Abstract
This study explores the dissolution behavior of an immediate-release tablet formulation containing a poorly soluble BCS Class drug, with an emphasis on the interaction between excipient’s mechanical properties and API’s solubility. We observed an unconventional trend where tablets with higher hardness exhibited faster dissolution rates. The study explores the brittle fracture nature of lactose monohydrate and the plastic deformation properties of microcrystalline cellulose (MCC), combined with the effects of a poorly soluble micronized API. According to our findings, the increased surface area caused by the brittle fracture of lactose at higher hardness levels improves the dissolving rate, particularly in our study where we assessed an API that is poorly soluble. This highlights the key role of excipient-API interactions and the mechanical properties of tablet ingredients in the design of immediate-release formulations. Additionally, our research highlights the utility of DoE software in retrospectively analyzing experimental results to predict future outcomes effectively. The DoE model was further validated through a confirmation run, to ensure the model’s ability for prediction. Examining the research work "Studies on Tableting Properties of Lactose" by Vromans et al. (1985)”. Our research also demonstrated that, in immediate release tablets containing lactose as a main diluent, a higher compaction force can lead to an increase in the surface area of lactose, which in turn can accelerates the rate of dissolution of poorly soluble API.
Keywords
Effect, Tablet, Hardness, Dissolution, Rate , Soluble, BSC class, Micronized, RSM
Introduction
Immediate-release (IR) tablets are designed to disintegrate quickly and release the active ingredient rapidly into the GI tract upon administration there by attaining the plasm concentrations at the earliest to achieve therapeutic efficacy. The dissolution rate of an IR tablet is a critical quality attribute that governs the rate and extent of bioavailability and ultimately influencing pharmacokinetic profile and clinical outcomes. Conventionally, high hardness tablets are expected to dissolve slower due to their denser structure which impedes the permeation of dissolution media into the core of the tablet matrix for dissolving the active substances and release the same from the tablet; however, our findings indicate the opposite. This research aims to interpret the primary mechanisms contributing to this unusual behavior, with a particular emphasis on the role of lactose monohydrate’s brittle fracture properties. The 1985 research paper titled “Studies on Tableting Properties of Lactose ” by Vromans et. al. spots as an outstanding work in pharmaceutical sciences in understanding the excipient mechanical properties under compaction, this paper highlights the brittle nature of lactose monohydrate in comparison to the plastic deformation of microcrystalline cellulose (MCC) and also presented the stress induced mechanical transformations of other commonly used fillers such as Dicalcium phosphate di hydrate (DCP). We validated the main finding that lactose monohydrate's brittleness may lead to an increased surface area upon compaction through our current research studies. Our study additionally confirmed that a larger compaction force can increase the surface area of lactose in immediate-release tablets which employ lactose as the primary diluent. This can increase the rate at which poorly soluble API dissolves. By integrating DoE for retrospective data analysis, we validate these classical results, bridging the gap between past and present in the field of pharmaceutical formulation. Lactose monohydrate, a frequently used diluent in tablet formulations, exhibits brittle fracture behavior when compacted. This brittleness is characterized by the material’s tendency to crack and fragment rather than deform plastically when subjected to compression forces. The fracture mechanics of lactose are critical in determining the tablet’s porosity and surface area following compression. When compressed to higher hardness, lactose particles are more susceptible to undergoing extensive fracturing, thereby increasing the tablet’s internal surface area. This increased surface area can significantly accelerate the rate of dissolution of the active ingredient, especially if the API is already micronized, has limited solubility, and requires improved surface contact with the dissolution media. Unlike lactose, Microcrystalline cellulose (MCC), shows a different physical property known as plastic deformation under compression. This particular characteristic of MCC enables it to form a more cohesive tablet matrix. Various tableting issues like poor hardness, capping, and lamination are caused by lactose brittleness. The plastic deformation nature of MCC, can help mitigate these effects, and ensures the structural integrity of the tablet, when used in combination with lactose. Hence, lactose and MCC combinations are the most preferred diluents for manufacturing poorly soluble APIs in tablet form, however to achieve the desired properties of tablets such as mechanical strength and dissolution, the careful balance of ratio between MCC and lactose must be managed. Excessive plastic deformation can lead to a decrease in tablet porosity which may result in a subsequent reduction in dissolution rate. In the current research, lactose is the main component, and the MCC content is low, therefore it is expected that the tablet's mechanical properties after compression would be guided predominantly by lactose brittleness nature rather than by MCC plastic deformation nature. Brittle fracture and Plastic deformation characteristics and their implications were discussed by RS Okor Deformation and Mechanical Characteristics of Compacted Binary Mixtures of Plastic (Microcrystalline Cellulose), Elastic (Sodium Starch Glycolate), and Brittle (Lactose Monohydrate) Pharmaceutical Excipients has been studied in a detailed manner by Zahraa et. al . where the relationship of stress induced transformation of lactose and MCC vs mechanical properties of the tablets prepared with these pure excipients and binary mixtures was proved.

