Abstract
Liposomes distinctive structural characteristics and biocompatibility have made them a revolutionary medication delivery technology. The solubility and bioavailability of hydrophilic and hydrophobic medications can be improved by encapsulating them in liposomes, which are made of phospholipid bilayers. The creation, characterisation, and use of liposomes in targeted drug administration are covered in this abstract, with a focus on how they may increase therapeutic efficacy while reducing adverse effects. Highlighted are developments in liposome formulation methods, including surface modification for improved cellular absorption and sustained release mechanisms. Additionally, the delivery of biologics, vaccines, and anticancer medicines using liposomes is examined, demonstrating its adaptability in handling challenging therapeutic issues. In general, liposomes offer a promising foundation for the creation of novel drug delivery systems that could completely alter the way that several medical specialties treat patients.
Keywords
Targeted drug delivery, Novel delivery, Controlled Release, Biocompatibility.
Introduction
Liposomes are tiny, spherical structures made up of one or more lipid (mostly manufactured and natural phospholipids) bilayers that enclose a little volume of the aqueous phase. They are sometimes referred to as lipid vesicles or just vesicles. Over time, liposomes have been used extensively as model biomembranes and delivery systems for a variety of bioactive compounds due to their size, amphiphilic nature, and biocompatibility (Lasic and Papahadjopoulos 1996, Torchilin 2005). They frequently encapsulate lipophilic substances in the membrane areas and hydrophilic compounds in their inner watery core (Torchilin 2005). Because lipophilic substances may firmly remain within membranes, their encapsulation efficiency (EE) in liposomes is often higher than that of hydrophilic ones. Many bioactive substances, including antibacterial and anticancer drugs, genetic materials, proteins, DNA, peptides, vaccines, and enzymes, to mention a few, have been studied for delivery systems employing liposomes (Carugo et al. 2016). Even though Gregoriadis and Ryman (1971) were the first to discuss the use of liposomes in drug delivery systems, it took over 30 years for the first liposomal medication, AmbisomeVR, to be released into the market (Gulati et al. 1998, Hann and Prentice 2001). There are currently a number of liposomal medications on the market that help patients get better therapeutic results (Chang and Yeh 2012, Allen and Cullis 2013). Two crucial elements for in vivo applications are liposome size and size distribution, particularly liposome size. impacts the therapeutic efficacy, drug loading, biodistribution, drug clearance rate from the body, and targeting efficacy to a particular organ. The thickness of the lipid bilayer is around 4 nm, and liposome sizes typically range from tens of nanometers to several tens of mms (Lasic 1993). For drug delivery applications, the ideal vesicle size is usually restricted to 50–150 nm.
