A series of novel 2-(substituted phenyl)-5-methyl-3-(pyridin-4-yl)-1,3-thiazolidin-4-ones (4a-g) were synthesized, structurally confirmed by elemental analysis, IR, 1H NMR and MS spectral analysis, further evaluated for their anti-HIV activity and cytotoxicity in MT-4 cells infected with wild-type HIV-1 strain IIIB and HIV-2 strain ROD in comparison with nevirapine (NVP), azidothymidine (AZT), dideoxycytidine (DDC) and dideoxyinosine (DDI), which were used as reference drugs. The experimental results indicated that none of the synthesized compounds inhibited the replication of HIV-1 (IIIB) and HIV-2 (ROD) in MT-4 cell cultures at subtoxic concentration.
Pyridin-4-amine, 1,3-thiazolidin-4-one, 2-sulfanylpropanoic acid, Anti-HIV activity, Cytotoxicity, MTT assay.
HIV-1 (human immunodeficiency virus type 1), a retrovirus of the lentivirus family, is the etiological agent of AIDS [1], an infection characterized by loss of helper T lymphocytes and heavy damage of lymphatic tissue. Global estimates of WHO/UNAIDS showed that 34 million people had been infected with HIV/AIDS at the end of 2010, with 2.7 million getting newly infected with the virus and 1.8 million reported deaths because of AIDS [2]. An estimated 4.0 million people are living with HIV in South-East Asia Region.
The current therapy against AIDS is based on seven classes of anti-HIV drugs: the nucleoside and nucleotide reverse transcriptase inhibitors (indicated as NRTIs and NtRTIs, respectively), the non-nucleoside reverse transcriptase inhibitors (NNRTIs), the protease inhibitors (PIs), the integrase inhibitors (INI), the chemokine (C-C motif) receptor 5 (CCR5) inhibitor and the fusion inhibitor (FI) [3]. NRTIs, NtRTIs, NNRTIs and PIs are combined in the highly active antiretroviral therapy (HAART), which dramatically reduces the incidence of AIDS infection and death.
Despite the fact that HAART combination regimens have significantly decreased the morbidity and mortality among patients with HIV infections, by bringing the viral replication to very low levels, they are still unable to eradicate the virus [4]. So, the continued suppression of the virus by long-term use of the anti-retroviral drugs induces the emergence of drug-resistant viral mutants and the undesirable metabolic side effects. Moreover, when individuals develop resistance to one antiretroviral agent within a class, there is often, but not always, development of cross-resistance to other agents of the same class.
In addition to the facts that millions of people still need HAART treatment, the utility of antiretroviral drugs is further limited by viral resistance and toxicity issues [5]. Unfortunately still there exists no safe, effective vaccine for prevention of HIV either upon pre-exposure or post-exposure prophylaxis. Hence the current need is availability of more potent, less toxic, easily available, cost-effective therapies not only to treat HIV, but also to prevent its transmission. This is particularly critical in regions of the world such as sub-Saharan Africa, where 67% of the world’s HIV infected individuals reside [6]. Reverse Transcriptase (RT) is a key enzyme which plays an essential and multifunctional role in the replication of the human immunodeficiency virus (HIV) [7] and thus represents an attractive target for the development of new drugs useful in AIDS therapy. RT is necessary for the catalytic transformation of single-stranded viral RNA into the double-stranded linear DNA which is integrated into host cell chromosomes. Drug targeted at HIV-RT can be divided into two categories: (i) nucleoside and nucleotide RT inhibitors, and (ii) non-nucleoside RT inhibitors (NNRTIs) [8]. However, in view of the increasing incidence of resistance to current drug regimens and the frequency of adverse events, the development of novel, selective, potent, safe, inexpensive antiviral agents, that are also effective against mutant HIV strains, remains a high priority for medical research.
Antiviral research in the past has primarily focused on the development of nucleoside analogues but of late, non-nucleoside derivatives [9] have also received considerable attention as an alternative therapy. Among the non-nucleoside analogues, 1,3-thiazolidin-4-one is an interesting molecule, which has been found to exhibit diverse biological activities.
The modeling studies carried out on 1H, 3H-thiazolo[3,4-a]benzimidazole (TBZ) analogues (Figure 1) [10], a class of NNRTIs, highlighted the importance of 2,6-dihalo substitution on the phenyl ring at C1 of the nucleus for the activity and also their ability to take “butterfly-like” shape on binding to the receptor site [11]. In this background TBZ analogues were modified by opening imidazole ring of TBZ (Figure 1) to generate 2,3-diaryl-1,3-thiazolidin-4-ones [12] as a new NNRTI scaffold to inhibit HIV-1 RT.
