Abstract
Introduction:
Anabolic androgenic steroids are testosterone derived molecules which are often used therapeutically, its misuse by athletes and non-athletes causes a broad spectrum of side effects. Neutrophil gelatinase-associated lipocalin (NGAL) is a 25 kDa protein belonging to the lipocalin superfamily. In clinical studies, NGAL has been found to be an early predictor for acute kidney injury (AKI).
Aim and Objective:
The present study was designed to analyze the Nandrolone decanoate induced nephrotoxicity in Wistar rats using biochemical and histopathological assessment and also to identify a reliable novel biomarker for anabolic androgenic steroid induced nephrotoxicity.
Materials and Methods:
Twelve male wistar rats (100 ± 50 g body weight) were randomly divided into two groups. Group 1- control rats received corn oil (1ml/kg body weight) intramuscularly and Group 2- ND group received 5mg/kg of Nandrolone decanoate intramuscularly. All treatments were given twice weekly for three months. One week after the last dose of injection, animals were sacrificed, blood and tissue were collected to investigate serum biomarkers and histopathology for nephrotoxicity.
Results:
Nandrolone decanoate group showed elevated levels of serum creatinine, urea, NGAL and severe alterations in the renal histology as compared with control. NGAL is a reliable novel biomarker for identifying the anabolic androgenic steroid-induced nephrotoxicity in rats.
Keywords
Nephtotoxicity, Nandrolone, Biomarker, Novel
Introduction
Anabolic androgenic steroids (AASs) are synthetic derivatives of testosterone, originally developed for clinical purposes to treat conditions resulting from hormone deficiency, such as delayed puberty, hypogonadism, bone marrow failure syndromes and muscle wasting disorders [1], but currently used not only among high-profile and non-professional athletes as performance enhancing drugs but also among the adolescents to improve physical performance and increase self-esteem due to its beneficial properties such as tissue building booster, maintenance of muscle mass, strength and bone health [2]. AAS misuse is accompanied with the frequent and dose dependent side effects such as neurotoxicity, nephrotoxicity and hepatotoxicity and histopathological changes in various organs. In our earlier work, we revealed the potentials of water emulsion of Withania Somnifera root powder against Nandrolone decanoate (ND) induced toxicity in rats by assessing various biochemical parameters [3] . The adverse effects of AAS became a significant public health concern, outside of sports. Among a vast number of flourishing anabolic steroid, Nandrolone decanoate (decadurabolin), the 19-nor-testosterone derivative, is the widely abused AAS in the world [4]. Neutrophil gelatinase–associated lipocalin (NGAL) is a 25 kDa protein belonging to the lipocalin superfamily initially found in activated neutrophils, in accordance with its role as an innate antibacterial factor [5]. However, it was shown that many other types of cells, including the kidney tubule, may produce NGAL in response to various injuries [6]. NGAL is expressed in human tissues such as lung, liver and kidney, in pathologic states. Preclinical studies identified NGAL to be one of the most upregulated genes and proteins in the kidney very early after acute kidney injury (AKI) in animal models [7]. NGAL protein expression was detected predominantly in renal tubule epithelial cells that were undergoing proliferation and regeneration. However, a major advantage of the use of NGAL is that its level increases earlier than other conventional biomarkers [8,9,10,11].
MATERIALS AND METHODS:
Materials
Drugs and Reagents:
Nandrolone decanoate (decadurabolin, 4-oestren-17?-ol-3-one-17-decanoate) was purchased from Cadila Health care Ltd., India (100mg/mL ampuoles), corn oil was purchased from local pharmacy, Chennai. Other chemicals of analytical grade were purchased commercially.
Animals:
Twelve male Wistar rats weighing 180-250g procured from Biogen Laboratory Animal Facility (CPCSEA Reg.No.971/Po/RcBibt/S.2006), Bangalore and maintained at Centre for Laboratory Animal Research, Saveetha Institute of Medical and Technical Sciences, Chennai were used. The animals were housed three per cage in a controlled environment with free access to food and water ad libitum with temperature of 25±2°C, humidity 40-60% and natural light/dark cycles. All animal procedures were approved by Institutional animal ethics committee of Saveetha Medical College, Chennai (IAEC- SU/CLAR/RD/004/2018) and animal experiments were performed in accordance with the guidelines of Committee for Control and Supervision of Experimentation on Animals (CPCSEA, New Delhi, India).