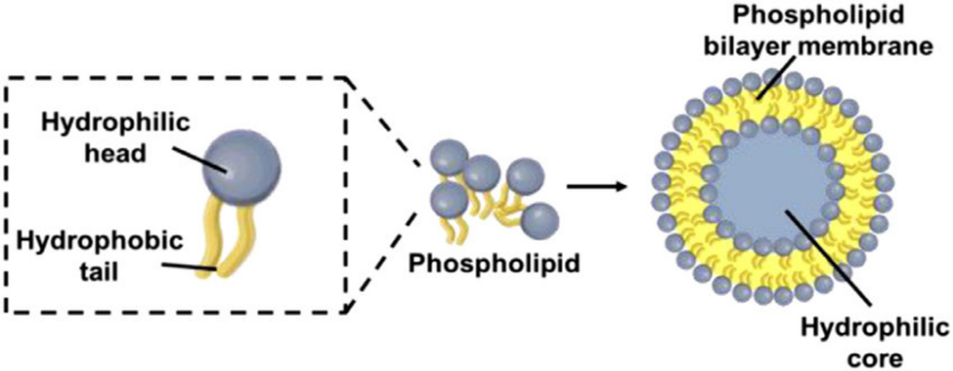
Fig No 1. The Basic Structure Of A Liposome
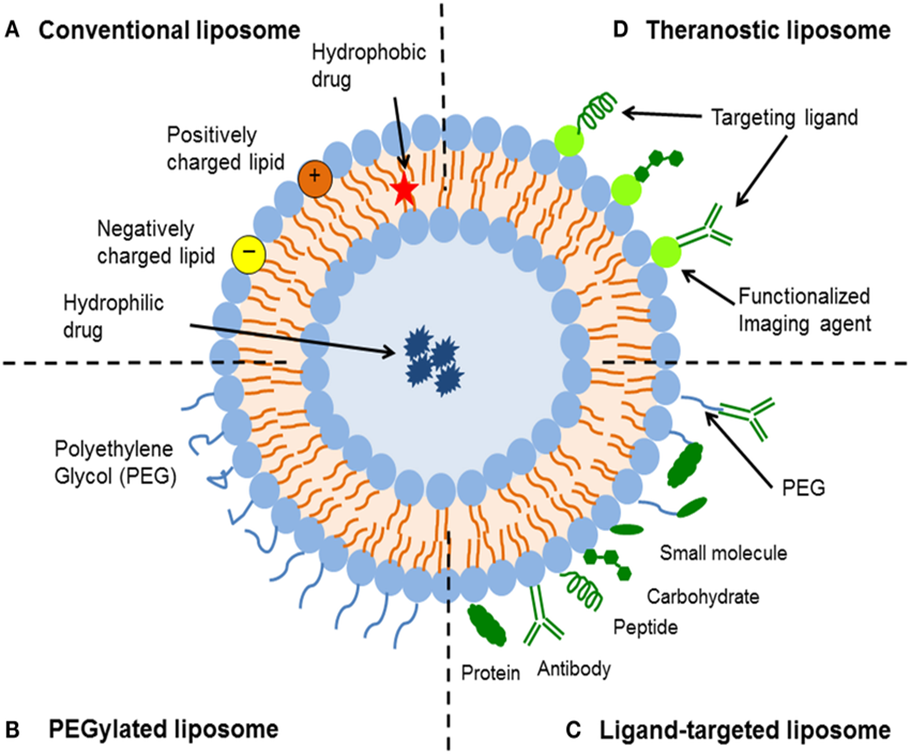
Fig No 2. A Illustration Of Liposome And Structural Components
Components Of Liposomes: -
The most common components are
• Phospholipids.
• Cholesterol.
1.Phospholipids:
A significant part of every cell membrane is made up of phospholipids, a class of lipids. They have a phosphate group joined to a polar head, two fatty acid tails, and a glycerol backbone. Because of their ability to form bilayers in aqueous settings, they are crucial for the integrity and proper operation of cells.
Characteristics: -
Amphipathic Nature:
The synthesis of the bilayers that comprise cell membranes is made possible by the hydrophilic (which attracts water) and hydrophobic (which repels water) components of phospholipids.
Types:
Phospholipids with distinct functions in membrane dynamics and signaling include phosphatidylcholine, phosphatidylethanolamine, and phosphatidylserine.
Functions:
Phospholipids have roles in cell signaling, membrane fluidity, and the synthesis of bioactive chemicals in addition to building membranes.
2.Cholesterol: -
One kind of lipid molecule that is necessary for animal cell membranes is cholesterol. It is a sterol with a four-ring structure that is essential to many aspects of cellular activity.
Characteristics: -
A. Membrane Fluidity: By keeping fatty acid chains from packing too tightly together, cholesterol contributes to the fluidity and stability of membranes, which is essential for healthy membrane function.
B. Hormone Precursor:
It acts as a precursor for the production of vitamin D, bile acids, and steroid hormones, all of which are essential for many physiological functions.
C. Rafts of Lipid:
Lipid rafts, which are microdomains in cell membranes that arrange protein interactions and signaling pathways, are formed in part by cholesterol.
D. Food Sources:
Although cholesterol can be acquired through diet, mostly from animal products, the body also produces it, mostly in the liver.
Advantages of Liposome: -
Improved Drug Delivery: By making it easier for medications to pass through biological membranes, liposomes increase their bioavailability. This is especially helpful for medications that are poorly soluble.
Targeted Delivery: Liposomes can be made to target particular tissues or cells by altering their surface, which lowers side effects and boosts therapeutic effectiveness.
regulated Release: Liposomes have the ability to release medications in a regulated manner, resulting in long-lasting therapeutic benefits.
Decreased Toxicity: Toxic medications can be encapsulated in liposomes to minimize systemic exposure and hence reduce unwanted effects.
Versatility: Liposomes have a number of uses, such as gene delivery, vaccinations, and cosmetics.