Figure 1. 1-Aryl-1H, 3H-thiazolo[3,4-a]benzimidazole (TBZ) analogues

1,3-thiazolidin-4-one derivatives have been found to exhibit diverse biological activities such as analgesic[13], anti-inflammatory[14], antiangiogenic[15], anti-HIV[16], in vitro anti-Toxoplasma gondii[17], antimicrobial[17], antimycobacterial[18], antimalarial[19], trypanocidal[20], antischistosomal[21], anticonvulsant[22], antihistaminic[23], antidiabetic[24], antiarrhythmic[25] and antihypertensive[26] properties.
To search for more specific and novel 1,3-thiazolidin-4-one analogues with a wide therapeutic window and anti-HIV activity, we synthesized some novel 2-(substituted phenyl)-5-methyl-3-(pyridin-4-yl)-1,3-thiazolidin-4-ones and evaluated them for their anti-HIV activity and cytotoxicity in MT-4 cells infected with wild-type HIV-1 strain IIIB and HIV-2 strain ROD by MTT assay method.
EXPERIMENTAL:
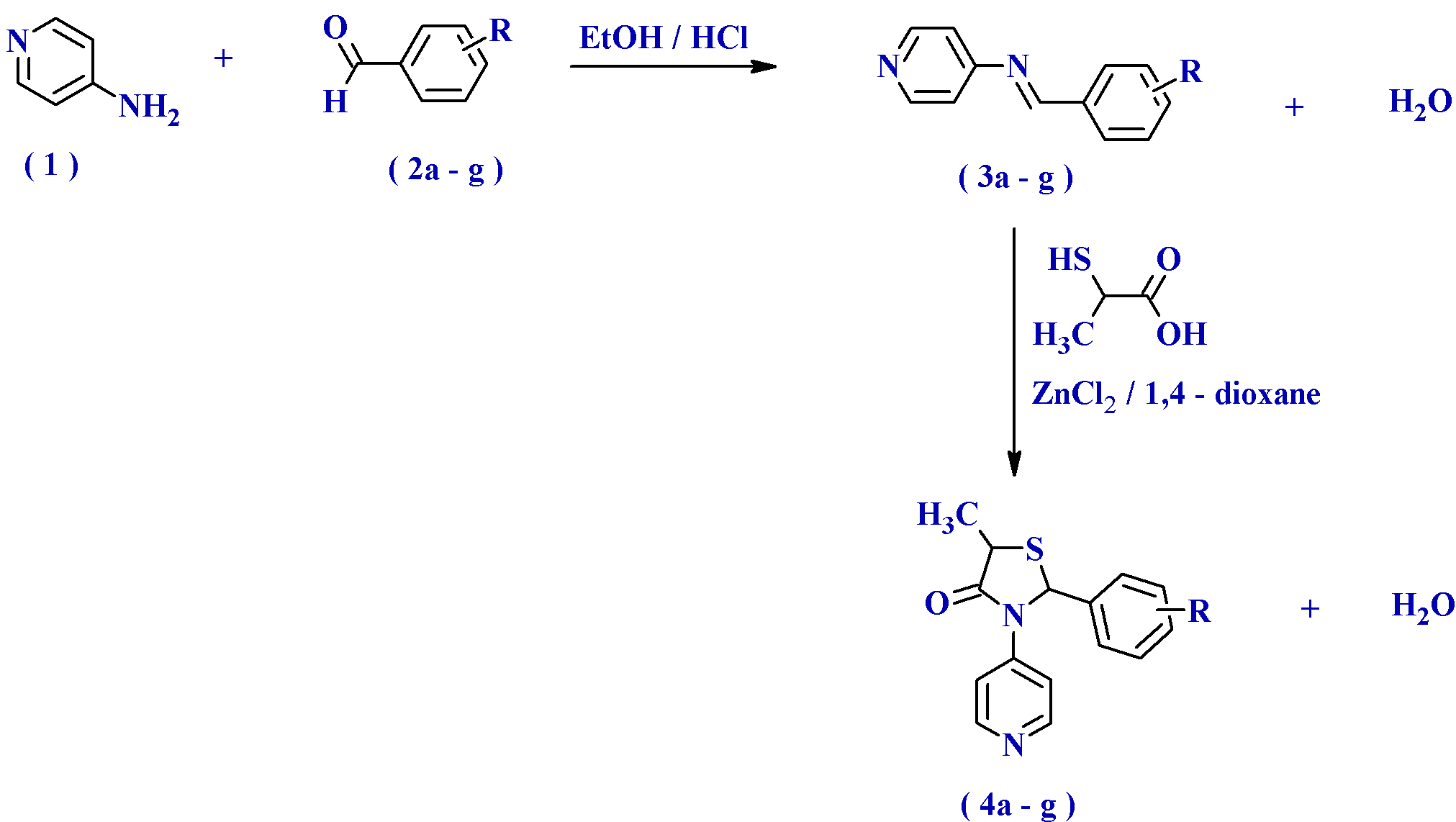
Chemistry: Pyridin-4-amine, 4-chlorobenzaldehyde, 2,4-dichlorobenzaldehyde, 2-fluorobenzaldehyde, 4-fluorobenzaldehyde, 3-nitrobenzaldehyde, 4-nitrobenzaldehyde and 4-bromobenzaldehyde and 2-sulfanylpropanoic acid, were commercially obtained from Aldrich (Milwaukee, WI) and dry 1,4-dioxane, anhydrous zinc chloride, dimethylformamide, chloroform, concentrated hydrochloric acid and silica gel-G, were purchased from Merck, Mumbai, India. Melting points were determined in open capillary tubes using Veego melting point apparatus (Model: VMP-DS) and are uncorrected. The purity of the compounds was checked by thin layer chromatography (TLC) on silica gel-G plates of 0.5 mm thickness using Chloroform: Methanol: Formic acid (10:2:0.2 v/v) and Benzene: Chloroform (1:1 v/v) as a solvent system and the spots being visualized under iodine vapours. Concentration of the solution after the reaction completion involved the use of a rotary evaporator (Eyela, Japan) operating under reduced pressure. Infrared (IR) spectra were recorded on a Jasco FTIR-4100 spectrophotometer (Jasco Ltd, Tokyo, Japan) using KBr pellet disc technique in the range of 4000-400 cm-1. 