Figure 1: The surface Morphology of Lactose monohydrate and Microcrystalline cellulose using SEM .
The brittle fracture index (BFI) of lactose-based formulations with different binders at fixed concentrations has been studied by Ebere I. Okoye et. al . Influence of different types of lactose on tablets compactibility has been studied by Karen Alejandra Velázquez González et.al. The API used in this investigation is poorly soluble and was micronized to improve its dissolution rate. Micronisation lowers particle size and hence increases the surface area accessible for dissolution. The interaction between micronized API particles and the lactose matrix is an important element in the dissolution rate.

Table 1: Microcrystalline cellulose and Lactose Properties under compression stress
As lactose undergoes brittle fracture at higher hardness levels, it exposes more of the API’s surface area to the dissolution medium, facilitating a faster dissolution rate. This effect is especially apparent for poorly soluble APIs, where the dissolution rate is greatly dependent on the available surface area. Conventionally, DoE requires the planning and execution of structured experiments to generate data for analysis. However, in many practical scenarios, researchers are confronted with an ample of data collected from past experiments, which may not adhere to a standard DoE framework. The challenge is resolved by our study, which uses the DoE software's Response Surface Methodology (RSM) for analyzing such historical data retrospectively.
MATERIALS AND METHODS
The studied formulation has a micronized BCS class II API with a D90 of approximately 5 microns. The excipients used are lactose monohydrate (200M), microcrystalline cellulose (MCC), sodium starch glycolate (SSG type A), hydroxypropyl cellulose (HPC), Maize starch, colloidal silicon dioxide, and magnesium stearate.

Table 2: Quantitative composition of Formulation under study
We used a Collette granulator equipped with a torque sensor to carry out the wet granulation process, the raw materials are sifted, dry mixed, followed by gradual binder addition (water) and kneading, and we employed the torque sensor for monitoring the granule’s resistance. Granule growth is monitored based on torque data, and the granulation endpoint is determined when the torque data reaches a plateau. Following discharge and drying, the granules are sized, sieved, mixed, and lubricated. The lubricated granules were then compressed into tablets at different hardness levels, coated, and their dissolution rates were measured.

Figure 2: Process Flow of Drug product manufacturing

Figure 3: Granulation end point Binder addition time vs Torque.
The tablet compression trials were carried out using Fette 102i compression machine by changing pre and main compression forces to produce tablets of varying hardness, specifically targeting 4 to 8 kilopond (Kp). The hardness of the tablets was measured using a standard hardness tester (Sotax).

Figure 4: Compression Force vs hardness

Figure 5: Hardness vs Drug release
RESULTS
The dissolution tests were performed on tablets of different hardness levels. It was observed that tablets with a hardness of 7 to 8 KP exhibited significantly faster dissolution rates compared to those with a hardness of 4 to 6 KP. This trend was consistent across multiple trials, indicating a clear relationship between tablet hardness and dissolution rate.