Disadvantages of Liposome: -
Problems with Stability: In biological settings, liposomes may exhibit instability, which could result in an early release of the medicine they contain and decreased effectiveness.
Complexity of Production: Liposomes' extensive use may be restricted by their complicated and expensive manufacturing method.
Immunogenicity: Certain liposomes have the ability to trigger immunological reactions, which may result in decreased effectiveness or more adverse effects.
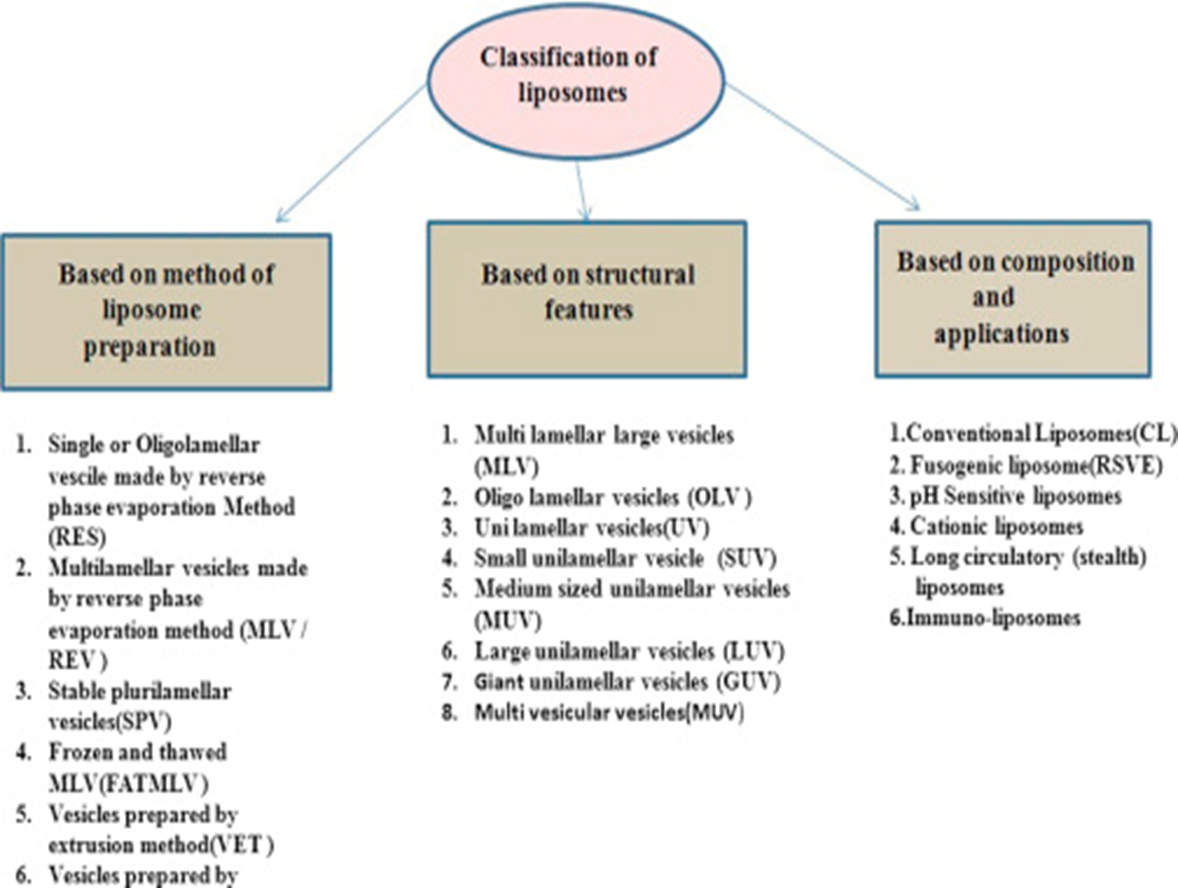
Fig No 3. Classification of Liposome
Mechanism of Formation of Liposomes: -
Lipid Selection and Film Formation:
Phospholipids, which are amphiphilic molecules with hydrophilic head groups and hydrophobic tails, make up the majority of liposomes. To create a thin lipid film on a container's walls, the lipid mixture is mixed in an organic solvent (such as chloroform) and then evaporated.
Hydration:
Multilamellar vesicles (MLVs) are created when an aqueous buffer is added to the dry lipid film. Because the lipids are amphiphilic that is, their hydrophilic heads interact with water while their hydrophobic tails avoid it they self-assemble when hydrated, forming a spherical bilayer structure.
