecules:Delivers growth factors, hormones, or other molecules to promote renal regeneration.
Kidney Regeneration Technologies:
- Kidney Organoids: Miniature, 3D kidney structures derived from stem cells.
- Renal Cell Cartridges:Bioartificial devices containing renal cells for implantation.
- 3D Printing: Creates customized kidney tissue and organs.
5.3D Bioprinting:
- Creates functional kidney tissue using living cells and biomaterials.
- Utilizes various bioprinting techniques (e.g., extrusion, inkjet, laser).
- Replicates kidney structure and function.
Mechanism of Action:
- Cellular differentiation and organization.
- Tissue maturation and vascularization.
- Integration with host tissue.
Benefits in CKD:
- Functional kidney tissue replacement.
- Reduced dialysis need.
- Enhanced quality of life.
- Potential for cure.
Organ-on-a-Chip (OoC):
- Microfluidic device mimicking kidney function.
- Recapitulates kidney structure and physiology.
- Allows for real-time monitoring and testing.
Mechanism of Action:
- Mimics kidney filtration and reabsorption.
- Models kidney disease progression.
- Enables drug testing and toxicity assessment.
Benefits in CKD:
- Personalized medicine.
- Improved drug development.
- Reduced animal testing.
- Enhanced disease understanding.
FUTURE SCOPE:
TECHOLOGICAL ADVANCEMENTS
- Wearable sensors: Monitoring kidney function and fluids status in real time.
- Telemedicine and remote monitoring: Enhancing patient engagement and care co ordination.
- Artifical kidneys: Developing implanatable and wearable devices for renal replacement.
- Point of care diagnostics: Rapid and accurate CKD diagnosis and monitoring. 5. 3D Printing: creating customized kidney models and prosthetics
CONCLUSION
Chronic kidney disease is a progressive, irreversible loss in renal function over a period of time resulting in a end stage condition i.e. decreased renal nephron function, decreased glomular filtration rate and decreased endocrine functions of the kidney. It is characterized by presence of kidney damage or an estimated glomular filtration rate is less than 60ml/min.
It is the 16th leading cause of years of lost of life in World wide. hence the need of advanced treatment of chronic kidney disease is very crucial so that Food And Drug administration (FDA) was approved the advanced drugs having the good therapeutic action and some new advanced treatments are introduced to treat CKD this may leads to increase the quality of life in people having CKD.
REFERENCES
- Romagnani P, Remuzzi G, Glassock R, Levin A, Jager KJ, Tonelli M, Massy Z, Wanner C, Anders HJ. Chronic kidney disease. Nature reviews Disease primers. 2017 Nov 23;3(1):1-24.
- Kalantar-Zadeh K, Jafar TH, Nitsch D, Neuen BL, Perkovic V. Chronic kidney disease. The lancet. 2021 Aug 28;398(10302):786-802.
- Peter WS. Introduction: chronic kidney disease: a burgeoning health epidemic. Journal of Managed Care Pharmacy. 2007 Dec;13(9 Supp D):2-5.
- Levey AS, Coresh J. Chronic kidney disease. The lancet. 2012 Jan 14;379(9811):165-80.
- Webster AC, Nagler EV, Morton RL, Masson P. Chronic kidney disease. The lancet. 2017 Mar 25;389(10075):1238-52.
- Almutary H, Bonner A, Douglas C. Symptom burden in chronic kidney disease: a review of recent literature. Journal of Renal care. 2013 Sep;39(3):140-50.
- Weisbord SD. Symptoms and their correlates in chronic kidney disease. Advances in chronic kidney disease. 2007 Oct 1;14(4):319-27.
- Goh ZS, Griva K. Anxiety and depression in patients with end-stage renal disease: impact and management challenges–a narrative review. International journal of nephrology and renovascular disease. 2018 Mar 12:93-102.
- Chen TK, Knicely DH, Grams ME. Chronic kidney disease diagnosis and management: a review. Jama. 2019 Oct 1;322(13):1294-304.
