Abstract
The hyphenated techniques refers to the combination or synthesis of distinct analytical methodologies. Spectroscopic techniques are mostly paired with chromatographic techniques. After that, an interphase will allow the mixture's separated components from the chromatographic approach to enter the spectroscopic technique. In GC-MS After separation from gas chromatography, ionization and mass spectrometry are added to MS. analysis and measurement of the mass-to-charge ratios of the ions that the mass spectrometer generates from each analysis. GC and MS can be connected by a membrane separator, jet/orifice separator, or effusion separator. The analytical flow cell in LC-NMR coupling was originally designed for continuous flow to NMR. But the requirement for thorough structural evaluation of novel products has resulted in the use of LCMS in stopped-flow mode. The use of LC-MS-MS is increasing continuously at a rapid rate. Hyphenated methods like UV-coupled HPLC. The combination of mass spectrometry (LC-UV-MS) with biological screening has proven to be quite beneficial for a quick analysis of products. Hyphenated procedures pertain to the process of separating, identifying, and the hyphenated procedures demonstrate improved sample analysis in terms of specificity, accuracy, and precision. (1).
Keywords
Liquid Chromatography, Gas Chromatography, Mass Spectroscopy, NMR, Accuracy, Precision.
Introduction
Hirsch Feld employed the term "hyphenation" in 1980 to refer to the potential fusion of two or more instrumental analytical techniques in a single run. In comparison to the results of using a single analytical approach, the coupling aims to provide a comprehensive detection for both identification and quantification. Pairing or coupling two distinct analytical approaches with the aid of an appropriate interface is known as a hyphenated methodology. Spectroscopic techniques are primarily paired with chromatographic procedures .The pure or almost pure portions of the chemical components in a combination were separated and Selective information for identification using standards or library spectra is produced by spectroscopy. The pairing hyphenated product as a result of the separation method and an online spectroscopic detection system. Hyphenated procedures consist of utilizing or coupling two distinct analytical techniques together with the aid of an appropriate interface. Hyphenated approaches include a number of methods, such as combining separation and strategies for separation, separation and identification.(2)
Advantages :
1. For fast and accurate analysis
2. A Higher degree of automation.
3. Higher sample throughput
4. Better reproducibility.
5. Reduction of contamination due to its closed system.
6. Separation of quantification at the same time. (3)
Types of hyphenated techniques :
1. Double hyphenated techniques.
2. Triple hyphenated techniques.
1. LC-MS :
Instrumentation and working
LC-MS is a chemistry technique that combines the physical separation of liquid chromatography (or HPLC) with the mass spectroscopy. A typical automated LC-MS system (Figure 3) consists of double three-way diverter inline with an autosampler, LC system, the Mass spectrometer. The diverter generally operates as an automatic switching valve to divert undesired portions of eluting from the LC system to waste before the sample enters the MS.