Self-Assembly:
Hydrophobic interactions propel the self-assembly, which is impacted by ionic strength, temperature, and lipid concentration. Larger vesicles arise during hydration as the lipid content rises; these can subsequently be processed to produce smaller, unilamellar vesicles.
Dimensions Reduction and Uniformity:
Liposomes are made smaller and more uniform by using methods like sonication and extrusion. Extrusion pushes the liposomal suspension through a membrane filter, whereas sonication uses ultrasonic vibrations to break apart bigger vesicles.
Encapsulation:
When liposomes are hydrated, they can hold hydrophilic medications in their aqueous core and hydrophobic medications in the lipid bilayer. Because of this characteristic, liposomes are adaptable drug delivery vehicles.
Application of Liposome:-
1. Drug Delivery: Liposomes are efficient transporters of hydrophilic and hydrophobic medications, increasing their bioavailability and lowering their toxicity.
2. Vaccine Development: By stabilizing antigens and improving delivery, liposomes are used as adjuvants to boost the immune response to vaccines.
3. Gene Delivery: Liposomes are essential for gene therapy applications because they make it easier to transport nucleic acids (DNA/RNA).
4. Imaging and Diagnostics: To enhance picture clarity in imaging procedures like MRI and ultrasound, liposomes are used as contrast agents.
5. Targeted Therapy: By enabling targeted delivery to particular tissues or cells, modifications to liposomes improve the efficacy of cancer treatments.
6. Cosmetic Applications: Liposomes improve the transport of active compounds in cosmetics, increasing their efficacy and skin absorption.
7. Antifungal and Antibacterial Treatments: Antifungal and antibacterial drugs can be encapsulated in liposomes, which enhances their transport and anti-infection efficacy.
8. Anticancer Therapy: By targeting cancer cells, liposomes containing chemotherapeutic drugs lessen systemic side effects and improve therapy effectiveness.
9. Ocular Drug Delivery: By facilitating better drug delivery to the eye, liposomes can help treat diseases like dry eye syndrome and glaucoma.
10. Neurotherapeutics: Because liposomes may penetrate the blood-brain barrier, medications for neurological conditions can be delivered precisely.
11. Pain Management: Analgesics can be delivered via liposomes, increasing their effectiveness and reducing their adverse effects.
12. Hormonal Therapy: In therapeutic applications, liposomes can enhance the stability and bioavailability of hormones by improving their distribution.
13. Nutritional supplements: Liposomes are being utilized more and more to improve the bioavailability of vitamins and other nutritional supplements.
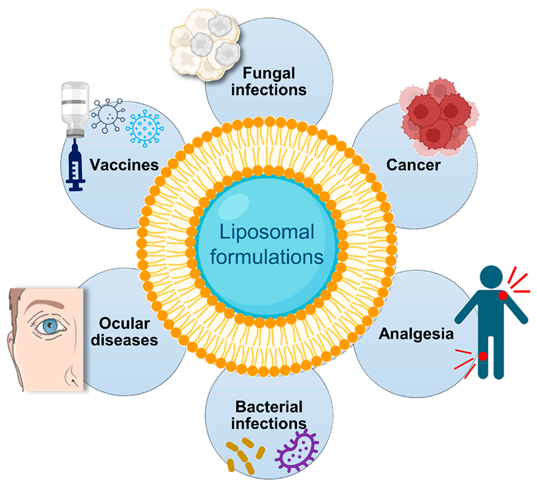
Fig No 4. Liposomal Formulation
Methods of Preparation: -

Fig No 5. Methods Of Preparation Of Liposome
1.Thin-Film Hydration Method:
One of the most popular techniques for making liposomes is this one.
Steps: -
To make a lipid solution, dissolve lipids (cholesterol and phospholipids) in an organic solvent such as methanol or chloroform.
To create a thin lipid coating on the walls of a glass vial or round-bottom flask, evaporate the solvent at a lower pressure.
Add an aqueous solution (such as buffer or distilled water) and/or sonicate to hydrate the lipid film. To create MLVs, or Multilamellar vesicles.
It is possible to use extrusion or sonication to shrink MLVs into smaller unilamellar vesicles (SUVs).
2. Reverse Phase Evaporation Method:
This technique is used for preparing liposomes with high encapsulation efficiency.