Figure 6: Dissolution profile of Compression trials at different Compaction force.
Dissolution testing revealed a direct correlation between tablet hardness and dissolution rate. Tablets with higher hardness displayed a faster dissolution rate than those with lower hardness. This finding is contrary to conventional expectations and suggests that factors other than tablet density are influencing the dissolution rate. Experimental Design and Data Collection We conducted an in-depth examination into the dissolution behavior and hardness of immediate release tablets. Our approach involved the utilization of Design-Expert® software, for analyzing responses. Here are the key steps we followed:
Historical Data Design:
We leveraged historical data, which allowed us to retrospectively analyze the responses. While this approach deviates from the conventional experimental design, it provided valuable insights into the interplay between tablet properties and dissolution kinetics.
Table 3: Trials and Results used in the historical data analysis in RSM using Designexpert® software

Factor A (Pre-compression):
The force applied during pre-compression, which influences the initial compactness of the granules.
Factor B (Main Compression):
The force applied during the main compression step, affecting the final tablet hardness.
Response Surface Design:
We employed a response surface design type, aiming to model the relationship between the factors (A and B) and the responses (dissolution and hardness). The choice of backward selection allowed us to identify significant terms while maintaining model simplicity.
Table 4: DoE Summary

Response 1- Hardness:
Based on the data, Design Expert suggested a quadratic model for hardness. This choice accommodated potential curvature effects. The model included terms for A, B, AB (interaction), and B2 (quadratic effect). Critical P-values (< 0>
Table 5: ANOVA Response -1 Hardness

R2 values indicated a good fit, capturing most of the variability in hardness. Diagnostic plots supported model adequacy, although influence plots revealed some outliers due to collinearity. Given the retrospective nature of our design, these outliers were expected and did not compromise the overall analysis.
Table 6: Regression Data for Response 1 - Hardness
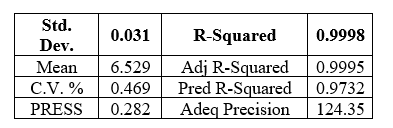
Model equation Actual terms:
Hardness = -12.79811 -1.22409 * Pre compression force +2.86318* Main Compression force
+0.092008 * Pre compression force * Main Compression force -0.10134* Main Compression force2

Figure 7: Model Graph for Response 1 - Hardness
Response 2-Dissolution Model (15 Minutes)
For dissolution at 15 minutes, a linear model was recommended. Factor B (main compression) emerged as the sole significant term (critical P-value < 0>
Table 7: ANOVA Response -2 Dissolution at 15 minutes

R-squared values confirmed the model’s reliability. Diagnostic and influence plots showed no outliers, indicating robustness.
Table 8: ANOVA Response -2 Dissolution at 15 minutes

Model equation Actual terms
Dissolution 15 min =+36.48699 +4.05838 * Main Compression force
Figure 8: Model Graph for Response2 – Dissolution at 15 min

Optimization and Validation:
We performed numerical and graphical optimization to identify optimal combinations of pre-compression and main compression forces. The overlay plot revealed the desired response region, where both hardness and dissolution met our criteria. Our model predicted that pre-compression at approximately 2.8 kn and main compression at around 15 kn would yield tablets with hardness > 7.7 kp and dissolution > 85% Remarkably, our actual trial results closely matched the predictions, validating the efficacy of our DoE model.
Desirability and Overlay Plots:


Table 9: Doe Model Validation With New Trial
*Observed results are within the 95% CI and PI, proving the model’s capability for prediction
In summary, our comprehensive analysis sheds light on the intricate balance between excipient mechanics, API solubility, and tablet properties. By understanding these interactions, we can optimize immediate release formulations for desired dissolution profiles.
DISCUSSION
The brittle fracture characteristic of lactose monohydrate is hypothesized to play an important role in this phenomenon. At lower hardness, lactose particles may not undergo sufficient fracture, resulting in a decreased surface area for the API to interact with the dissolution medium. Conversely, at higher hardness, the lactose particles may fracture extensively, increasing the surface area and facilitating faster dissolution. This hypothesis is supported by the micronized nature of the API, which would facilitate the increased surface area resulting from the fracturing of lactose particles. The findings of the compression trials indicate that the optimal hardness for achieving the fastest dissolution rate in our formulation is between 7-8 KP range. This finding aligns with our hypothesis regarding the brittle fracture nature of lactose monohydrate. At lower hardness levels (4-6 KP), the lactose may not undergo sufficient fracturing, leading to a reduced surface area for the dissolution medium to interact with the API. However, at higher hardness levels (7-8 KP), the lactose appears to undergo complete fracturing, resulting in a greater surface area and, consequently, a faster dissolution rate. These findings highlight the significance of tablet hardness as a critical process parameter in the formulation of immediate-release tablets containing micronized APIs. The research study offers consideration of the compaction characteristics of lactose monohydrate-containing formulations and emphasizes the importance of meticulously adjusting tablet hardness to obtain the optimum dissolving profile. By integrating DoE with historical data analysis, we demonstrate that meaningful and predictive insights can be gleaned without the constraints of traditional experimental designs.
CONCLUSION
The dissolution of immediate-release tablets comprises a comprehensive relationship between excipient mechanics and API solubility. The brittle fracture nature of lactose monohydrate, when paired with the increased surface area created by a micronized, poorly soluble API, can result in a faster dissolving rate at higher tablet hardness. Understanding these interactions is critical for rationally designing tablet formulations with optimal dissolve rates. Our data reveal that the mechanical features of excipients, notably the brittle fracture behavior of lactose monohydrate, have a considerable impact on the dissolution rate of IR tablets. This study emphasizes the need-to-know excipient-API interactions as well as the mechanical features of tablet formulation to optimize dissolving characteristics. The findings of this study significantly contribute to the evolving narrative of tablet formulation in the pharmaceutical industry. By employing Design of Experiments (DoE) with historical data, we have demonstrated that the conventional understanding of tablet hardness and dissolution rate is not universally applicable. Our research underscores the potential for higher tablet hardness to result in faster dissolution rates, a notion that contradicts traditional expectations. This approach not only conserves resources but also accelerates the formulation development process by enabling the use of existing data to inform future trials. Our study’s predictive model, validated through subsequent trials, confirms the reliability of DoE model in forecasting outcomes. This validation is a testament to the robustness of our methodology and its potential for broader application within the field.
REFERENCES
- Studies on tableting properties of lactose; Part 2. Consolidation and compaction of different types of crystalline lactose, H. VRomas* et al; vol. 7 - 1985 Pharmaceutisch Weekblad Scientific Edition
- RS Okor, Brittle fracture during tableting – a problem for the pharmaceutical industry, Tropical Journal of Pharmaceutical Research, December 2005; 4 (2): 481-482
- Deformation and Mechanical Characteristics of Compacted Binary Mixtures of Plastic (Microcrystalline Cellulose), Elastic (Sodium Starch Glycolate), and Brittle (Lactose Monohydrate) Pharmaceutical Excipients Zahraa A et.al. 2013
- The effect of excipient particle size on the reduction of compactability after roller compaction International Journal of Pharmaceutics X · December 2022, Pauline H.M. Janssen, DFE Pharma
- Brittle fracture index (BFI) as a tool in the classification, grouping and ranking of some binders used in tablet formulation: Lactose tablets, by Ebere I. Okoye et. al, Scientific Research and Essays Vol. 5 (5), pp. 500-506, 4 March, 2010.
- Influence of different types of lactose on tablets compactibility, Karen Alejandra Velázquez González, Eduardo Ramírez Flores, Leopoldo Villafuerte Robles, Department of Pharmacy, National School of Biological Sciences,
- National Polytechnic Institute of Mexico, 2015.
- Deformation properties of pharmaceutical excipients determined using an in-die and out-die method International Journal of Pharmaceutics · February 2013, Ilija German Ili?


 SRK RAJU SAGIRAJU*
SRK RAJU SAGIRAJU*
 Erdi EKMEKC?
Erdi EKMEKC?
 Emre Erol ALDEN?Z
Emre Erol ALDEN?Z
 Udaya DUDE
Udaya DUDE
 Bogachan CAGRI
Bogachan CAGRI

















 10.5281/zenodo.11204296
10.5281/zenodo.11204296