(4) The ionization techniques used in LC-MS are generally soft techniques that mainly display the molecular ion species with only a few fragment ions. The information obtained from a single LC-MS run is not sufficient for confirmation of the identity of the compound. Nevertheless, the problem has now been solved by the introduction of tandem mass spectrometry (MS-MS), which provides fragments through collision-induced dissociation of the molecular ions produced. Use of LC-MS-MS is increasing speedily day by day. Hyphenated techniques such as HPLC coupled to UV and mass spectrometry (LC-UV-MS) have been proved to be extremely useful in combination with biological screening for a rapid survey of natural products. Nowadays, various types of LC-MS systems incorporating different types of interfaces are available commercially. The interfaces are designed in such a way that they offer adequate nebulization and vaporization of the liquid, ionization of the sample, removal of the excess solvent vapour, and extraction of the ions into the mass analyser. The two most widely used interfaces, especially in relation to natural product analysis, are electrospray ionization (ESI) and atmospheric pressure chemical ionization (APCI). The latter is considered as ''the chromatographer's LC-MS interface'' because of its high solvent flow rate capability, sensitivity, response linearity, and fields of applicability. With these interfaces, various types of analysers, e.g., quadruple, ion trap, or TOF, can be used. Each of these analyser’s however, offers a varying degree of mass accuracy and resolution. In the LC-UV-MS mode, thermospray (LC-TSP-MS) and continuous-flow FAB (LC-CF-FAB) interfaces can also be applied. For phytochemical analysis, the TSP has been found to be the most suitable interface as it allows introduction of aqueous phase into MS system at a flow rate (1-2 ml/min) compatible with that usually used in phytochemical analysis.(5)
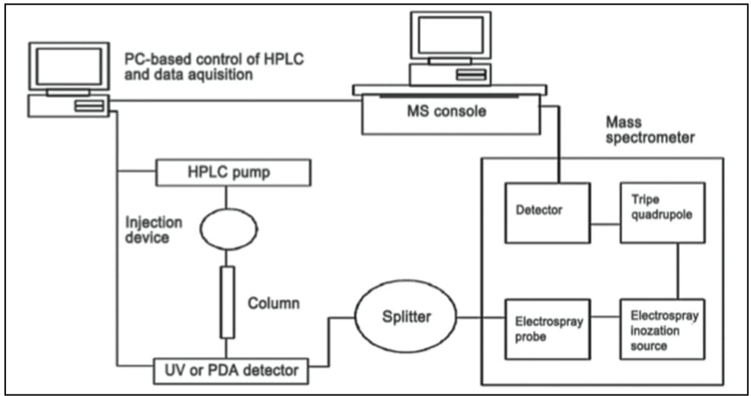
Fig. 1 LC MS
2. LC-NMR :
Instrumentation and working :
NMR is probably the least sensitive, and yet it provides the most useful structural 240 Sarker and Nahar information toward the structure elucidation of natural products. (12)Technological developments have allowed the direct parallel coupling of HPLC systems to NMR, giving rise to the new practical technique HPLC-NMR or LC-NMR, which has been widely known for more than last 15 years. The first on-line HPLC-NMR experiment using superconducting magnets was reported in the early 1980s. However, the use of this hyphenated technique in the analytical laboratories started in the latter part of the 1990s only. LC-NMR promises to be of great value in the analysis of complex mixtures of all types, particularly the analysis of natural products and drug-related metabolites in biofluids.
LC-NMR experiments can be performed in both continuous-flow and stop-flow modes. A wide range of bioanalytical problems can be addressed using 500, 600, and 800 MHz systems with 1H, 13C, 2H, 19F, and 31P probes. The main prerequisites for on-line LC-NMR, in addition to the NMR and HPLC instrumentation, are the continuous-flow probe and a valve installed before the probe for recording either continuous-flow or stoppedflow NMR spectra. A UV–vis detector is also used as a primary detector for LC operation. Magnetic field strengths higher than 9.4 T are recommended, i.e., the 1H resonance frequency of 400 MHz for a standard HPLC-NMR coupling. The analytical flow cell was initially constructed for continuous-flow NMR acquisition. However, the need for full structural assignment of unknown compounds, especially novel natural products, has led to the application in the stopped-flow mode.
In most of the LC-NMR operations, reversed-phase columns are used, employing a binary or tertiary solvent mixture with isocratic or gradient elution. The protons of the solvents of the mobile phase cause severe problems for obtaining an adequate NMR spectrum. The receiver of the NMR spectrometer is not quite able to handle the intense solvent signals and the weak substance signals at the same time. To overcome this problem, solvent signal suppression can be achieved by one of the three major methods: pre-saturation, soft-pulse multiple irradiations or water suppression enhancement through T1 effects (WET) pre-saturation employing a z-gradient.
