There is a numerous study that have been reported as of the immense biological and pharmacological properties, there has always been an attraction from heterocyclic compound in the sector of medicinal chemistry. The triazole compounds are a key element in the area of heterocyclic chemistry and plays a role in as building blocks in both organic and medicinal chemistry. Triazole has become one of the most important heterocyclic compounds that possess characteristics of both of the natural products and medicinal compounds. Triazoles are supposed to be one of the most abundantly present compounds in many of the synthetic drugs. Current review focuses on the brief introduction of triazole and its derivatives as well as synthetic method and biological importances. The review thus explains the utilisation and importance of triazole moiety as a promising heterocyclic derivative in future with the help of various literature review that significantly shows the pharmacological activities of triazole nucleus.
triazole, heterocyclic, derivatives, moiety, pharmacological activity
Heterocyclic chemistry is branch of organic chemistry with long history and future promise. In the current scenario heterocyclic compounds has brought up with usual activity in synthesis of drug [1]. The five membered heterocyclic compounds have played a significant and concerned role in pharmaceutical field especially tetrazole, triazoles and their derivatives [2]. Nitrogen containing five membered heterocycles are important structural moiety and considered to be active biologically, corrosion inhibitors, pesticides, dyes, acid base indicator and other industrial based chemicals [3].
The triazole term was first coined by Bladin in 1885 for the five membered three nitrogen containing heterocyclic aromatic ring system having molecular formula C2H2N3. When the triazole was discovered, the chemistry was developed gradually and speedily due to the development of various synthetic method and its versatility with the biological systems [4].
Chemistry of Triazole
1,2,3 triazole, an unsaturated, five membered heterocycle, ?-excessive with delocalized electron ring system gives it an aromatic character. All the five atoms (three nitrogen and 2 carbon) are sp2 hybridized. Out of three nitrogen one is pyrrole kind and other one is pyridine kind. The primary classes to triazoles are monocyclic 1,2,3 triazoles, 1,2,3- triazolium salt and benzotriazoles. 1,2,3 triazoles are classified into three sub classes 1H- 1,2,3-triazoles and 2H-1,2,3-triazoles and 4H-1,2,3-triazoles. The first two are aromatic and equilibrium with each other in solution and gas phase while 4H-1,2,3-triazoles is non aromatic in nature [5].

Fig 1: Structure of Triazole
Properties of Triazoles
- In aqueous solution, 2H-1,2,3- Triazole can exist as major as compared to another tautomer.
- The primary triazole 1H-1,2,3-Triazole has bp of 203 0C and exist as clear liquid, it is also soluble in water [6].
- Mostly the 1,2,3 triazoles derivatives are synthesized from azides, and the presence of one pyrrole type and two pyridine type nitrogen atom makes the ring very stable
- The electrophilic substitution at carbon and at nitrogen undergoes easily.
- In case of 1,2,4-triazoles the parent form 1H is a white powder solid bp 2600C and mp 120-1210C, it is soluble in water and in most of the organic solvents.
- The two tautomers 1H and 4H- are of 1,2,4-triazoles are in equilibrium and 1H-1,2,4 -triazole is more stable than 4H.
- 1H-1,2,4- triazole exhibit both nucleophilic and electrophilic substitution reaction, chemically.
- Because of the high electron density electrophilic substitution occurs at nitrogen atoms and nucleophilic substitution occurs at both the carbon atom ring under mild conditions [6].
Synthetic Approaches for Triazoles
Synthesis of 1,4 and 2,4-disubstituted 1,2,3-triazoles
Kalisiak in 2008 reported an efficient three component synthetic pathway for the synthesizing 2-hydroxymethyl -2H-1,2,3-Triazole through Cu catalysed in one pot [7].

Fig 2: 2-hydroxymethyl -2H-1,2,3-Triazole
One pot three component synthesis of N2-substituted -1,2,3-triazoles
In 2012, a method was proposed by chen et al with chalcone, sodium azide, halogenated aromatics as raw material [8].

In 2015, a technique as described using Cu catalyzed nitrogenation of alkynes or alkenes for the rapid synthesis of triazole with Sulphur [9].

In 2015, water mediated cycloaddition reaction of enaminone and tosilazide was reported [10].

In 2015, a reaction between hydrazine and formamide under microwave condition was reported indicating highly functional group tolerance [11].

In 2018, a highly effective method for the synthesis of 1,5-disubstituted-1,2,4-triazole was reported using aryl diazonium salts and isocyanide [12].

Pharmacological Significance of Triazoles
The triazole moiety is very versatile and is said to be featured in variety of drug used clinically because of the moiety. The study revealed that triazoles have immense pharmacological activity classified into following categories:
It is always challenging when it comes to treatment of infectious disease due to the combination of factors which includes development to resistance in current therapies, etc [13]. Current triazole drugs used are fluconazole, itraconazole, voriconazole, Posaconazole are frequently used as antifungal, they have broad spectrum activity and reduce toxicity as compared to imidazole [14].
- Analgesic and anti-inflammatory activity
There has been study for the series of 5-aryl -3-alkylthio-1,2,4-triazoles and corresponding sulphones for better development of analgesic and anti-inflammatory compound by limiting ulcerogenic risk [15].
Triazole Containing Marketed Drug
Fluconazole: Antifungal drug

Ribavirin: broad spectrum antiviral agent
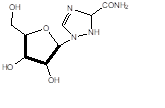
Vorozole: anticancer

Letrozole: anticancer [16]

Biological Activity of Triazole
Table 1: biological activity of triazole

THERAPEUTIC APPLICATION


 Hricha Joshi*
Hricha Joshi*











 10.5281/zenodo.11550285
10.5281/zenodo.11550285