1H NMR spectra were recorded on a Bruker DPX 300 (operating at 300 MHz) NMR spectrometer using CDCl3 and DMSO-d6 as solvent and TMS as internal standard (chemical shifts in ?, ppm). Spin multiplets are given as s (singlet), br s (broad singlet), d (doublet), t (triplet), q (quartet) and m (multiplet). Mass spectra (MS) were recorded on a Q-TOF micromass spectrometer by using electronspray ionization (ESI) technique. The respective physico-chemical characteristics of all the synthesized compounds have been presented in Table 1.
Fig. 1. Synthetic route for the preparation of novel 2-(substitutedphenyl)-5-methyl-3-(pyridin-4-yl)-1,3-thiazolidin-4-ones (4a-g)
|
Compound
|
R
|
|
4a
4b
4c
4d
4e
4f
4g
|
4-Cl
2,4-(Cl)2
2-F
4-F
3-NO2
4-NO2
4-Br
|
Synthesis of N-[(Z)-(substitutedphenyl)methylidene]pyridin-4-amine (3a-g): A mixture of pyridin-4-amine (1) (0.01 mol) and different aromatic aldehydes (2a-g) (0.01 mol) (4-chlorobenzaldehyde (2a), 2,4-dichlorobenzaldehyde (2b), 2-fluorobenzaldehyde (2c), 4-fluorobenzaldehyde (2d), 3-nitrobenzaldehyde (2e), 4-nitrobenzaldehyde (2f) and 4-bromobenzaldehyde (2g)) dissolved in absolute ethanol (20 ml) in presence of catalytic amount of conc. hydrochloric acid (0.5 ml) was refluxed for 5-6 h. The progress of the reaction was monitored by TLC using Chloroform: Methanol: Formic acid (10:2:0.2 v/v) as eluents. After the completion of the reaction, the reaction mixture was cooled, concentrated under rotary vacuum. Then the resulting residue was poured into crushed ice and the product separated was filtered, washed with cold water, dried and crystallized from chloroform. Adopting the above procedure seven different schiff’s bases (3a-g) was synthesized. Percentage yield, melting point and Rf value of the synthesized compound (3a-g) were determined and presented in Table 1.
