Abstract
Pharmacovigilance and Quality Assurance (QA) are essential fields in pharmaceuticals dedicated to maintaining drug safety, efficacy, and regulatory compliance throughout a product's lifecycle. Pharmacovigilance emphasizes continuous monitoring, identification, and management of adverse drug reactions (ADRs), incorporating processes such as signal detection, causality assessment, and risk management to safeguard patient safety. QA complements these efforts by enforcing high standards in drug development and manufacturing through Good Manufacturing Practices (GMP), consistent quality checks, and compliance with regulatory standards. Emerging technologies, including artificial intelligence, machine learning, and blockchain, is enhancing the efficiency of adverse event detection and data integrity, while global harmonization efforts seek to unify regulatory frameworks for greater consistency across regions. Together, these fields address the complexities of modern drug development, such as personalized medicine and biologic therapies, supporting proactive risk management and fostering public trust in pharmaceuticals. This review highlights the integrated role of pharmacovigilance and QA in promoting safe, effective, and high-quality drug use, with a forward-looking perspective on digital transformation and global collaboration in advancing patient care.
Keywords
Pharmacovigilance, Quality Assurance, ADR, GMP.
Introduction
Pharmacovigilance is the science of monitoring, detecting, and preventing adverse effects of pharmaceutical products [1, 2]. It involves collecting data from various sources, including pre-clinical studies, clinical trials, and post-marketing surveillance [1]. The process includes signal detection, evaluation, and taking action to manage risks [1]. Regulatory aspects and international collaboration are crucial in Pharmacovigilance [1, 3]. Ethical considerations and stakeholder perspectives are important factors [1]. Quality assurance in pharmaceuticals ensures products are safe and effective [4]. It involves following good manufacturing practices and implementing comprehensive inspection policies [4]. Product Quality Review is a mechanism to identify trends and support continuous improvement [4]. Both Pharmacovigilance and quality assurance are essential for maintaining public confidence and producing high-quality, safe pharmaceutical products [2, 4]. Pharmacovigilance plays a crucial role in healthcare by monitoring drug effects and ensuring patient safety [5, 6]. It involves collecting, detecting, and assessing adverse drug reactions (ADRs) to promote rational drug use and improve patient care [7]. The field has grown significantly, with India becoming the third-largest pharmaceutical producer globally [5]. However, challenges persist, including underreporting of ADRs and increased hospitalization rates due to adverse effects [7]. Pharmacovigilance encompasses various aspects, including good manufacturing practices, ICH guidelines, and ADR assessment scales [6, 7]. A robust Pharmacovigilance system is essential for identifying hazards quickly and ensuring drug safety [5]. The responsibility for effective Pharmacovigilance is shared among scientists, clinicians, pharmaceutical manufacturers, regulators, policymakers, and the public [8]. As the field continues to evolve, it remains vital in addressing the challenges posed by an expanding range of medicines. Pharmacovigilance, the science of drug safety monitoring, has evolved significantly over the past 170 years [9]. It emerged in response to safety concerns in the 1950s and was formalized in the 1962 Kefauver-Harris Amendments [10]. The scope of Pharmacovigilance has expanded to encompass various activities, including adverse event detection, signal management, benefit-risk assessment, and quality assurance in drug manufacturing [10]. The field continues to advance with the integration of new technologies like artificial intelligence and machine learning [10]. In Europe, the SCOPE Joint Action was initiated in 2013 to strengthen Pharmacovigilance capabilities across member states, focusing on areas such as adverse drug reaction collection, signal management, and risk communication [11]. This collaborative effort has produced guidance, training materials, and tools to support best practices in Pharmacovigilance for regulatory authorities and other stakeholders [11]. One of the earliest signs of a formal drug safety monitoring system was the formation of a committee by The Lancet, tasked with documenting anesthesia-related deaths in Britain and its colonies. This initiative was prompted by the 1848 death of a 15-year-old girl after receiving chloroform anesthesia during an ingrown toenail removal [12, 13]. In 1950, reports emerged in the United States linking chloramphenicol to cases of aplastic anemia [14]. Consequently, the American Medical Association’s Council on Drugs established a Blood Dyscrasia Registry [15]. By 1961, the FDA had begun systematically collecting adverse drug reaction (ADR) reports, particularly through the Hospital Reporting Program. A significant moment in drug safety monitoring was triggered by a letter from Dr. W.G. McBride, published in The Lancet which suggested a link between thalidomide and congenital malformations in newborns, acting as a major catalyst for improved safety oversight. Thalidomide was first synthesized in 1954 and introduced to the public in 1956, where it was widely prescribed as a seemingly safe remedy for morning sickness and nausea. However, on November 25, 1961, the manufacturer withdrew thalidomide from the market. It is estimated that between 6,000 and 12,000 children were born with severe congenital malformations due to maternal use of the drug [16]. By 1968, ten countries, including Australia, Canada, Czechoslovakia, Germany, the Netherlands, Ireland, New Zealand, Sweden, the United Kingdom, and the USA, established national drug monitoring centers and joined the World Health Organization’s (WHO) Pilot Research Project for International Drug Monitoring [15]. In 1972, a report was released that laid the foundation for the current global network of national centers working within the WHO program [16-18]. The creation of a pharmacovigilance system is crucial for supporting public health policies. In a study by Olsson et al., data on pharmacovigilance activities from 55 low- and middle-income countries showed that the information collected was used in different ways by these countries. This included supporting regulatory functions, advising consumer groups, and aiding in the development of essential medicine lists and drug therapy guidelines [19]. Pharmaceutical Quality Assurance ensures that quality standards are met for products or services within the pharmaceutical industry. Its purpose is to build and sustain customer trust in the product by detecting or preventing defects early on. In the constantly changing field of Pharmacovigilance, Quality Assurance serves as the foundation for ensuring drug safety and efficacy [19, 20]. It plays a critical role, making it essential in the pursuit of excellence within the pharmaceutical sector. Quality Assurance professionals serve as vigilant overseers of pharmaceutical safety and effectiveness. Their responsibilities span from drug development through post-market surveillance, ensuring that each stage adheres to strict quality standards. They implement strong quality control systems and protocols to prevent deviations that could affect patient safety. Quality Assurance and pharmacovigilance work hand-in-hand to protect patients; while pharmacovigilance centers on the monitoring and reporting of adverse events, Quality Assurance focuses on reducing the occurrence of these events through preventive actions [20].