Recent advances in both hardware and software for the direct coupling of LC and NMR have given a new life to this hyphenated technique. These developments include a new coil and flow cell design for high sensitivity, new RF system for multiple solvent suppression and improved dynamic range gradient elution capability, and automatic peak-picking/storing capabilities. As a result, this method is a powerful tool used in many areas such as natural products, organic molecules, biomolecules, drug impurities, by-products, reaction mixtures, and drug degradation products. The potential of HPLC-NMR for the investigation and structural elucidation of novel natural products has been enormously extended by the advent of powerful solvent suppression schemes, and their combination with a series of homo- and heteronuclear 2D NMR experiments such as 2D total correlation spectroscopy (TOCSY) or 2D nuclear Over Hauser enhancement spectroscopy (NOESY). LC-NMR, despite being known for about last two decades, has not quite become a widely accepted technique, mainly because of its lower level of sensitivity and higher cost compared to other available hyphenated techniques. However, the recent advances in technology, especially in relation to the developments in pulse field gradients and solvent suppressions methods, the improvement in probe technology, and the introduction of high-field magnets (800– 900 MHz) have offered new impetus to this technique. (6)
Fig.2 LC-NMR
3. LC-IR :
The hyphenated technique developed from the coupling of an LC and the detection method infrared spectrometry (IR) or FTIR is known as LC-IR or HPLC-IR (Figure 5). While HPLC is one of the most powerful separation techniques available today, the IR or FTIR is a useful spectroscopic technique for the identification of organic compounds, because in the mid-IR region the structures of organic compounds have many absorption bands that are characteristic of particular functionalities, e.g., -OH, -COOH, and so on. However, a combination of HPLC and IR is difficult and the progress in this hyphenated technique is extremely slow because the hyphenated technique's 237 absorption bands of the mobile phase solvent are so huge in the mid-IR region that they often obscure the small signal generated by the sample components. [7] Because FT-IR is an absorbance process, the geometry of the sample during the measurement process matters. For a fixed mass or volume of the analyte, reducing the diameter by a factor of two creates a deposit with four times the thickness and four times the optical density. Because the IR detector is total light limited, this deposit diameter reduction of two improves the signalto-noise ratio by four times. Therefore, to achieve a useful instrument that produces full mid-infrared spectra, the LC–IR hyphenation process must.