Synthesis of 2-(substitutedphenyl)-5-methyl-3-(pyridin-4-yl)-1,3-thiazolidin-4-one (4a-g): A mixture of N-[(Z)-(substitutedphenyl)methylidene]pyridin-4-amine (3a-g) (0.01 mol), 2-sulfanylpropanoic acid (0.015 mol) and anhydrous zinc chloride (0.5 g) in dry 1,4-dioxane (30 ml) was refluxed for 10-12 h. The progress of the reaction was monitored by TLC using Benzene: Chloroform (1:1 v/v) as eluents. After the completion of TLC, 1,4-dioxane was removed under reduced pressure. The final residue obtained was poured into crushed ice and the separated solid was neutralized by adding 10% sodium bicarbonate solution, for the removal of unreacted 2-sulfanylpropanoic acid. The neutralized solid product was filtered, washed with cold water, dried and crystallized from DMF. Adopting the above procedure seven different 1,3-thiazolidin-4-one analogues (4a-g) was synthesized. Percentage yield, melting point and Rf value of the synthesized compound (4a-g) were determined and presented in Table 1.
Table 1. Physical data of N-[(Z)-(substitutedphenyl)methylidene]pyridin-4-amine (3a-g) and 2-(substitutedphenyl)-5-methyl-3-(pyridin-4-yl)-1,3-thiazolidin-4-ones (4a-g)
N-[(Z)-(4-chlorophenyl)methylidene]pyridin-4-amine (3a): IR (KBr, cm-1): 3141.47 (aromatic C-H), 1602.56 (C=N), 815.742, 711.604 (C-Cl), 1602.56, 1521.56 (C=N, C=C ring stretch), 1315.21, 1213.01, 1078.98 (In-plane ring C-H bend); 1H NMR (DMSO-d6, ? ppm): 7.286-7.593 (m, 8H, Ar-H, PyH), 8.079 (s, 1H, N=CH-Ar). Anal. calcd. for C12H9ClN2: C, 66.52; H, 4.19; N, 12.93. Found: C, 66.56; H, 4.25; N, 12.91.
N-[(Z)-(2,4-dichlorophenyl)methylidene]pyridin-4-amine (3b): IR (KBr, cm-1): 3147.26, 3081.69, 3023.84 (aromatic C-H), 1686.44 (C=N), 854.311, 820.563, 754.031 (C-Cl), 1686.44, 1583.27, 1462.74 (C=N, C=C ring stretch), 1375.96, 1247.72, 1195.65, 1131.05, 1096.33, 1051.01 (In-plane ring C-H bend); 1H NMR (CDCl3, ? ppm): 7.270-7.633 (m, 7H, Ar-H, PyH), 8.263 (s, 1H, N=CH-Ar). Anal. calcd. for C12H8Cl2N2: C, 57.40; H, 3.21; N, 11.16. Found: C, 57.42; H, 3.25; N, 11.18.
N-[(Z)-(2-fluorophenyl)methylidene]pyridin-4-amine (3c): IR (KBr, cm-1): 3144.37, 3027.69 (aromatic C-H), 1603.52 (C=N), 1313.29, 1220.72, 1153.22, 1059.69 (C-F), 1603.52, 1522.52 (C=N, C=C ring stretch), 900.594, 809.956, 755.959 (out-of-plane ring C-H bend); 1H NMR (DMSO-d6, ? ppm): 6.707 (br s, 2H, Ar-H), 7.200-7.415 (m, 4H, Ar-H, PyH), 7.590-7.634 (m, 1H, PyH), 8.097 (br s, 2H, N=CH-Ar, PyH). Anal. calcd. for C12H9FN2: C, 71.99; H, 4.53; N, 13.99. Found: C, 72.04; H, 4.62; N, 14.02.
N-[(Z)-(4-fluorophenyl)methylidene]pyridin-4-amine (3d): IR (KBr, cm-1): 3144.37, 3027.69 (aromatic C-H), 1603.52 (C=N), 1313.29, 1220.72, 1154.19, 1059.69 (C-F), 1603.52, 1522.52 (C=N, C=C ring stretch), 899.63, 809.956, 755.959 (out-of-plane ring C-H bend); 1H NMR (DMSO-d6, ? ppm): 6.704-6.723 (d, 2H, Ar-H), 7.192-7.453 (m, 4H, Ar-H, PyH), 7.587-7.633 (m, 1H, PyH), 8.090-8.108 (d, 2H, N=CH-Ar, PyH). Anal. calcd. for C12H9FN2: C, 71.99; H, 4.53; N, 13.99. Found: C, 72.02; H, 4.58; N, 13.96.