The aim of this review is to provide a comprehensive analysis of the interconnected roles of Pharmacovigilance and Pharmaceutical Quality Assurance in ensuring drug safety, efficacy, and patient well-being. The objective is to explore the evolution and significance of Pharmacovigilance as a scientific discipline that monitors, detects, and prevents adverse drug reactions (ADRs), highlighting its crucial role in public health policy and regulatory frameworks. Additionally, the review will examine the role of Quality Assurance in the pharmaceutical industry, focusing on its contribution to maintaining high standards of product safety and effectiveness by implementing good manufacturing practices and proactive quality control measures. By synthesizing historical milestones, current practices, international collaborations, and challenges in both fields, this review aims to provide a holistic understanding of how these disciplines complement each other to safeguard public health. Furthermore, it will analyze the impact of advancements in technology, such as artificial intelligence, on improving pharmacovigilance practices, while also addressing the ethical considerations and stakeholder perspectives essential for continuous improvement in pharmaceutical safety systems.
Pharmacovigilance: A Quality Assurance Perspective
Pharmacovigilance plays a critical role in quality assurance within the pharmaceutical industry by systematically monitoring, evaluating, and preventing adverse effects associated with drug use. It encompasses the collection and analysis of data regarding the safety and efficacy of medications, ensuring that any potential risks are identified and communicated effectively to healthcare professionals and patients. By integrating pharmacovigilance practices into the drug development lifecycle, companies can enhance the overall safety profile of their products, leading to improved patient outcomes and greater public trust. Furthermore, a robust pharmacovigilance system supports compliance with regulatory requirements, thereby mitigating legal risks and fostering a culture of continuous improvement in product quality and patient safety [17].
Market Trends and Patients Expectations
The pharmaceutical industry is undergoing significant change, driven by multiple factors that require a more proactive and flexible approach to drug safety. As we delve into the evolving landscape of pharmacovigilance, it's crucial to understand the underlying dynamics. There's a growing emphasis on personalized medicine, with patients increasingly expecting treatments customized to their individual genetic profiles and medical histories. Market trends indicate a rise in biologics, gene therapies, and precision medicines, which add new complexities to monitoring drug safety. Additionally, patients now have access to extensive medical information, raising their expectations for both the safety and effectiveness of treatments [18].
Complexity in drug development
Drug development has become increasingly complex, driven by advancements in biotechnology, genomics, and artificial intelligence. While these innovations offer immense potential, they also introduce new challenges. The emergence of therapies for rare diseases and orphan drugs requires tailored pharmacovigilance strategies, as these treatments are designed for smaller, more specific patient groups. Additionally, the use of combination therapies and polypharmacy complicates safety evaluations, making it crucial to closely monitor for potential drug interactions [18].
Adaptation and Innovations
To succeed in this rapidly changing environment, pharmacovigilance must evolve and embrace innovation. Traditional post-market surveillance methods are no longer enough to guarantee drug safety. Real-world evidence (RWE) and data analytics are becoming essential for assessing the safety and effectiveness of drugs across varied patient groups. By integrating pharmacovigilance with digital health technologies, such as wearables and telemedicine, real-time monitoring and early detection of adverse events become possible. In this transformative era, pharmacovigilance professionals need to be agile, responsive, and forward-thinking. The challenges posed by shifting market trends, complex drug development, and rising patient expectations call for proactive approaches and a dedication to leading in safety and quality assurance. As we explore further in this blog, we'll examine the critical role of Quality Assurance in addressing these changes [19].
Crucial Role of Quality Assurance
In the constantly changing field of pharmacovigilance, Quality Assurance serves as the foundation for ensuring drug safety and effectiveness. Let's explore the crucial role Quality Assurance plays and why it is essential in achieving excellence in the pharmaceutical industry [19].
Guide for Safety and Efficacy
Quality Assurance professionals are the diligent protectors of pharmaceutical safety and effectiveness. Their responsibilities span from drug development to post-market monitoring, ensuring that each stage adheres to strict quality standards. They implement strong quality control systems and protocols, preventing any deviations that might jeopardize patient health [20].