1. Remove the solvent without thermally damaging the analyses or overloading the vacuum system with diluent gas.
2. Have efficient transmission of analyses to the spectrometer.
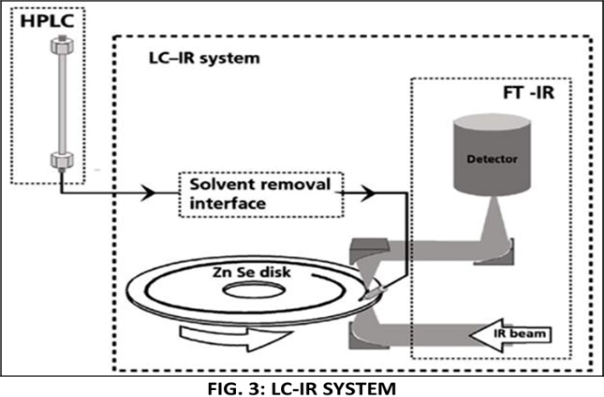
3. Present analyses to the FT-IR in a thick deposit.
4. Preserve the chromatographic resolution (8)
4. CE-MS
Instrumentation and working :
Capillary zone electrophoresis (CZE) is widely recognized as a powerful analytical technique in its own right, known for its high separation efficiency, short analysis times and low-volume sample requirements. These characteristics made CZE a popular method for the analysis of peptide mixtures, protein digests, drug substances and biotechnological products. The coupling of CZE with electrospray ionization mass spectrometry (ESI-MS). However, it can be said that the major advantage of such coupling is that the migration time is not the only parameter used for identifying the eluted components. These times are subjected to variations between runs, yet such variations become irrelevant when, in the same run, highly diagnostic mass spectra are obtained It goes without saying that every analytical technique has its limitations and CZE/MS is no exception. One of the main limitations of this experimental arrangement is its relatively poor sample concentration/ion sensitivity. Approaches to reduce such limitations included online preconcentration, sample stacking, and the increasing use of time-of-flight (TOF) analysers which use ESI and TOF analysers with and without a quadruple in between. The innovative feature of this class of instruments is their fast scanning, which allows the acquisition of a number of full spectra per second. Additionally, as all ions in each spectrum are sampled at the same moment in time, spectra are free of mass discrimination or peak skew typical of slow scanning systems that must scan over a narrow chromatographic/electro phonetic peaks. Capillary electrochromatography (CEC) is another technique which is currently undergoing a rapid phase of advancement and development. This technique was revived by Jorgenson and Lukas in 1981; these authors used 0.005 mol L1 phosphate buffer170 m packed column and 30 kV separation voltage to separate 9-methylanthracene and polyline. This technique has recently become more diffuse because of a number of advances in both CE instruments and detection techniques including electrospray Mass spectrometry. However, on-column UV detection and in-column laser-induced Fluorescence detection remain the most commonly used methods. Despite its high sensitivity, the latter method is subjected to interferences by buffer Fluorescence. (18) In MS detection, the column is commonly packed right up to the point where the sample is injected into the mass spectrometer. The combination of CEC with mass spectrometry provides reliable molecular weights and in many cases structural information, which makes it highly attractive for a wide range of applications. (9)

5. GC-FTIR :
Instrumentation and working :
For novel structures or new chemical entities, it is possible that no matched reference spectra can be found in MS databases. Manual interpretation of mass spectra requires sound knowledge on organic mass spectrometry and dedicated experience and often not possible to suggest any candidate structures. With the help of the molecular spectroscopy FT-IR, information on functional groups or structure moieties with specific infrared absorptions is complementary to MS and can be very valuable for structure elucidation, as already being used as stand-alone. FTIR coupling, the effluent from the GC flows through a heated transfer-line into the light pipe. A schematic drawing of a typical GC-FTIR is shown in Figure. The interferograms are scanned continuously to record either ‘on-the-fly’ gas-phase vapour IR spectra or trapped component spectra. In the earlier days of this technique, there were many discussions and studies about its sensitivity followed by the possible overloading of the columns when a capillary GC is used. Nowadays a GC-FTIR system having ng sensitivity of absolute substance amount has been introduced. GC-FTIR has been applied for analysis of polychlrorinateddibenzo-p-dioxins, dibenzofurans (Sommer et al., 1997), aromatic polymers (Oguchi et al., 1991),., respectively. The combination of a gas chromatograph with both FT-IR and MS detectors on one instrument allows the simultaneous measurement of one peak by two supplementary detections. In fact, at each retention time, two different chromatograms were obtained. The sample passes the IR detector without destruction and is registered by the subsequent MS detector. (10)

Fig 5. GC-FTIR
6. GC-MS :
As its name suggested, a GC-MS instrument is composed of at least the following two major building blocks: a gas chromatograph and a mass spectrometer. GC-MS separates chemical mixtures into individual components and identifies / quantifies the components at a molecular level (using a MS detector). It is one of the most accurate and efficient tools for analyzing volatile organic samples. The separation occurs in the gas chromatographic column (such as capillary) when vaporized analytes are carried through by the inert heated mobile phase (so-called carrier gas such as helium). The driven force for the separation is the distinguishable interactions of analytes with the stationary phaseand the mobile phase respectively. For gas-liquid chromatography, it depends on the column's dimensions (length, diameter, film thickness), type of carrier gas, column temperature as well as the properties of the stationary phase.