Experimental Protocol :
The animals were randomly divided into 2 groups (n = 6 each for control group and ND group).
Group 1:
Control [C] - Rats received 1mL/kg body weight of corn oil intramuscularly (i.m) in thigh region twice weekly for 3 months as vehicle.
Group 2:
Nandrolone decanoate group (ND) - Rats received intramuscular (i.m) injection of ND (5mg/kg) in thigh region in a volume of 1mL/kg body weight twice weekly for 3 months. ND was diluted in corn oil. The animals were given ND i.m every Monday and Wednesday and four days after the last doses of injection, all groups of animals were anesthetized by isofluorane and blood was collected by from retro orbital plexus and then sacrificed by cervical dislocation. After allowing the blood to clot for 30min at room temperature, it was centrifuged (1000g, 10min) and serum was collected and stored at -20°C for biochemical analysis. Kidney was excised and washed in ice?cold saline, weighed, and fixed in 10% phosphate?buffered formalin (pH 7.4) for histopathological examination.
Procedure
Estimation of neutrophil gelatinase-associated lipocalin (NGAL)
The NGAL levels were determined using stored sandwich-type assay based on anti-NGAL antibodies. Microtiter plates were coated overnight at 4°C with monoclonal NGAL antibody dissolved in phosphate-buffered saline (PBS). Plates were blocked for 2 h at room temperature with assay buffer (1% [w/v] bovine serum albumin and 0.05% [v/v] Tween 20 in PBS [PBST]) and washed 3 times with 0.05% (v/v) PBST. A 100 ?l of sample was added to the coated plates. Between each step of the assay, the plates were washed 3 times with PBST. Samples and controls were diluted 1:200 in assay buffer and incubated overnight at 4°C. Plates were then incubated with biotinylated monoclonal NGAL antibody in assay buffer and then incubated with streptavidin solution. Enhancement solution was added, and ELISA-horseradish peroxidase substrate and tetramethylbenzidine with hydrogen peroxide were added to the vials. ELISA stop solution was added and plates were measured in an ELISA reader. The samples were analyzed in duplicates.
STATISTICAL ANALYSIS:
All data were analyzed by one?way ANOVA. The values are presented as mean±Standard error of mean. The mean value differences between the groups were analyzed by Student–Newman–Keuls method. P <0>
RESULT:
Biochemical Analysis
The levels of serum urea and serum creatinine and NGAL were estimated. Serum urea, creatinine and NGAL were significantly elevated in experimental group when compared with control group. The creatinine levels of control and ND are 22.4 ± 0.65, 43.5 ± 1.7 mg/dL, respectively. The creatinine levels of ND group increased by 94.2% as compared with control, and these changes are beyond the reference range of rat species. The urea levels of control and ND are 0.69 ± 0.08, 1.3 ± 0.13 mg/dL, respectively. The urea levels of ND group increased by 88.4% as compared with control and such changes are beyond the reference range of rat species. Similarly, the NGAL was also elevated nearly to 109.3 % than the control group [Table 1]
From Table 1 and figure 1, it is understood that the percentage increase of serum NGAL is greater than that of serum creatinine and urea. Hence, NGAL can be a novel biomarker for ND-induced nephrotoxicity. All the above estimations were carried out using semi?autoanalyzer (Robonik Prietest) as per the standard protocols.