- Luyckx VA, Tuttle KR, Garcia-Garcia G, Gharbi MB, Heerspink HJ, Johnson DW, Liu ZH, Massy ZA, Moe O, Nelson RG, Sola L. Reducing major risk factors for chronic kidney disease. Kidney international supplements. 2017 Oct 1;7(2):71-87.
- Haroun MK, Jaar BG, Hoffman SC, Comstock GW, Klag MJ, Coresh J. Risk factors for chronic kidney disease: a prospective study of 23,534 men and women in Washington County, Maryland. Journal of the American Society of Nephrology. 2003 Nov 1;14(11):2934-41.
- Yamagata K, Ishida K, Sairenchi T, Takahashi H, Ohba S, Shiigai T, Narita M, Koyama A. Risk factors for chronic kidney disease in a community-based population: a 10-year follow-up study. Kidney international. 2007 Jan 2;71(2):159-66.
- Kazancio?lu R. Risk factors for chronic kidney disease: an update. Kidney international supplements. 2013 Dec 1;3(4):368-71.
- Luyckx VA, Tuttle KR, Garcia-Garcia G, Gharbi MB, Heerspink HJ, Johnson DW, Liu ZH, Massy ZA, Moe O, Nelson RG, Sola L. Reducing major risk factors for chronic kidney disease. Kidney international supplements. 2017 Oct 1;7(2):71-87.
- Tan F, Li X, Wang Z, Li J, Shahzad K, Zheng J. Clinical applications of stem cell-derived exosomes. Signal Transduction and Targeted Therapy. 2024 Jan 12;9(1):17.
- Li L, Fu H, Liu Y. The fibrogenic niche in kidney fibrosis: components and mechanisms. Nature Reviews Nephrology. 2022 Sep;18(9):545-57.
- Cockwell P, Fisher LA. The global burden of chronic kidney disease. The Lancet. 2020 Feb 29;395(10225):662-4.
- Jafar TH. FDA approval of dapagliflozin for chronic kidney disease: a remarkable achievement? The Lancet. 2021 Jul 24;398(10297):283-4.
- Lu M, Cohen MH, Rieves D, Pazdur R. FDA report: Ferumoxytol for intravenous iron therapy in adult patients with chronic kidney disease. American journal of hematology. 2010 May;85(5):315-9.
- Bhutani P, Joshi G, Raja N, Bachhav N, Rajanna PK, Bhutani H, Paul AT, Kumar R. US FDA approved drugs from 2015–June 2020: a perspective. Journal of Medicinal Chemistry. 2021 Feb 22;64(5):2339-81.
- Ndumele CE, Neeland IJ, Tuttle KR, Chow SL, Mathew RO, Khan SS, Coresh J, BakerSmith CM, Carnethon MR, Després JP, Ho JE. A synopsis of the evidence for the science and clinical management of cardiovascular-kidney-metabolic (CKM) syndrome: a scientific statement from the American Heart Association. Circulation. 2023 Nov 14;148(20):1636-64.
- Passi I, Salwan S, Kumar B. US-FDA approved drugs in 2020 and 2021: a review. Mini reviews in medicinal chemistry. 2023 Jul 1;23(12):1273-97.
- Star RA. Treatment of acute renal failure. Kidney international. 1998 Jan 1;54(6):1817-31.
- Hermanson T, Bennett CL, Macdougall IC. Peginesatide for the treatment of anemia due to chronic kidney disease–an unfulfilled promise. Expert opinion on drug safety. 2016 Oct 2;15(10):1421-6.
- Aursulesei V, Costache II. Anticoagulation in chronic kidney disease: from guidelines to clinical practice. Clinical cardiology. 2019 Aug;42(8):774-82.


 B. Akhila*
B. Akhila*
 N. Navyasree
N. Navyasree
 K. Mounika
K. Mounika
 M. Sreya
M. Sreya
 E. Anusha
E. Anusha
 B. Manasa
B. Manasa




 10.5281/zenodo.14339742
10.5281/zenodo.14339742