As the separated substances emerge from the column opening, they flow further into the MS through an interface. This is followed by ionization, mass-analysis and detection of mass-to charge ratios of ions generated from each analyte by the mass spectrometer. Dependent on the ionization modes, the ionization interface for GC-MS can not only ionize the analytes but also break them into ionized fragments, and also detect these fragments together with the molecular ions such as, in positive mode, radical cations using electron impact ionization (EI) or (de-)protonated molecules using chemical ionization (CI). All ions from an analyte together (molecular ions or fragment ions) form a fingerprint mass spectrum, which is unique for this analyte. The combination of the two essential components, gas chromatograph and mass spectrometer in a GC-MS, allows a much accurate chemical identification than either technique used separately. It is not easy to make an accurate identification of a particular molecule by gas chromatography or mass spectrometry alone, since they may require a very pure sample or standard. Sometimes two different analytes can also have a similar pattern of ionized fragments in their standard mass spectra. Combining the two processes reduces the possibility of identification error.(11)

Fig. 6 GC-MS
7. GC-NMR:
Nuclear Magnetic Resonance (NMR) (Rabi, 1938) spectroscopy is considered one of the most powerful analytical techniques for structural elucidation and identification of unknown compounds. It utilizes a physical phenomenon in which magnetic nuclei in a magnetic field absorb and re-emit electromagnetic radiation. Although the most commonly studied nuclei have specific chemical shifts, the abundances of the useful naturally occurring isotopes are generally low. That has significantly limited the sensitivity of NMR. Often mg of pure substance is required in order to obtain a meaningful spectrum. However, organic compounds of synthetic or natural products are often not in the pure form but are found as mixtures. The investigation on the coupling of GC and NMR has been very promising development because of the valuable information provided by NMR on molecular structure for each separated component.Unlike liquid or solid samples commonly analysed by NMR, the carrier gas in GC causes experimental difficulties in handling and results in low signal-to-noise ratio of the NMR signals obtained at atmospheric pressure. With the applications of Fourier-transform and averaging techniques, and gases can now be studied at fairly low pressures. With the aid of stronger magnets and the newly developed microprobes, the first online GC-NMR spectra was recorded recently.These experiments revealed the high potential of this technique, but it was also shown that the peaks in the GC separation elute up to 10 min, so that the advantage of the high separation performance of GC was lost in the experiment. The required amount for a GC-NMR run was between 1 and 2 mg for each analyte.Using stopped-flow measurements, very low sample amounts (~ 100-300 ?g at 400 MHz), the potential applications of the hyphenation of high performance capillary GC to microprobe 1H NMR detection with the help of a spectra database has been demonstrated and an identification of stereoisomers in a complex mixture has been achieved. Since the hyphenation of enantioselective capillary gas chromatography and mass spectrometry is not always sufficient to distinguish between structural isomers, thus requiring peak identification by NMR spectroscopy. NMR allows constitutional and configurational isomers (diastereomers and enantiomers) to be distinguished. Enantiomers display identical spectra at different retention times.(12)
8. GC-AAS :
GC-AAS is a combination of GC with AAS is a hyphenated technique, in which separation technique of GC is coupled with diverse selective and sensitive detection methods of AAS. GC performs the separation of the components and with the help of AAS the elemental identification of the component is performed. For the determination of specific organometallic compounds in various environmental samples, coupled gas chromatography-atomic absorption spectroscopy(GC-AAS) has proved to be a useful instrumental combination. It is widely used in the determination of arsenic, antimony, mercury, lead and thallium. In this technique coupling of a gas chromatography directly to the burner head of a conventional, commercially available atomic absorption is made. The controlling of the operating condition of a gas chromatograph with the AAS atomizer makes this technique useful since the analytes are already present in the gas phase. However , several conditions need to be optimized in order to obtain good sensitivity and selectivity for specific analytical problems. Efforts have been made GC-AAS technology can perform morphological analysis on complex mixtures containing metals and certain non-metal elements. Ø GC-AAS technology can directly introduce the gaseous components separated by gas chromatography and the carrier gas into the atomic spectrum for direct analysis and determination.to optimize the process by using the quartz furnace , heating with flame or a thermostat, or using the graphite furnace as the atomization device.(13)