Steps:
Lipids and an aqueous solution containing the material to be encapsulated should be dissolved in an organic solvent.
To create a water-in-oil emulsion, evaporate the organic solvent at a lower pressure.
Liposomes carrying the encapsulated material remain after the organic phase is removed.
3.Extrusion Method
This technique is used to prepare liposomes with uniform size and narrow size distribution.
Steps:
To make MLVs, prepare liposomes by the thin-film hydration technique.
Using a high-pressure extruder or a hand-held extruder, pass the liposome suspension through a succession of polycarbonate membrane filters with specified pore diameters. Smaller, uniformly sized liposomes are the end product of this process.
4.Sonication Method:
This technique is used to create small unilamellar vesicles (SUVs) or reduce the size of liposomes.
Steps:
The thin-film hydration method or any suitable technique can be used to manufacture liposomes.
To make the liposomes smaller, sonicate (or apply high-frequency ultrasound) to the liposome suspension.
5 Detergent Removal Method:
This technique is used to create liposomes that contain hydrophobic materials.
Steps:
Use a detergent solution to dissolve the hydrophobic material and lipids.
To create liposomes containing the encapsulated material, remove the detergent using methods such as chromatography or dialysis.
6.Freeze-Thawing Method:
This technique is used to create liposomes that are more stable.
Steps:
Lower the temperature at which the liposome suspension is frozen, usually below the lipid’s phase transition temperature.
Repeat the cycle several times to thaw the frozen suspension at a higher temperature. This procedure decreases leakage and traps materials inside liposomes.
CONCLUSION: -
To sum up, liposomes offer improved medication solubility, targeted administration, and fewer adverse effects, making them a major breakthrough in drug delivery technologies. They are appropriate for a variety of uses due to their exceptional capacity to encapsulate various therapeutic substances, especially in the fields of biopharmaceuticals and oncology. Even though there are obstacles including inconsistent manufacturing and legal restrictions, research is still being done to improve liposome formulations, which could eventually result in safer and more effective treatment choices.
REFERENCES
- Has C, Sunthar P. A comprehensive review on recent preparation techniques of liposomes. J Liposome Res. 2020 Dec;30(4):336-365. doi: 10.1080/08982104.2019.1668010. Epub 2019 Sep 27. PMID: 31558079.
- Lodish, H., Berk, A., Kaiser, C. A., et al. (2000). Molecular Cell Biology. 4th ed. New York: W.H. Freeman.
- Ghosh, R., et al. (2015). “Phospholipids in cell signaling.” Biochemistry, 54(31), 4884-4895.
- Wenk, M. R. (2010). “Lipids: The Missing Link in Membrane Biology.” Nature Reviews Molecular Cell Biology, 11(12), 811-818.
- Meyer, H. W., et al. (2004). “Cholesterol and membrane fluidity.” Biochemistry, 43(2), 553-560.
- Sullivan, D. A., et al. (2004). “Cholesterol: A crucial role in cellular processes.” Journal of Lipid Research, 45(7), 1237-1246.
- Simons, K., & Toomre, D. (2000). “Lipids and membrane dynamics.” Nature Reviews Molecular Cell Biology, 1(1), 31-39.
- Goldstein, J. L., & Brown, M. S. (1990). “Regulation of the mevalonate pathway.” Annual Review of Biochemistry, 59, 311-343.
- Allen, T. M., & Cullis, P. R. (2013). Liposomal drug delivery systems: From concept to clinical applications. Advanced Drug Delivery Reviews, 65(1), 36-48.
- Gabizon, A., Shmeeda, H., & Barenholz, Y. (2003). Pharmacokinetics of pegylated liposomal Doxorubicin: From the bench to the clinic. Clinical Pharmacokinetics, 42(5), 419-436.
- D’Souza, G., & Dewangan, S. (2016). Liposomes: A review of the state of the art. American Journal of PharmTech Research, 6(3), 152-161.
- Barenholz, Y. (2012). Doxil®—the first FDA-approved nano-drug: Lessons learned. Nature Reviews Drug Discovery, 9(8), 789-796.
- Lasic, D. D. (1998). Drug delivery with liposomes. Nature, 392(6679), 1-5.
- Barenholz, Y. (2012). Doxil®—the first FDA-approved nano-drug: Lessons learned. Nature Reviews Drug Discovery, 9(8), 789-796.