Building Trust and Alignment with Pharmacovigilance
Quality Assurance extends beyond technical precision; it cultivates trust among patients, healthcare providers, and regulatory authorities. Consistently delivering safe and effective products builds this trust, helping to protect a company’s reputation from harm. Regulatory bodies like the FDA and EMA depend on the strength of Quality Assurance systems to ensure compliance with strict standards. This trust facilitates smoother approvals and minimizes the risk of penalties. Quality Assurance is more than just a function—it is a core philosophy woven into the essence of pharmaceutical excellence. As we explore the complexities of pharmacovigilance in its evolving landscape, it’s important to recognize that the diligent efforts of Quality Assurance professionals form the cornerstone of patient safety and the pharmaceutical industry’s reputation. Quality Assurance and pharmacovigilance work hand in hand in the shared goal of patient safety. While pharmacovigilance is dedicated to the systematic monitoring and reporting of adverse events, Quality Assurance focuses on minimizing these events through preventive measures. By continuously improving processes, identifying potential risks, and addressing issues before they arise, Quality Assurance aligns closely with pharmacovigilance efforts [20].
Navigating Regulatory Challenges
In the constantly changing pharmaceutical industry, regulatory requirements play a critical role in determining the success of drug development and safeguarding patient safety. Let's examine the challenges pharmaceutical companies face in keeping up with these evolving regulations and discuss strategies for navigating this complex regulatory environment while mitigating concerns such as penalties and potential reputational harm [20].
Shift of Regulatory Landscape
The pharmaceutical industry functions within a constantly shifting regulatory landscape. Regulatory changes, both at the national and international levels, demand ongoing attention and flexibility. Keeping up with updates like the ICH E6 (R3) guideline for clinical trials or the FDA’s Drug Competition Action Plan is essential for maintaining compliance [20].
Compliance Strategy and Reputational Damage
Compliance starts with a proactive approach to Quality Assurance, which includes thorough documentation, strict adherence to standard operating procedures, and comprehensive training programs. Utilizing Quality Management Systems (QMS) software and Electronic Document Management Systems (EDMS) helps streamline compliance efforts, ensuring that documentation is precise, easily accessible, and ready for audits. Quality professionals’ concerns about regulatory penalties or reputational damage are valid, as non-compliance can lead to hefty fines, product recalls, and public criticism. Quality Assurance acts as a safeguard against these risks. By consistently meeting and exceeding regulatory requirements, companies can minimize the likelihood of compliance violations and associated penalties [20].
Transparency and Accountability
Regulatory compliance is more than just a formality; it represents a commitment to transparency and accountability. Companies must be ready to provide thorough documentation and demonstrate due diligence during audits and inspections. Utilizing audit management software can streamline the audit process, allowing for prompt and efficient responses to regulatory inquiries. Addressing regulatory challenges in the pharmaceutical industry requires a comprehensive approach that includes proactive Quality Assurance, the adoption of technology, and cultivating a culture of compliance from the ground up. By taking these steps, pharmaceutical companies can not only adhere to changing regulatory standards but also alleviate concerns associated with non-compliance, thereby ensuring patient safety and maintaining their reputations [19, 20].
Leverage Technology for Efficiency
In an age characterized by swift advancements, technology serves as a powerful ally in achieving efficient and effective pharmacovigilance. Let’s examine how technology such as Quality Management Systems (QMS), electronic document management, and other tools enhances pharmacovigilance processes. We’ll discuss the numerous advantages of automation and data-driven decision-making, as well as practical tips for effectively implementing technology solutions [20].
Quality Assurance with Technology
Quality Management Systems (QMS) software is essential for ensuring Quality Assurance. It provides a centralized platform for overseeing and managing quality control processes, including document control and corrective and preventive actions (CAPA). Electronic Document Management Systems (EDMS) facilitate documentation, ensuring that it is easily accessible and ready for audits. These systems guarantee that important documents are kept current and maintained in real time [20].
Automation Benefits
Automation alleviates the workload of manual tasks, enabling Quality Assurance professionals to concentrate on more essential activities. By automating routine processes like data entry, reporting, and compliance checks, it enhances efficiency and reduces the likelihood of human error [20].
Data and Technology
Technology offers access to extensive data, enabling data-driven decision-making in pharmacovigilance. Analytical tools can uncover trends, patterns, and potential safety signals, allowing for the early detection of adverse events and proactive risk management. When implementing technology solutions, it's essential to begin with a clear understanding of your organization’s specific needs and challenges. Invest in user training to ensure your team can fully utilize the technology. Additionally, regularly update and maintain your technology stack to keep pace with evolving requirements and emerging threats. In today’s dynamic landscape, leveraging technology for efficiency in pharmacovigilance is not merely an option but a necessity. Quality Management Systems, electronic document management, and data-driven decision-making equip Quality Assurance professionals to maintain the highest standards of drug safety and regulatory compliance. By adopting these technological advancements and applying them effectively, pharmaceutical companies can navigate the complexities of pharmacovigilance with greater accuracy and assurance [18-20].
Mitigates Risk and Ensure Transparency
In the field of pharmacovigilance, risk mitigation strategies act as a protective barrier against potential harm, while transparency and accountability establish the basis for trust. Let’s examine the vital significance of risk mitigation, transparency, and accountability, and provide practical tips for maintaining a strong quality control system while effectively managing costs [20].
Risk, Transparency and Accountability
Risk mitigation is fundamental to pharmacovigilance. It entails recognizing potential risks and proactively implementing measures to prevent quality incidents and adverse events. Strategies can include strong quality control practices, real-time monitoring, and continuous risk assessments. For instance, using Failure Mode and Effects Analysis (FMEA) can help pinpoint and prioritize potential risks. Transparency in quality control is more than just a trendy term; it signifies a commitment to clear and honest communication regarding the safety and effectiveness of pharmaceutical products. Accountability involves taking responsibility for any deviations or quality incidents and addressing them swiftly and decisively [20].