N-[(Z)-(3-nitrophenyl)methylidene]pyridin-4-amine (3e): IR (KBr, cm-1): 3068.02 (aromatic C-H), 1615.90 (C=N), 1582.56, 1534.23 (asymmetric (ArNO2) (N=O)2), 1352.37, 1275.33 (symmetric (ArNO2) (N=O)2), 811.24 (C-N, ArNO2), 1615.90, 1582.56, 1534.23, 1471.71, 1446.15, 1399.03 (C=N, C=C ring stretch), 933.21, 917.82, 811.24, 729.42, 677.58 (out-of-plane ring C-H bend), 1202.58, 1101.74, 1076.51, 1008.18 (In-plane ring C-H bend); 1H NMR (CDCl3, ? ppm): 6.831-6.862 (m, 2H, Ar-H), 7.260-7.544 (m, 5H, Ar-H, PyH), 8.280 (s, 2H, N=CH-Ar, PyH). Anal. calcd. for C12H9N3O2: C, 63.43; H, 3.99; N, 18.49. Found: C, 63.51; H, 4.08; N, 18.52.
N-[(Z)-(4-nitrophenyl)methylidene]pyridin-4-amine (3f): IR (KBr, cm-1): 1518.67 (asymmetric (ArNO2) (N=O)2), 1372.1, 1341.25, 1273.75 (symmetric (ArNO2) (N=O)2), 860.096 (C-N, ArNO2), 2981.41 (aromatic C-H), 1597.73 (C=N), 1597.73, 1518.67, 1460.81, 1405.85 (C=N, C=C ring stretch), 913.129, 860.096, 771.387, 693.284 (out-of-plane ring C-H bend); 1H NMR (DMSO-d6, ? ppm): 8.078-8.240 (m, 7H, Ar-H, PyH), 8.390 (s, 1H, PyH), 8.416 (s, 1H, N=CH-Ar).
N-[(Z)-(4-bromophenyl)methylidene]pyridin-4-amine (3g): IR (KBr, cm-1): 3136.65 (aromatic C-H), 1601.59 (C=N), 529.364 (C-Br), 1601.59, 1520.6 (C=N, C=C ring stretch), 897.701, 812.849 (out-of-plane ring C-H bend), 1314.25, 1212.04, 1071.26 (In-plane ring C-H bend); 1H NMR (CDCl3, ? ppm): 6.442-6.589 (m, 3H, Ar-H), 7.360-7.387 (m, 2H, Ar-H, PyH), 7.535-7.590 (m, 2H, PyH), 8.262 (s, 2H, N=CH-Ar, PyH). Anal. calcd. for C12H9BrN2: C, 55.20; H, 3.47; N, 10.73. Found: C, 55.24; H, 3.52; N, 10.76.
2-(4-chlorophenyl)-5-methyl-3-(pyridin-4-yl)-1,3-thiazolidin-4-one (4a): IR (KBr, cm-1): 2921.63 (methyl C-H, ?as CH3), 2853.17 (methyl C-H, ?s CH3), 1701.87 (C=O, thiazolidin-4-one), 1402 (C-N, tertiary aromatic amine), 2921.63 (aromatic C-H), 1627.63, 1488.78, 1402 (C=N, C=C ring stretch), 1264.11, 1091.51, 1015.34 (In-plane ring C-H bend), 828.277 (C-Cl); 1H NMR (CDCl3, ? ppm): 7.021-7.422 (m, 8H, Ar-H, PyH), 3.577 (s, 1H, N-CH-Ar), 3.970-4.045 (q, 1H, CH-CH3), 1.628-1.731 (d, 3H, CH-CH3); ESI-MS: m/z 306 [M + 1]+. Anal. calcd. for C15H13ClN2OS: C, 59.11; H, 4.30; N, 9.19. Found: C, 59.16; H, 4.36; N, 9.17.