Table 1: Correlation of mean value of different biomarkers between ND and control groups
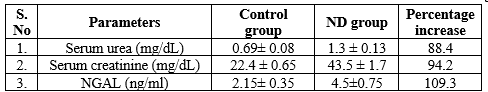
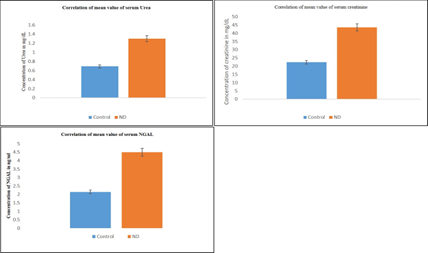
Figure 1: Correlation of mean values of serum Urea (mg/dL), serum Creatinine (mg/dL) and serum NGAL (ng/ml)
Values are mean ± SE (n= 6 each for Control and ND group). ND- Nandrolone decanoate, NGAL- Neutrophil gelatinase associated lipocalin
Histopathological assessment
Kidney tissues were fixed in 10% phosphate?buffered formalin (pH 7.4) and processed by routine histological methods and embedded in paraffin blocks. Five micrometer thick sections were cut coronally. All sections were stained with hematoxylin and eosin and examined under light microscope (Olympus iNEA). Kidney of control group rats showed parenchyma with well?defined glomerulus and tubules. ND group showed marked congestion in capillaries and atrophy of glomerulus, multifocal cytoplasmic congestion, and degenerative changes in the proximal convoluted tubules [Figure 2].
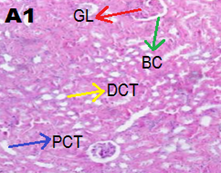
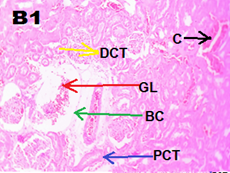
Figure 2. Representative images of kidney tissue stained with haematoxylin and eosin (40 x magnification)
(A1) Control group showed normal renal architecture, (B1) ND group showed marked congestion in glomerulus (red arrow), capillaries (black arrow) and PCT degenerative changes (blue arrow).
DISCUSSION:
The adverse effects of AASs on the kidney were recently analyzed through a literature review [12], the authors were suggested that AASs assumption can affect the kidney in different ways, inducing or aggravating acute kidney injury, chronic kidney disease and glomerular toxicity. Although a dose-related nephrotoxic effect has been proposed [13], to the best of our knowledge, no studies have been published about Novel biomarker against, AAS induced kidney damages. The side effects caused by AAS such as ND are shown in many convincing works [13,14]. The key findings revealed elevation of serum levels of creatinine, and urea in ND group as compared with control. The present findings are in agreement with our earlier works that showed increased serum levels of the creatinine and urea after ND administration to rats [3]. It was shown that elevated activities of cytochrome p?450 in the liver and kidney tissues of ND?induced mice could be indications for the tissue level alterations caused by anabolic steroids [16]. In the present study, histopathology data revealed severe alterations in the architecture of kidney of ND?induced rats. The histopathological assessment clearly indicates the cellular damages such as karyolysis, congestion of blood vessels and sinusoids in ND group and degenerative changes in PCT [17,18] and such cellular damages may be due to decreased total antioxidant capacity [19]. Moreover, it was reported that the thickness of renal parenchyma and renal volume were drastically increased in bodybuilders after anabolic steroid usage implicating kidney dysfunctions [20]. The primary organs that are affected by ND are the liver and kidney. Among these, the kidney is more prone for toxicity mainly due to drug, their identification in early stage is difficult so we used a novel biomarker for identifying the acute kidney injury. In the present study, rats were administrated with ND and their serum samples were analyzed with standardized biomarker such as urea and creatinine due to lack of early sensitivity; we adjoin a novel biomarker such as NGAL [21], which was also used to analyze and identify the early renal damage. The increase in serum urea and creatinine were indicating the renal damage. The upregulation of NGAL, a protein-based biomarker which also shows early damage in the kidney. As per previous literature, the ND reduces the antioxidant level and produces oxidative damage of cells with the help of synthesis of free radicals and lipid peroxidation [19]. Lipid peroxidation alters the structural integrity of cell membrane and functions. The changes like tubular inflammatory infiltration, necrosis, and atrophy are predominately seen in the proximal tubules of the kidney and minimally there is multifocal congestion in glomeruli are observed in microscopic examination [3]. Previous studies have showed that the level of serum NGAL increases significantly after 24 hours of acute kidney injury and much earlier than the serum creatinine level [22]. It was reported that serum NGAL starts to rise within 6 hours after kidney injury and it continues to raise up to 48 hours, whereas the rise of serum creatinine is significant only after 48 hours [23]. Reports revealed that NGAL, apart from being more accurate biomarker for acute kidney injury, it can be used as a differential diagnostic tool between various renal diseases such as acute kidney disease and hepatorenal syndrome-related kidney injury [24]. Similarly, the level of NGAL in chronic kidney injury also plays a significant role in estimating the onset, diagnosis, severity, and prognosis of it. In chronic kidney injury, the active renal tubular injury leads to increased NGAL synthesis, which results in increased NGAL concentrations in urine [25]. Renal replacement therapy is becoming a much needed procedure for patients with kidney failure. Plasma NGAL <400>
[Figure 2].