Cost-effective Quality Control
Balancing quality control with cost efficiency poses a challenge for pharmaceutical companies. Here are some practical suggestions:
- Invest in preventive measures: It is often more cost-effective to implement strong quality control systems from the beginning rather than dealing with the fallout from a quality incident.
- Leverage technology: Quality Management Systems (QMS) and Electronic Document Management Systems (EDMS) can optimize processes and lower operational costs.
- Continuous improvement: Foster a culture of continuous improvement to uncover opportunities for cost savings while sustaining and enhancing quality.
By adopting risk mitigation strategies, ensuring transparency, and taking responsibility for quality control, pharmaceutical companies can minimize the likelihood of quality incidents, regulatory fines, and damage to their reputations. Although cost efficiency is an important consideration, it should never compromise the safety and effectiveness of pharmaceutical products. With a balanced approach, the industry can continue to deliver high-quality, life-saving medications to patients around the globe [18-20].
Objectives of Pharmacovigilance and Quality Assurance
Effective drug safety monitoring involves a continuous process of identifying, assessing, and managing adverse drug reactions (ADRs) to ensure patient safety. This includes evaluating the risk-to-benefit ratio of medications, systematically gathering data on potential adverse effects, and implementing risk mitigation strategies. Ongoing education for healthcare professionals and patients is vital to promote the safe use of medications, alongside effective communication and compliance with regulatory standards. Post-marketing surveillance further ensures that any rare or long-term adverse effects are detected and addressed, fostering informed decision-making in medication use as shown in Figure 1 [21].
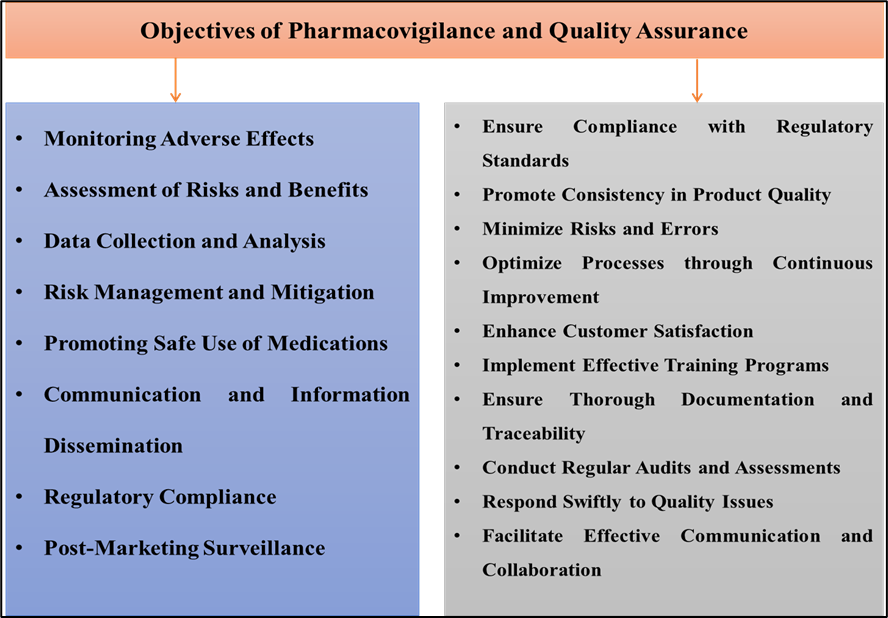
Figure 1: Objectives of Pharmacovigilance and Quality Assurance
1. Monitoring Adverse Effects
Continuous monitoring of adverse drug reactions (ADRs) is at the core of drug safety. ADRs refer to any harmful or unintended reactions that occur when a drug is administered correctly. The importance of surveillance lies in its role in identifying and evaluating these adverse reactions early in medication use. This process helps detect patterns or unexpected side effects that could potentially compromise patient safety. By continuously monitoring ADRs, healthcare providers and regulatory bodies can quickly recognize emerging safety concerns, which, in turn, allows them to make timely and appropriate interventions, whether through updating drug guidelines, adjusting recommended dosages, or enhancing monitoring for certain patient populations [21].
2. Assessment of Risks and Benefits
Evaluating the risk-to-benefit ratio of medications is essential to ensure that a drug’s therapeutic benefits outweigh any potential risks or adverse effects. Risk-benefit analysis is particularly critical during clinical trials but continues to be relevant post-approval, especially as new patient populations use the medication. This assessment involves determining the likelihood of adverse effects relative to the drug’s intended therapeutic impact. For example, a life-saving medication may be deemed acceptable despite having potential side effects, as its benefits could outweigh its risks. However, for drugs with moderate or minimal therapeutic effects, a higher tolerance for adverse effects may not be justifiable. This thorough examination helps healthcare professionals make well-informed choices and tailor treatment plans that maximize safety and efficacy for individual patients [21].
3. Data Collection and Analysis
To effectively monitor drug safety, it is crucial to collect and analyze information from diverse sources. This data includes reports from healthcare providers, patients, and findings from clinical trials. By systematically gathering real-world information, pharmacovigilance teams can better understand the range and frequency of adverse effects associated with specific medications. Once collected, this data undergoes rigorous analysis, which helps identify trends, potential risk factors, and mechanisms underlying adverse reactions. Analyzing these patterns allows for a more comprehensive understanding of how the drug behaves in different populations, which is vital for optimizing drug safety. Furthermore, this data provides valuable insights that can guide future research and contribute to safer medication development [21].