2-(2,4-dichlorophenyl)-5-methyl-3-(pyridin-4-yl)-1,3-thiazolidin-4-one (4b): IR (KBr, cm-1): 1702.84 (C=O, thiazolidin-4-one), 1401.03 (C-N, tertiary aromatic amine), 2921.63 (methyl C-H, ?as CH3), 2853.17 (methyl C-H, ?s CH3), 2921.63 (aromatic C-H), 1637.27, 1488.78, 1401.03 (C=N, C=C ring stretch), 1266.04, 1091.51, 1010.52 (In-plane ring C-H bend), 827.312 (C-Cl); 1H NMR (CDCl3, ? ppm): 7.020-7.422 (m, 7H, Ar-H, PyH), 3.577 (s, 1H, N-CH-Ar), 3.968-4.044 (q, 1H, CH-CH3), 1.597-1.724 (d, 3H, CH-CH3); Anal. calcd. for C15H12Cl2N2OS: C, 53.11; H, 3.57; N, 8.26. Found: C, 53.17; H, 3.61; N, 8.28.
2-(2-fluorophenyl)-5-methyl-3-(pyridin-4-yl)-1,3-thiazolidin-4-one (4c): IR (KBr, cm-1): 2921.63 (methyl C-H, ?as CH3), 2853.17 (methyl C-H, ?s CH3), 1702.84 (C=O, thiazolidin-4-one), 1401.03 (C-N, tertiary aromatic amine), 2921.63 (aromatic C-H), 1613.16, 1489.74, 1401.03 (C=N, C=C ring stretch), 1401.03, 1267, 1090.55, 1015.34 (C-F), 827.312 (out-of-plane ring C-H bend); 1H NMR (CDCl3, ? ppm): 6.975-7.423 (m, 8H, Ar-H, PyH), 3.577 (s, 1H, N-CH-Ar), 3.970-4.045 (q, 1H, CH-CH3), 1.254-1.730 (d, 3H, CH-CH3); Anal. calcd. for C15H13FN2OS: C, 62.48; H, 4.54; N, 9.72. Found: C, 62.56; H, 4.63; N, 9.75.
2-(4-fluorophenyl)-5-methyl-3-(pyridin-4-yl)-1,3-thiazolidin-4-one (4d): IR (KBr, cm-1): 2979.56 (methyl C-H, ?as CH3), 1714.35 (C=O, thiazolidin-4-one), 1366.58, 1342.65 (C-N, tertiary aromatic amine), 2979.56 (aromatic C-H), 1644, 1601.71, 1525.42, 1461.32, 1410.52 (C=N, C=C ring stretch), 1461.32, 1410.52, 1366.58, 1342.65, 1273.58, 1172.36, 1101.08, 1012.31 (C-F), 771.99, 720.25, 690.30 (C-S); 1H NMR (CDCl3, ? ppm): 7.018-7.412 (m, 8H, Ar-H, PyH), 2.968 (s, 1H, N-CH-Ar), 3.491-3.722 (q, 1H, CH-CH3), 1.254-1.412 (d, 3H, CH-CH3).
5-methyl-2-(3-nitrophenyl)-3-(pyridin-4-yl)-1,3-thiazolidin-4-one (4e): IR (KBr, cm-1): 2980.45 (methyl C-H, ?as CH3), 2920.66 (methyl C-H, ?s CH3), 1715.37 (C=O, thiazolidin-4-one), 1405.85 (C-N, tertiary aromatic amine), 2980.45 (aromatic C-H), 1599.66, 1460.81, 1405.85 (C=N, C=C ring stretch), 773.315, 694.248 (C-S), 1599.66 (asymmetric (ArNO2) (N=O)2), 1273.75 (symmetric (ArNO2) (N=O)2), 862.025 (C-N, ArNO2); 1H NMR (CDCl3, ? ppm): 8.183-8.403 (m, 8H, Ar-H, PyH), 3.922 (m, 2H, N-CH-Ar, CH-CH3), 1.402-1.462 (d, 3H, CH-CH3); Anal. calcd. for C15H13N3O3S: C, 57.13; H, 4.16; N, 13.33. Found: C, 57.21; H, 4.24; N, 13.36.
