Abstract
Sustained release tablets represent a vital advancement in pharmaceutical technology, providing a controlled and prolonged release of active pharmaceutical ingredients (APIs). This approach ensures steady therapeutic drug levels, reduced dosing frequency, and improved patient compliance, particularly for chronic conditions requiring long-term treatment. Formulating these tablets involves the strategic use of polymers, hydrophilic and hydrophobic matrix systems, and advanced manufacturing techniques such as direct compression and hot-melt extrusion. Evaluation focuses on critical quality attributes such as drug release rate, stability, and in vitro-in vivo correlation. This review discusses the principles of sustained release, mechanisms of drug release, formulation strategies, excipient selection, and evaluation methodologies, providing a comprehensive understanding of the design and development of sustained release tablets.
Keywords
Sustained release tablet, drug delivery, matrix systems, polymers, patient compliance, drug release, evaluation techniques, therapeutic efficacy
Introduction
Oral drug delivery remains the most preferred and convenient method for administering pharmaceuticals due to its ease of use, cost efficiency, and high patient compliance. Conventional tablets, despite their widespread use, often present challenges such as frequent dosing and fluctuating plasma drug concentrations, which can lead to reduced therapeutic efficacy and patient non-adherence. To address these issues, advanced drug delivery systems like sustained release tablets have been developed.
Sustained release tablets are designed to release the drug at a controlled and predictable rate over an extended period, maintaining consistent therapeutic drug levels and improving overall treatment outcomes. These systems reduce the need for frequent dosing, minimize side effects associated with peak drug concentrations, and enhance the convenience and compliance for patients managing chronic conditions like hypertension, diabetes, and arthritis.
Principles of Sustained Release Systems
The fundamental principle of sustained release drug delivery is to maintain a consistent plasma drug concentration within the therapeutic window over an extended period. Common drug release mechanisms include diffusion-controlled, erosion-controlled, and osmotic pressure systems.
Polymers play a critical role in controlling drug release. Hydrophilic polymers such as hydroxypropyl methylcellulose (HPMC) and hydrophobic polymers like ethyl cellulose are widely used.
Formulation Strategies
Matrix systems are simple and cost-effective formulations that incorporate hydrophilic or hydrophobic polymers to control drug release. Hydrophilic matrix systems use swellable polymers, while hydrophobic systems use insoluble materials to retard drug release.
Polymeric coatings on tablets or granules regulate drug release by creating a diffusion barrier. Osmotic systems rely on osmotic pressure to deliver drugs at a controlled rate. These systems are independent of pH and gastrointestinal motility.
Evaluation of Sustained Release Tablets
The performance of sustained release tablets is evaluated through various in vitro and in vivo methods to ensure safety, efficacy, and reproducibility.
In vitro evaluations include dissolution testing, drug content uniformity, and stability studies under accelerated conditions. In vivo evaluations involve pharmacokinetic studies and in vitro-in vivo correlation (IVIVC) to establish a link between in vitro release and in vivo performance.
Challenges and Future Perspectives
Despite their advantages, sustained release systems face challenges such as solubility issues, formulation complexity, and stringent regulatory requirements. Future directions include the use of biodegradable polymers, nanotechnology, and personalized medicine .
Sustained Release Tablet –

Process
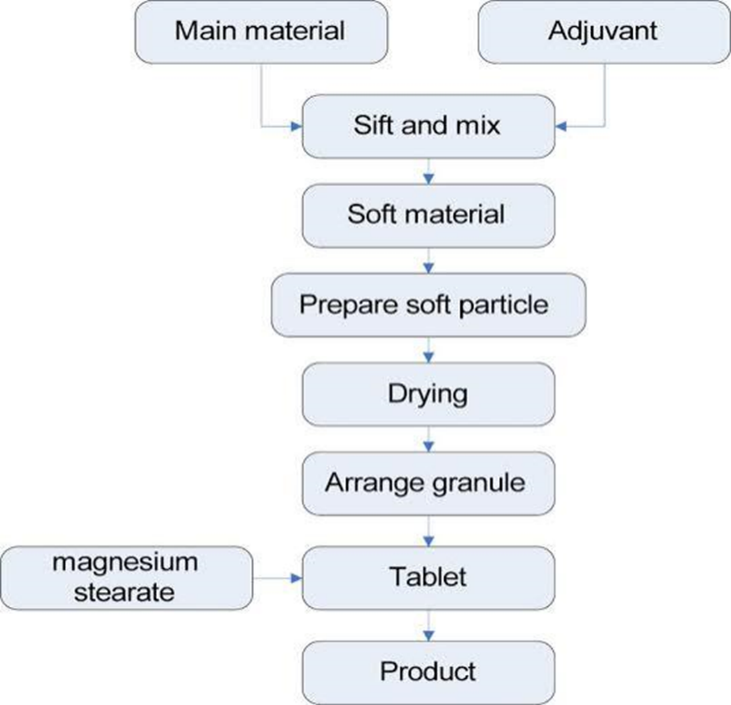
CONCLUSION
Sustained release tablets represent a significant advancement in oral drug delivery. By providing consistent drug levels and reducing dosing frequency, they improve therapeutic outcomes and patient adherence. Continued research in polymers, formulation technologies, and evaluation methods will drive innovation and expand the applicability of sustained release systems.
REFERENCES
- Aulton, M. E., & Taylor, K. (2017). Pharmaceutics: The Science of Dosage Form Design. Elsevier.
- Chien, Y. W. (2005). Novel Drug Delivery Systems. CRC Press.
- Siepmann, J., & Peppas, N. A. (2001). Modeling of drug release from delivery systems based on hydroxypropyl methylcellulose (HPMC). Advanced Drug Delivery Reviews, 48(2-3), 139-157.
- Banker, G. S., & Anderson, N. R. (2002). Tablet Formulation and Manufacturing. Marcel Dekker.
- Jain, N. K. (2008). Controlled and Novel Drug Delivery. CBS Publishers.
- Colombo, P., Bettini, R., & Santi, P. (2000). Oral controlled release systems for systemic delivery. Advanced Drug Delivery Reviews, 47(2-3), 229-247.
- Peppas, N. A., & Sahlin, J. J. (1989). A simple equation for the description of solute release. International Journal of Pharmaceutics, 57(1), 169-172.
- Costa, P., & Sousa Lobo, J. M. (2001). Modeling and comparison of dissolution profiles. European Journal of Pharmaceutical Sciences, 13(2), 123-133.
- Higuchi, T. (1963). Mechanism of sustained-action medication. Journal of Pharmaceutical Sciences, 52(11), 1145-1149.
- Alderman, D. A. (1984). A review of cellulose ethers in hydrophilic matrices for oral controlled-release dosage forms. International Journal of Pharmaceutics, 1(2), 1-21.


 Ghansham Pharande *
Ghansham Pharande *
 Someshwar More
Someshwar More
 Dr. V. M. satpute
Dr. V. M. satpute
 S. R. Ghodake
S. R. Ghodake


 10.5281/zenodo.14643179
10.5281/zenodo.14643179