4. Risk Management and Mitigation
Risk management involves developing strategies to minimize or manage risks associated with medications. Once adverse effects are identified, steps must be taken to mitigate these risks. This may include updating the medication’s labeling to reflect new information about side effects, contraindications, or specific populations at higher risk. Additionally, risk minimization plans may be implemented, such as restricted distribution programs, dosage adjustments, or additional monitoring requirements. In some cases, if a medication’s risks are deemed too high, it may even be withdrawn from the market. Risk management is a dynamic process that requires close collaboration between regulatory agencies, healthcare providers, and pharmaceutical companies to ensure that any identified risks are effectively managed and that patient safety remains a top priority [21].
- Promoting Safe Use of Medications
Education plays a pivotal role in drug safety, as both healthcare providers and patients must understand how to use medications safely and effectively. This includes guidance on appropriate dosing, administration methods, and monitoring for potential side effects. Healthcare professionals are responsible for communicating these guidelines clearly and ensuring that patients are informed about the importance of adhering to the prescribed treatment plan. Patient education is particularly important for self-administered medications, where improper use can lead to increased risks of adverse effects. By promoting safe medication use, healthcare providers help minimize the risk of avoidable complications and encourage patients to take an active role in their treatment and safety [21].
6. Communication and Information Dissemination
Clear and effective communication of drug safety information is essential to keep healthcare providers, regulatory agencies, and the public informed. Pharmacovigilance systems facilitate the dissemination of safety information through various channels, including official publications, alerts, and digital platforms. Regular updates on drug safety profiles, newly identified risks, or changes in usage guidelines are essential for informed decision-making. For example, if a new adverse effect is discovered, healthcare providers need immediate access to this information to modify treatment plans as needed. Additionally, transparency in drug safety communication builds trust and helps maintain public confidence in healthcare systems and regulatory bodies [22].
7. Regulatory Compliance
Regulatory compliance ensures that pharmaceutical companies and healthcare providers adhere to the legal requirements governing drug safety monitoring and reporting. Different countries have specific regulations that mandate the reporting of ADRs and require pharmaceutical companies to maintain and submit post-market surveillance data. Compliance with these regulations helps standardize pharmacovigilance practices across different regions, making it easier to compare safety data and coordinate global efforts to protect patient safety. Moreover, regulatory agencies such as the U.S. Food and Drug Administration (FDA) and the European Medicines Agency (EMA) play an active role in overseeing drug safety and enforcing compliance to maintain the integrity of the healthcare system [22].
8. Post-Marketing Surveillance
Post-marketing surveillance is an essential component of pharmacovigilance, as it involves monitoring drug safety after a medication is approved and made available to the public. This phase is particularly important because clinical trials may not fully capture rare or long-term adverse effects due to limited sample sizes and shorter study durations. Once the medication is widely used in diverse populations, real-world data can reveal adverse effects that were not evident during clinical trials. Post-marketing surveillance allows regulatory agencies to continue monitoring the safety of approved medications, ensuring that any newly discovered risks are promptly addressed. This process ultimately supports informed decision-making by healthcare providers and helps build confidence in the safety and effectiveness of medications in real-world settings [22]. Quality Assurance (QA) in healthcare and pharmaceutical fields focuses on maintaining high standards to ensure the safety, efficacy, and reliability of products and services as shown in Figure 1. Below are objectives commonly associated with a robust quality assurance framework in Pharmaceuticals:
1. Ensure Compliance with Regulatory Standards: Adhere to all applicable regulatory and legal standards for safety, efficacy, and quality as mandated by authorities such as the FDA, EMA, or WHO.
2. Promote Consistency in Product Quality: Establish and maintain uniform procedures to ensure that all products meet quality standards across production batches, minimizing variability and ensuring reliability.
3. Minimize Risks and Errors: Identify, evaluate, and reduce potential risks or errors in processes, materials, and procedures to enhance product and service safety for end-users.
4. Optimize Processes through Continuous Improvement: Regularly assess and refine production, testing, and documentation processes to achieve ongoing improvement and efficiency.
5. Enhance Customer Satisfaction: Ensure products and services meet or exceed customer expectations for quality, safety, and performance, contributing to overall satisfaction and trust.
6. Implement Effective Training Programs: Provide comprehensive training for all staff to ensure understanding and adherence to quality standards and procedures, fostering a culture of quality.
7. Ensure Thorough Documentation and Traceability: Maintain detailed records for all processes, enabling traceability and accountability for each stage of production and quality control.
8. Conduct Regular Audits and Assessments: Perform internal and external audits to evaluate compliance with quality standards and identify areas for improvement.
9. Respond Swiftly to Quality Issues: Establish protocols for identifying, reporting, and resolving quality issues promptly to prevent reoccurrence and maintain product integrity.
10. Facilitate Effective Communication and Collaboration: Promote clear communication and collaboration across all departments involved in the quality process to ensure alignment with quality objectives [23-25].
Functions of Pharmacovigilance and Quality Assurance
Pharmacovigilance is a vital discipline in healthcare focused on the detection, assessment, understanding, and prevention of adverse effects or any other drug-related problems. It ensures that medications remain safe and effective for patients by monitoring adverse drug reactions (ADRs), documenting them thoroughly, and reporting significant findings to regulatory authorities. These functions collectively help build a safer healthcare environment, inform medical practices, and support regulatory actions as needed. The primary functions of Pharmacovigilance identifying, documenting, and reporting ADRs—form the foundation of this field. Here’s a detailed explanation of each of these core functions and their significance in maintaining drug safety [25].