Advantages:
- extremely low limits of detection and quantification,
2) insignificant influence of interferences on the determination process
3) very high precision and repeatability of determinations
9. HPLC-DAD :
The hyphenated HPLC-DAD-MS-SPE-NMR system comprises HPLC, UV-Vis, MS, SPE and NMR shown schematically in Fig. 1. The complex mixture of diverse constituents with variable concentrations is separated and isolated as individual components using HPLC columns; then the eluted components from the column are split into two streams using an adjustable high pressure stream splitter, for example, 95% of the eluants go to a DAD (UV-VIS) detector and 5% to the MS. The minor portion that departed to the MS agrees with its high sensitivity and this configuration allows MS to be operated at the higher ionization efficiency without com promising the NMR sensitivity. After the DAD detector, water is added to the eluant stream with a make-up pump, to decrease the organic solvent proportions of the elution and to promote method for analyzing such complex mixtures and hence is well suited for such a task. Many new compounds and even trace metabolites have been successfully discovered since the arrival of this technique. For example, recently, the chemical composition of rosemary[23] has been characterized using the combination of 2D NMR of the mixture and the HPLC-DAD-MS-SPE-NMR hyphenated technique.[29] A total of 33 metabolites including sugars, amino acids, organic acids, polyphenolic acids and diterpenes have been identified.(14)

Applications :
Plant derived medicine has gained increasing attention in the last few decades, and approximately 20% of the global population have become regular users. However, there have been many reports of different side effects of phytomedicines owing to active ingredients, contaminants and/or interactions with other drugs. Therefore, the safety of phytomedicines is of greater concern than ever by the commercial sectors, academic researchers and by regulatory authorities. Total or partial characterization of the chemical compositions of a phytomedicine is the first step towards management of safety and efficacy of a phytomedicine.
10. ICP-MS :
The most common set up for the analysis of metalloid species is the direct coupling of an HPLC to an ICPMS via small diameter PEEK tubing. The high sampling and data acquisition rates of the ICPMS allow adjacent chromatography peaks to be separated and quantified. However, typically only 1% of the sample is transferred into the ICPMS plasma. An example of this set up for measuring mercury species.The sensitivity of HPLC–ICPMS measurements can be substantially increased by the incorporation of a hydride generation system (HG; Fig. 3A). Many metalloid species form volatile hydrides e.g. AsH3, CH3AsH2, (CH3)2AsH etc. allowing 70–80% of the sample to be transferred to the ICPMS plasma. Hydride generation efficiency critically depends on the species being analyzed. For example, arsenobetaine and arsenocholine do not form hydride species, while arsenoribosides form volatile analytes but the efficiency is poor (~5%) unless the hydride generation system is specifically optimized for arsenoribosides (21–28%). This set up incorporates the trapping of the hydride forming metalloid species using a packed column and cooling with liquid nitrogen (Fig. 4A). After trapping, the column is heated and volatile species flushed into the ICPMS for analysis [37]. This set up is useful when extreme sensitivity is required and is particularly suitable for aqueous samples and extracts. A typical separation of germanium species using this system utilizing different column packings is shown in Figure.(15)

Fig. 10 ICP-MS
11. ICP-AAS / AFS:
The field of selenium speciation has been studied for decades and the growing interest in this field seems never to reach a plateau. Although powerful techniques based on mass spectrometry are nowadays used for selenium determination/speciation, few laboratories can support the high cost of such techniques. The hyphenation of chromatography to atomic absorption or atomic fluorescence spectrometry (AAS or AFS) is still a reliable and low-cost alternative for routine laboratories. In this work we present the most important parameters dealing with selenium speciation along with the latest trends in this subject, namely in the items related with sample treatment and hyphenation techniques with AAS and AFS detection. Research in Se speciation is still an attractive area since, on the one hand, lower detection limits can be achieved with recent powerful analytical tools, such as ICP–MS or LC–MS–MS and, on the other hand, there are several Se-containing organic molecules that remain unidentified. Although modern techniques using mass detection can help to a better understanding of the experimental data and to almost certain species identification, many analytical laboratories cannot support such equipment because of their high price and expensive maintenance. Hence, alternatives for Se speciation in routine laboratories are mandatory. The hyphenation of common techniques such as chromatography and atomic absorption or fluorescence spectrometry (AAS or AFS) is a substitute of mass spectrometry (MS) techniques of great interest.