- Allen, T. M., & Cullis, P. R. (2013). Liposomal drug delivery systems: From concept to clinical applications. Advanced Drug Delivery Reviews, 65(1), 36-48.
- Shimizu, T., & Yoshikawa, T. (2009). Liposomal drug delivery systems: Current status and future perspectives. Drug Development and Industrial Pharmacy, 35(9), 1065-1071.
- Bangham, A. D., Horne, R. W., & Spohr, H. A. (1965). “Negative staining of phospholipid vesicles in electron microscopy.” Journal of Molecular Biology, 13(1), 238-252.
- Düzgüne?, N., et al. (1999). “Liposomes as drug delivery systems.” Journal of Pharmaceutical Sciences, 88(10), 1059-1072.
- Ulrich, A. S. (2005). “Structure and function of membrane-active peptides.” Biophysical Journal, 88(5), 3701-3714.
- Wassef, L., et al. (2000). “Production of liposomes by extrusion.” Methods in Molecular Biology, 144, 203-215.
- Lasic, D. D. (1998). “Liposomes: From physics to applications.” Trends in Biotechnology, 16(7), 307-313.
- Allen, T. M., & Cullis, P. R. (2013). Liposomal drug delivery systems: From concept to clinical applications. Advanced Drug Delivery Reviews, 65(1), 36-48. Doi:10.1016/j.addr.2012.09.037
- O’Hagan, D. T., & Rappuoli, R. (2004). Vaccine adjuvants: A bridge between science and industry. Nature Reviews Drug Discovery, 3(6), 475-482. Doi:10.1038/nrd1397
- Naderi, M., et al. (2015). Liposome-mediated delivery of oligonucleotides. Biomaterials, 56, 17-30. Doi:10.1016/j.biomaterials.2015.04.034
- Kullacka, M., & Schaefer, U. (2015). Liposomes as drug delivery systems in imaging: An overview. Frontiers in Pharmacology, 6, 239. Doi:10.3389/fphar.2015.00239
- Gabizon, A., & Barenholz, Y. (2003). Pharmacokinetics of pegylated liposomal Doxorubicin: Review of animal and human studies. Clinical Pharmacokinetics, 42(5), 419-436. Doi:10.2165/00003088-200342050-00001
- Cevc, G., & Blume, G. (1992). Lipid vesicles as carriers for skin penetration: A mechanistic study. Biochimica et Biophysica Acta (BBA) – Biomembranes, 1104(1), 1-10. Doi:10.1016/0005-2736(92)90011-4
- Raghavendra, M. et al. (2014). Liposomal formulation of antifungal drugs: A review. Journal of Liposome Research, 24(1), 1-8. Doi:10.3109/08982104.2013.835363
- Allen, T. M., & McMurray, J. (1998). The role of liposomes in the treatment of cancer. Clinical Cancer Research, 4(4), 1579-1585. PMID: 9581883
- Lobenberg, R., & Amiji, M. (2000). Liposomal drug delivery for the treatment of ocular diseases. Journal of Controlled Release, 66(2-3), 229-238. Doi:10.1016/S0168-3659(00)00296-9
- Lajter, I., et al. (2020). Liposomal drug delivery for the treatment of central nervous system disorders. Frontiers in Pharmacology, 11, 224. Doi:10.3389/fphar.2020.00224
- Chandrasekaran, S., et al. (2017). Liposome formulations for drug delivery in pain management. Pain Physician, 20(2), 79-90. PMID: 28226340
- Mura, S., et al. (2013). Liposomes in hormone therapy: A review. Journal of Controlled Release, 165(1), 1-12. Doi:10.1016/j.jconrel.2013.09.006
- Naczk, M., & Shahidi, F. (2006). Liposome applications in nutraceuticals. Food Research International, 39(5), 613-623. Doi:10.1016/j.foodres.2006.03.008
- Shoaib Shaikh Hamid M, Hatwar PR, Bakal RL, Kohale NB. A comprehensive review on liposomes: As a novel drug delivery system. GSC Biol Pharm Sci. 2024;27(01):199–210. Doi:10.30574/gscbps.2024.27.1.0121.


 Sanap Tushar Khanderao * 1
Sanap Tushar Khanderao * 1





 10.5281/zenodo.14029074
10.5281/zenodo.14029074