1. Identifying Adverse Drug Reactions (ADRs)
The first step in pharmacovigilance is identifying adverse drug reactions, which refers to the unexpected and harmful effects that may arise when patients use a medication. ADR identification is not limited to initial clinical trials; it is an ongoing process that continues long after a drug enters the market. This ongoing vigilance is essential because not all ADRs can be identified during clinical trials, as these studies often have controlled environments, limited sample sizes, and shorter durations. Consequently, rare or long-term side effects may only become apparent once the drug is used by a larger and more diverse population. Identifying ADRs is a meticulous process that often involves healthcare providers, patients, and regulatory agencies. Healthcare providers play a critical role by observing and recording any unusual symptoms or health changes in patients taking medications. These observations are then shared with pharmacovigilance teams, who further assess the information to establish a potential link between the drug and the adverse reaction. A key aspect of this identification process is causality assessment, which determines the likelihood that a drug caused the observed reaction. Causality assessment involves evaluating the timing of the reaction (temporal relationship), ruling out other possible causes, and analyzing previous data on the drug’s safety profile. The World Health Organization (WHO) provides a widely used causality assessment tool that categorizes ADRs as “certain,” “probable,” “possible,” or “unlikely.” These categories help pharmacovigilance professionals understand the degree of association between the drug and the adverse effect, which in turn guides further actions. Establishing causality is crucial because it ensures that any interventions, warnings, or regulatory actions are based on credible evidence. By accurately identifying ADRs and establishing causality, pharmacovigilance teams can take proactive steps to safeguard patients, whether through adjusting dosage guidelines, issuing warnings, or in extreme cases, recommending the withdrawal of a drug from the market. This function of identifying ADRs is fundamental to maintaining the overall safety of medications and protecting public health [25, 26].
2. Documentation of Adverse Drug Reactions
Documentation is the second essential function of pharmacovigilance, and it serves as the backbone of the entire process. Accurate and detailed documentation of ADRs is necessary for effective monitoring, assessment, and subsequent decision-making. When healthcare providers or patients report an ADR, pharmacovigilance professionals record essential details about the incident. This documentation typically includes patient demographics (age, gender, weight), medical history, and information on the drug involved (dose, route of administration, duration of use). Additionally, it includes a description of the adverse reaction, the timing of its onset, and any subsequent medical interventions taken to address the reaction. The quality of documentation has a direct impact on the utility of pharmacovigilance data. Comprehensive records help create a clear picture of the circumstances surrounding each ADR, which is critical for assessing patterns and trends. For instance, if a particular adverse reaction is more common among patients with certain medical histories or demographic profiles, these insights can inform risk management strategies and guide healthcare providers in making informed prescribing decisions. Additionally, well-documented cases allow researchers to analyze data from multiple sources and identify potential risk factors that may not have been apparent in individual reports. Documentation also plays a crucial role in international pharmacovigilance. Adverse reaction data is often shared across countries and health organizations, making it possible to identify global trends and enhance drug safety on an international scale. For example, if multiple countries report similar ADRs for a specific drug, this could prompt regulatory agencies to issue warnings or conduct further studies. Clear and accurate documentation is essential for enabling this level of collaboration and ensuring that healthcare providers worldwide can make safe choices for their patients. Moreover, documentation is a legal requirement in many regions, as regulatory agencies mandate that pharmaceutical companies and healthcare providers report ADRs in a standardized format. This documentation not only supports drug safety efforts but also ensures accountability and traceability in the event of a safety review or legal inquiry. Through comprehensive documentation, pharmacovigilance teams can maintain reliable records that uphold the integrity of drug safety monitoring [26, 27].
3. Reporting Adverse Drug Reactions
Reporting adverse drug reactions is the third core function of pharmacovigilance, and it is instrumental in ensuring that regulatory authorities and healthcare organizations are informed about emerging safety issues. Reporting typically involves submitting ADR data to local pharmacovigilance centers, national regulatory agencies, or international bodies such as the WHO. These reports contribute to a centralized database where safety information from multiple sources is aggregated and analyzed. This broader data pool helps identify trends, detect rare adverse effects, and ultimately strengthen global drug safety.
Timely and accurate reporting is particularly important for serious ADRs, which can have life-threatening consequences or result in hospitalization. Such reactions require immediate attention and intervention, as they may necessitate a review of prescribing guidelines or even prompt a temporary suspension of the drug. Prompt reporting enables regulatory agencies to respond swiftly, helping to prevent further harm and protect public health. Reporting also allows pharmaceutical companies to review and revise their risk management plans, ensuring that appropriate safety measures are in place for high-risk medications. In addition to serious ADRs, reports of less severe or unexpected adverse reactions are also valuable. While individual cases of mild side effects may not appear concerning, they can signal potential issues when viewed collectively. Over time, these cumulative reports allow pharmacovigilance professionals to detect patterns that might indicate a higher-than-expected risk associated with the drug. These findings may lead to regulatory actions such as updating drug labels with new safety information or issuing special warnings for specific patient populations.