CONCLUSION:
The remarkable improvements in hyphenated analytical methods over the last two decades have significantly broadened their applications in the analysis of compounds. In this article, recent advances in the applications of various hyphenated techniques, along with applications of hyphenated techniques in the different fields like Environment, Forensic, Pharmaceuticals, and Petrochemicals etc. are discussed with appropriate examples. Hyphenated techniques such as LC-MS, GC-MS, LC-NMR, CE-MS and ICP-MS have been developed to solve various complex analytical problems in different fields. These techniques solve such problems in time efficient manner. Sample requirement for hyphenated technique is less as compare to conventional techniques. (16)
REFERENCES
- World Journal of Pharmaceutical Research SJIF Impact Factor 5.045 Volume 4, Issue 2, 214-225. Review Article ISSN 2277– 7105.
- Applications of hyphenated LC-MS techniques in pharmaceutical analysis Joachim Ermer* and Martin Vogel Aventis Pharma Deutschland GmbH, Global Pharmaceutical Development Analytical Sciences, Frankfurt am Main, Germany.
- Christian W. Klampfl Institute of Analytical Chemistry, Johannes Kepler University Linz, Linz, Austria ,CE with MS detection: A rapidly developing hyphenated technique.
- L. C. Robosky,D. G. Robertson, J. D. Baker,S. Rane,M. D. Reily,Comb. Chem. High. Throughput Screen. 2002, 5, 651.
- Hyphenated Techniques- A Comprehensive Review S. Nagajyothi, Y. Swetha, J. Neeharika, P. V Suresh, N. Ramarao.
- HYPHENATED TECHNIQUES: AN OVERVIEW Sheetal V. Patil*, Dr.Shashikant D. Barhate Shree Sureshdada Jain Institute of Pharmaceutical Education and Research, Jamner.
- Overview of hyphenated techniques using an ICP-MS detector with an emphasis on extraction techniques for measurement of metalloids by HPLC–ICPMS William Maher a , Frank Krikowa a, Michael Ellwood b , Simon Foster a , RajaniJagtap a , George Raber c
- Hyphenated Techniques in Gas Chromatography XinghuaGuo* and Ernst Lankmayr Institute of Analytical Chemistry and Food Chemistry, Graz University of Technology, Austria
- 16. Pan, C. et al. The use of LC/MS, GC/ MS, and LC/NMR hyphenated techniques to identify a drug degradation product in pharmaceutical development. J. Phaemaceutical Biomed. Anal. 2019; 40: 581–590.
- Sakhreliya, B. D., Kansara, S. LC-NMR?: A powerful tool for analyzing and characterizing complex chemical mixtures without the need of chemical separation. J. Pharm. Sci. Biosci. Res. 2013; 3: 115-121.
- Hamid Khan, J. A. Identification of Impurities and Degradation Products in Pharmaceutical Products- Role of Hyphenated Techniques. Asian J. Res. Chem. 2017; 7: 31–35.
- Rabie S. Farag, S. R. A. A.-S. Confirmatory Method for Determination of 11-Nor- 9 - Tetrahydrocannabinol-9-Carboxylic Acid in Urine Samples Using Gas Chromatography – Mass Spectrometry (GC/MS). Asian J. Res. Chem. 2011; 4: 373–376.
- Pallavi S. Thombare, R. N. K. Review on Hyphenated Techniques. World J. Pharm. Res. 2020; 9: 370–377.
- Maher, W. et al. Overview of hyphenated techniques using an ICP-MS detector with an emphasis on extraction techniques for measurement of metalloids by HPLC–ICPMS. Microchem. J. 2012; 105: 15–31.
- Patel, K. N., Patel, J. K., Patel, M. P., Rajput, G. C. and Patel, H. A. Introduction to hyphenated techniques and their applications in pharmacy. Pharm. Methods. 2010;1: 2–13.
- Thamizhanban, D., Rani, T., Pravalika, P. A Review on Hyphenated Separation Techniques Used in Pharmaceutical Analysis. J. Pharm. Biol. Sci. 2016; 11: 65–74.


 Shounak R. Mande*
Shounak R. Mande*








 10.5281/zenodo.14762916
10.5281/zenodo.14762916