FUNDING SUPPORT
The author declare they have no funding support for this study.
CONFLICT OF INTEREST
The authors declare no conflict of interest regarding this investigation.
REFERENCES
- Shahidi NT. A Review of chemistry, biological action, and clinical applications of anabolic-androgenic steroids. Clin.Ther. 2001; 23:1355-1390.
- Gadallah AA, El-Ghawet HA, and Sabry DA. Deca Durabolin (Nandrolone Decanoate) Impair Structure and Function of Liver, Prostate and Pituitary of Male Wistar Rats. J Pharma Reports. 2017; 2:3.
- Vasavan SS, Jagadesan V, Sivanesan S, Rajagopalan V. Protective effect of Withania somnifera On Nandrolone Decanoate induced Biochemical alterations and Hepatorenal Toxicity in Wistar Rats. Pharmacogn Mag. 2020; 16:S218-23.
- Busardò FP, Frati P, Sanzo MD, Napoletano S, Pinchi E, Zaami S, Fineschi V. The impact of nandrolone decanoate on the central nervous system. Curr Neuropharmacol. 2015;13(1):122-31. doi: 10.2174/1570159X13666141210225822. PMID: 26074747; PMCID: PMC4462037.
- Bolignano D, Donato V, Coppolino G, Campo S, Buemi A, Lacquaniti A, Buemi M. Neutrophil gelatinase-associated lipocalin (NGAL) as a marker of kidney damage. Am J Kidney Dis. 2008; 52(3):595-605. doi: 10.1053/j.ajkd.2008.01.020. Epub 2008 Apr 2. PMID: 18725016
- Paragas N, Qiu A, Zhang Q, Samstein B, Deng SX, Schmidt-Ott KM, Viltard M, Yu W, Forster CS, Gong G, Liu Y, Kulkarni R, Mori K, Kalandadze A, Ratner AJ, Devarajan P, Landry DW, D'Agati V, Lin CS, Barasch J. The Ngal reporter mouse detects the response of the kidney to injury in real time. Nat Med. 2011;17(2):216-22. doi: 10.1038/nm.2290. Epub 2011 Jan 16. PMID: 21240264; PMCID: PMC3059503.
- Supavekin S, Zhang W, Kucherlapati R, Kaskel FJ, Moore LC, Devarajan P. Differential gene expression following early renal ischemia/reperfusion. Kidney Int. 2003;63(5):1714-24. doi: 10.1046/j.1523-1755.2003.00928.x. PMID: 12675847.
- Mishra J, Ma Q, Prada A, Mitsnefes M, Zahedi K, Yang J, Barasch J, Devarajan P. Identification of neutrophil gelatinase-associated lipocalin as a novel early urinary biomarker for ischemic renal injury. J Am Soc Nephrol. 2003;14(10):2534-43. doi: 10.1097/01.asn.0000088027.54400.c6. PMID: 14514731.
- Mishra J, Mori K, Ma Q, Kelly C, Barasch J, Devarajan P. Neutrophil gelatinase-associated lipocalin: a novel early urinary biomarker for cisplatin nephrotoxicity. Am J Nephrol. 2004;24 (3):307-15. doi: 10.1159/000078452. Epub 2004 May 12. PMID: 15148457.
- Cai L, Rubin J, Han W, Venge P, Xu S. The origin of multiple molecular forms in urine of HNL/NGAL. Clin J Am Soc Nephrol. 2010 Dec;5(12):2229-35. doi: 10.2215/CJN.00980110. Epub 2010 Sep 9. PMID: 20829422; PMCID: PMC2994084
- Mårtensson J, Xu S, Bell M, Martling CR, Venge P. Immunoassays distinguishing between HNL/NGAL released in urine from kidney epithelial cells and neutrophils. Clin Chim Acta. 2012 Oct 9;413(19-20):1661-7. doi: 10.1016/j.cca.2012.05.010. Epub 2012 May 18. PMID: 22609864.