The reporting process is not only valuable for healthcare professionals but also empowers patients to play a role in pharmacovigilance. Many pharmacovigilance systems, such as the FDA’s MedWatch program, allow patients to report ADRs directly. Patient-reported outcomes provide valuable real-world insights, especially regarding side effects that may impact quality of life but are underreported by healthcare providers. This inclusive approach helps create a more comprehensive understanding of a drug’s safety profile. Through reporting, pharmacovigilance centers maintain an up-to-date database that informs regulatory policies, clinical practices, and pharmaceutical industry standards. This centralized information-sharing supports proactive decision-making and allows pharmacovigilance professionals to communicate risks effectively to the public, healthcare providers, and pharmaceutical companies [27, 28]. Quality Assurance (QA) in the pharmaceutical industry is essential to ensure that all products meet high standards of safety, efficacy, and reliability before reaching patients. One of the core functions of QA is compliance with regulatory standards, such as those set by the FDA, EMA, or WHO. These standards mandate rigorous guidelines for every stage of drug development and manufacturing, from raw material sourcing to final product release. QA teams develop standard operating procedures (SOPs) that outline each process step-by-step, ensuring that every batch produced is consistent, effective, and safe for consumption. In addition, QA ensures that all products undergo regular, standardized testing throughout manufacturing to detect any deviations or potential quality issues early on, allowing for corrective actions before products enter the market. This compliance safeguards patients and ensures that pharmaceutical companies maintain high standards in product development, gaining public and regulatory trust [29]. Another critical function of QA in pharmaceuticals is risk management and continuous improvement. By monitoring production, testing, and documentation practices, QA teams can identify potential risks or recurring issues in manufacturing processes, allowing them to implement corrective and preventive actions. Regular audits—both internal and external—are conducted to review quality systems and identify any gaps in compliance. Through these audits, QA can improve product consistency and safety, and enhance process efficiency by eliminating redundant or outdated steps. QA teams also focus on data integrity, making sure all data related to quality testing is accurate, complete, and available for traceability. This commitment to continuous improvement supports a culture of quality within pharmaceutical organizations, reinforcing safe manufacturing practices that reduce the likelihood of adverse events. Ultimately, QA functions as a cornerstone for safeguarding public health, while also ensuring that the pharmaceutical industry maintains ethical and consistent practices aligned with global standards [30]. Pharmacovigilance and Quality Assurance (QA) are essential functions in pharmaceuticals, working together to ensure drug safety, efficacy, and compliance with regulatory standards. Pharmacovigilance focuses on monitoring, identifying, documenting, and reporting adverse drug reactions (ADRs) and potential drug-related issues that may emerge after a medication enters the market, allowing for the assessment and management of risks to patient safety. Simultaneously, QA ensures that all stages of drug development, from raw materials to finished products, adhere to strict regulatory requirements, emphasizing consistency, safety, and efficacy across batches. QA teams establish standard operating procedures (SOPs) and conduct regular audits to maintain compliance, while pharmacovigilance reports ADR data to regulatory bodies, contributing to ongoing surveillance and patient protection. Together, these functions promote a culture of safety and reliability, ensuring that pharmaceuticals remain effective and safe for patients worldwide [29, 30].
Future Perspectives
The future of pharmacovigilance and quality assurance in pharmaceuticals holds promising advancements aimed at enhancing drug safety, efficiency, and regulatory compliance. With the integration of artificial intelligence (AI) and machine learning technologies, pharmacovigilance systems are evolving to process vast amounts of real-world data from electronic health records, social media, and adverse event databases more efficiently, enabling faster detection and response to safety concerns. These technologies can predict adverse drug reactions (ADRs) with higher precision and help regulatory bodies and healthcare providers make timely, data-driven decisions. Similarly, in quality assurance, digital technologies such as blockchain and cloud-based platforms are transforming data integrity, traceability, and transparency, fostering stronger collaboration between pharmaceutical companies, regulatory agencies, and healthcare professionals. Another key future perspective involves global harmonization of pharmacovigilance and quality assurance practices. As pharmaceutical markets and supply chains become increasingly global, there is a need for standardized regulatory frameworks that ensure consistent quality and safety across borders. Initiatives led by international organizations, such as the International Council for Harmonization (ICH), aim to streamline guidelines, improve risk management practices, and enable more efficient sharing of pharmacovigilance data across countries. Furthermore, with the growing importance of patient-centered healthcare, future pharmacovigilance and QA systems are expected to incorporate patient-reported outcomes more extensively, providing valuable insights into the real-world impact of drugs. Emphasizing patient engagement, digital innovations, and international collaboration will play an essential role in advancing pharmacovigilance and quality assurance to meet the challenges of a rapidly evolving pharmaceutical landscape.
CONCLUSION
In conclusion, pharmacovigilance and quality assurance are indispensable in upholding the safety, efficacy, and integrity of pharmaceutical products, providing a comprehensive framework that spans from preclinical studies through post-marketing surveillance. Pharmacovigilance safeguards patient health through continuous monitoring and prompt response to adverse drug reactions, while quality assurance ensures that drugs meet rigorous standards through compliance with Good Manufacturing Practices (GMP) and systematic quality reviews. Together, these fields address the growing complexities in drug development, particularly with the advent of personalized medicine and biologic therapies. Emerging digital technologies, such as artificial intelligence, machine learning, and blockchain, offer promising advancements, enhancing the speed and accuracy of adverse event detection and data transparency. Global regulatory harmonization and collaborative initiatives, supported by patient-centered approaches, are critical to fostering public trust and achieving safer healthcare outcomes. As these disciplines evolve, their combined focus on regulatory standards, technological innovation, and patient well-being will continue to play a crucial role in advancing the pharmaceutical industry.
ACKNOWLEDGEMENT: Authors are thankful to Shree Santkrupa College of Pharmacy Ghogaon, Shivaji University, Kolhapur 416004, Maharashtra, India for providing best of the facility to conduct this work.