5-methyl-2-(4-nitrophenyl)-3-(pyridin-4-yl)-1,3-thiazolidin-4-one (4f): IR (KBr, cm-1): 2982.37 (methyl C-H, ?as CH3), 1714.41 (C=O, thiazolidin-4-one), 1404.89 (C-N, tertiary aromatic amine), 2924.52 (methyl C-H, ?s CH3), 2982.37 (aromatic C-H), 1600.63, 1519.63, 1461.78, 1404.89 (C=N, C=C ring stretch), 1273.75, 1168.65, 1105.01, 1017.27 (In-plane ring C-H bend), 772.351, 693.284 (C-S), 1519.63 (asymmetric (ArNO2) (N=O)2), 1273.75 (symmetric (ArNO2) (N=O)2), 860.096 (C-N, ArNO2); 1H NMR (CDCl3, ? ppm): 8.149-8.403 (m, 8H, Ar-H, PyH), 4.378-4.474 (m, 2H, N-CH-Ar, CH-CH3), 1.401-1.462 (d, 3H, CH-CH3); Anal. calcd. for C15H13N3O3S: C, 57.13; H, 4.16; N, 13.33. Found: C, 57.19; H, 4.20; N, 13.34.
2-(4-bromophenyl)-5-methyl-3-(pyridin-4-yl)-1,3-thiazolidin-4-one (4g): IR (KBr, cm-1): 3140.68 (aromatic C-H), 2974.25 (methyl C-H, ?as CH3), 1702.06 (C=O, thiazolidin-4-one), 1335.63, 1316.96 (C-N, tertiary aromatic amine), 1601.68, 1526.06, 1485.96, 1429.94 (C=N, C=C ring stretch), 665.41, 631.11, 614.47, 585.28, 526.77 (C-Br), 716.30, 684.14, 665.41, 631.11, 614.47 (C-S); 1H NMR (CDCl3, ? ppm): 8.181-8.402 (m, 8H, Ar-H, PyH), 4.378-4.473 (m, 2H, N-CH-Ar, CH-CH3), 1.401-1.461 (d, 3H, CH-CH3); ESI-MS: m/z 350 [M + 1]+. Anal. Calcd. for C15H13BrN2OS: C, 51.59; H, 3.75; N, 8.02. Found: C, 51.63; H, 3.81; N, 8.05.
Anti-HIV Activity Assays: Cells: MT-4 cells were grown and maintained in RPMI 1640 supplemented with 10% heat-inactivated fetal calf serum, 2 mM L-glutamine, 0.1% sodium bicarbonate and 20 µg gentamicin per mL[27].
Evaluation of the antiviral activity of the compounds against HIV-1 strain (IIIB) and HIV-2 strain (ROD) in MT-4 cells was performed using the MTT assay as previously described[28]. Stock solutions (10 × final concentrations) of test compounds were added in 25 µL volumes of two series of triplicate wells to allow simultaneous evaluation of their effects on mock- and HIV-infected cells at the beginning of each experiment. Serial 5-fold dilutions of test compounds were made directly in flat-bottomed 96-well microtiter trays using a Biomek 3000 robot (Beckman Instruments, Fullerton, CA). Untreated control HIV- and mock-infected cell samples were included for each sample. HIV-1 (IIIB) or HIV-2 strain (ROD) [29] stock (50 µL) at 100-300 CCID50 (50?ll culture infectious dose) was added to either the infected or mock-infected wells of the microtiter tray. Mock-infected cells were used to evaluate the cytotoxicity of the test compound. Exponentially growing MT-4 cells [30] was centrifuged for 5 min at 1000 rpm and the supernatant was discarded. The MT-4 cells were resuspended at 6 × 105 cells/mL and 50 µL volumes were transferred to the microtiter tray wells. Five days after infection, the viability of mock- and HIV-infected cells was examined spectrophotometrically by the MTT assay.
The MTT assay is based on the reduction of yellow-colored 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) (Agros Organics, Geel, Belgium) by the enzyme mitochondrial dehydrogenase of metabolically active cells to a blue-purple formazan that can be measured spectrophotometrically [31]. The absorbances were read in an eight-channel computer-controlled photometer (Multiscan Ascent Reader, Labsystems, Helsinki, Finland) at two wavelengths (540 and 690 nm). All data were calculated using the median OD (optical density) value of three wells. EC50 was defined as the concentration of the drug required for 50% inhibition of virus-induced cytopathicity. CC50 was defined as the concentration of the drug required for reducing the viability of mock-infected cells by 50%. CC50, EC50, and the selectivity index (SI = CC50/ EC50) were then calculated and results analysed (Table 2).
Table 2. Anti-HIV activity, cytotoxicity and selectivity index of 2-(substitutedphenyl)-5-methyl-3-(pyridin-4-yl)-1,3-thiazolidin-4-ones in MT-4 cells.


 Dr. G. Nagalakshmi*
Dr. G. Nagalakshmi*


 10.5281/zenodo.14382288
10.5281/zenodo.14382288