- Davani-Davari D, Karimzadeh I, Khalili H. The potential effects of anabolic-androgenic steroids and growth hormone as commonly used sport supplements on the kidney: A systematic review. BMC Nephrol. 2019; 20 (1): 198. doi: 10.1186/s12882-019-1384-0.
- D’Errico S, Di Battista B, Di Paolo M, Fiore C, Pomara C. Renal Heat Shock Proteins Over-Expression Due to Anabolic Androgenic Steroids Abuse. Mini-Rev. Med. Chem.2011; 11(5): 446–450. doi: 10.2174/138955711795445934
- Almaiman AA, Almaiman SH, Elagamy EI, Al Wutayd O, Almarzuqi M, Alzunaidi R. Side effects of anabolic steroids used by athletes at Unaizah gyms, Saudi Arabia: A pilot study. J Sports Med Phys Fitness. 2019; 59(3): 489-495.
- Pozzi R, Fernandes KR, de Moura CF, Ferrari RA, Fernandes KP, Renno AC. Nandrolonedecanoate induces genetic damage in multiple organs of rats. Arch Environ Contam Toxicol. 2013;64:514?8.
- Acharjee BK, Mahanta R. Enhanced hepatic and kidney cytochrome p?450 activities in nandrolone decanoate treated albino mice. Drug Metab Lett. 2009;3:120?4.
- Saad Al?Dhuayan I. Possible protective role of whey protein on the rat’s liver tissues treated with nandrolone decanoate. Pak J Biol Sci. 2018;21:262?74.
- Kahal A, Allem R. Reversible effects of anabolic steroid abuse on cyto?architectures of the heart, kidneys and testis in adult male mice. Biomed Pharmacother. 2018;106:917?22.
- Smitha S Vasavan, Senthilkumar Sivanesan, Vijayakumar Jagadesan Antiperoxidative effect of Withania somnifera on Lipid peroxidation and Antioxidant capacity in the serum of nandrolone decanoate treated rats Research J. Pharm. and Tech. 2021; 14(2):1065-1068.
- Kantarci UH, Punduk Z, Senarslan O, Dirik A. Evaluation of anabolic steroid induced renaldamage with sonography in bodybuilders. J Sports Med Phys Fitness. 2018;58:1681?7.
- Hoste EA, Vandenberghe W. Plasma neutrophil gelatinase-associated lipocalin (NGAL) for timing of initiation of renal replacement therapy for acute kidney injury? J Thorac Dis 2018;10:S3989-93.
- Annamalai SK, Kapur NK. Contrast induced nephropathy after coronary or vascular intervention: More biomarkers than answers. Catheter Cardiovasc Interv. 2018;91:1192-3.
- Liao B, Nian W, Xi A, Zheng M. Evaluation of a diagnostic test of serum neutrophil gelatinase-associated lipocalin (NGAL) and urine KIM-1 in contrast-induced nephropathy (CIN). Med Sci Monit. 2019;25:565-70.
- Huelin P, Solà E, Elia C, Solé C, Risso A, Moreira R, et al. Neutrophil gelatinase-associated lipocalin for assessment of acute kidney injury in cirrhosis. A prospective study. Hepatology 2019;70(1):319-333.
- Soni SS, Cruz D, Bobek I, Chionh CY, Nalesso F, Lentini P, et al. NGAL: A biomarker of acute kidney injury and other systemic conditions. Int Urol Nephrol. 2010;42:141-50.
- Srisawat N, Laoveeravat P, Limphunudom P, Lumlertgul N, Peerapornratana S, Tiranathanagul K, et al. The effect of early renal replacement therapy guided by plasma neutrophil gelatinase associated lipocalin on outcome of acute kidney injury: A feasibility study. J Crit Care. 2018;43:36-41.


 Smitha S Vasavan* 1
Smitha S Vasavan* 1
 Vijeesh Vasavan 2
Vijeesh Vasavan 2




 10.5281/zenodo.13351255
10.5281/zenodo.13351255