CONFLICT OF INTEREST: Authors do not report any conflict of interest.
REFERENCES
- Waller, Patrick C.. “An Introduction to Pharmacovigilance.” (2009). DOI:10.1002/9781119289777
- Kengar, Manohar D., Kiran K. Patole, Akshay K. Ade, Sumesh M. Kumbhar, Chetana D. Patil and Ashutosh R. Ganjave. “Introduction to Pharmacovigilance and Monitoring.” Asian Journal of Pharmaceutical Research (2019): n. page. DOI:10.5958/2231-5691.2019.00019.4
- Malinovsky, Jean Marc. “An Introduction to Pharmacovigilance, 2nd ed.” Anesthesia & Analgesia (2018): n. page. DOI:10.1213/ANE.0000000000003638
- Kantilal Patil, Divyashree, Divyani Rajendra Patil and Sunila A. Pati. “A Review on Introduction to Quality Assurance.” Research Journal of Pharmacology and Pharmacodynamics (2023): n. page. DOI:10.52711/2321-5836.2023.00015
- Naik SA. Review on Pharmacovigilance. Asian Journal of Pharmaceutical Research. 2020;10(2):123-8. DOI:10.5958/2231-5691.2020.00024.6
- Rajgopal, Jayesh K.m., Kajal Shilpi and Arun Kumar Srivastav. “PHARMACOVIGILANCE: A REVIEW ARTICLE.” Journal of the Medical Sciences 4 (2016): 6-7.
- Sangita Fulchand Pawar and Vikram Limbaji Musale (2020); PHARMACOVIGILANCE: A REVIEW Int. J. of Adv. Res. 8 (Jan). 235-243. DOI:10.21474/ijar01/10289
- Dogra R, Garg R, Kumar V, Verma V, Tripathi V. Detection, assessment, understanding and prevention of adverse effects: Pharmacovigilance: A review. International journal of pharmaceutical sciences review and research. 2013;20(1):71-86.
- Fornasier, Giulia et al. “An historical overview over Pharmacovigilance.” International Journal of Clinical Pharmacy 40 (2018): 744 - 747. DOI:10.1007/s11096-018-0657-1
- Beninger, Paul. “Pharmacovigilance: An Overview.” Clinical therapeutics 40 12 (2018): 1991-2004. DOI:10.1016/j.clinthera.2018.07.012
- Radecka, Anna et al. “Enhancing Pharmacovigilance Capabilities in the EU Regulatory Network: The SCOPE Joint Action.” Drug Safety 41 (2018): 1285 - 1302. DOI:10.1007/s40264-018-0708-5
- Routledge P. 150 years of pharmacovigilance. Lancet 1998; 351: 1200– 1.
- van Grootheest K. The dawn of pharmacovigilance. Intern J Pharm Med 2003; 17: 195–200.
- Rich ML. Fatal case of aplastic anemia following chloramphenicol therapy. Ann Intern Med 1950; 33: 1459.
- Wallerstein RO, Condit PK, Kasper CK, Brown JW, Morrison FR. Statewide study of chloramphenicol therapy and fatal aplastic anemia. JAMA 1969; 208: 2045–50.
- Randall T. Thalidomide’s back in the news, but in more favorable circumstances. JAMA 1990; 263: 1467–8.
- Olsson S. The role of the WHO Programme on international drug monitoring in coordinating worldwide drug safety efforts. Drug Safe 1998; 19: 1–10.
- World Health Organization. International drug monitoring: the role of national centers. Geneva: World Health Organization; 1972.
- Olsson S, Pal S, Stergachis A, Couper M. Pharmacovigilance activities in 55 low- and middle-income countries. Drug Safe 2010; 33: 689–703.
- https://www.qualifyze.com/resources/blog/the-future-of-pharmacovigilance-a-quality-assurance-perspective/ Last Accessed 22 October 2024.
- Tripathi DK, Shiv S. Pharmacovigilance (Nirali Prakashan). and others, editor; 2017. p. 262.
- Dr R. history And Development of pharmacovigilance. and others, editor; p. 1–10.
- Nimesh S. Pharmacovigilance program of review article Acta scientific pharmaceutical sciences; 2022.
- Sahu RK, Yadav R. Adverse drug reaction monitoring prospects and impending challenges for Pharmacovigilance.
- Sutar MR, Gawne DR. Review article of study of drug regulatory approval process and comparative requirement of common technical document in Europe, USA and India in coordination with drug development process. Int J Pharm Sci. 2013;20(2):68–79.
- Sachdev Y. Pharmacovigilance safety matter, Indian pharmacology; 2008.
- Mohiuddin AK. Department Of pharmacy, world University of Bangladesh, green road, Dhaka , Bangladesh.
- Caffrey S, Paul C. Generic drugs – The Indian scenario. J Postgrad Med. 2019;65(2):67–9.
- Lakshmi I, Aashritha M. A review on pharmacovigilance and its importance. Teja A World J Pharm Pharm Sci. 2017;6(1):300–10
- Bharati A, Dubey T, Thakur R, Bhosale K, Bhoir P. A Review article of pharmacovigilance and studies of clinical research for health care. IP Int J Comprehensive Adv Pharmacol 2024;9(2):116-124.


 Chaitali Kulkarni1* 1
Chaitali Kulkarni1* 1
 Pratiksha .B Shinde 2
Pratiksha .B Shinde 2
 Aishwarya S Shinde 3
Aishwarya S Shinde 3

 10.5281/zenodo.14029139
10.5281/zenodo.14029139