Abstract
Liver cirrhosis is the final stage of progressive chronic liver pathologies. This is a histopathological alteration of the liver characterized by the loss of the hepatic parenchyma, thus generating the formation of fibrous septa and structurally abnormal regeneration nodules, resulting in a distortion of the normal hepatic architecture and an alteration of the anatomy of the hepatic vascularization and microcirculation. Currently for the treatment of liver cirrhosis and its complications, there are different drugs of which the main ones are lactulose, L-ornithine, L-aspartate (LOLA) and certain antibiotics, especially rifaximin. Methodology: a bibliographic search was carried out in databases, selecting original articles, case reports and bibliographic reviews from 2009 to 2021, using the documents that deal with rifaximin as an alternative treatment for hepatic encephalopathy, obtaining 21 articles for the preparation of this document. Results: In a 2015 meta-analysis, a significant comparison was made in the use of rifaximin and some other treatments for HD, where it was evidenced that rifaximin has a greater reduction in plasma ammonium levels (the cause of this complication), likewise, several studies have been conducted comparing rifaximin with placebo, other antibiotics and non- absorbable disaccharide laxatives. Conclusion: rifaximin is a dominant alternative in the treatment of acute episodes of hepatic encephalopathy and to prevent relapses.
Keywords
rifaximin, hepatic encephalopathy, cirrhosis, hepatic carcinoma.
Introduction
Liver cirrhosis is the final stage of progressive chronic liver pathologies. This is a histopathological alteration of the liver characterized by the loss of liver parenchyma, thus generating the formation of structurally abnormal fibrous septa and regeneration nodules, resulting in a distortion of the normal hepatic architecture and an alteration of the vascularization anatomy.
In the past it was considered that cirrhosis was never reversible, however, in recent years the term cirrhosis has changed from a static to a dynamic stage. At present it is known that by eliminating the fundamental aggression that has produced cirrhosis, fibrosis could be resolved; this can be observed in patients with hemochromatosis treated satisfactorily with phlebotomies; patients with alcoholic hepatopathy in alcohol abstinence; patients with cirrhosis of autoimmune etiology treated with immunosuppressors and chronic hepatitis C with cirrhosis stage with sustained virological response to antiviral treatment (1).
Liver cirrhosis is a very frequent pathology worldwide, and the prevalence of this is variable between countries depending on etiological factors. Liver cirrhosis tends to manifest itself usually towards the fourth or fifth decade of life, however, there are cases of young people and even pediatric, this pathology is a more frequent disease in the male sex, probably because infection by hepatitis viruses and alcoholism are more frequent in men. The causes of cirrhosis are multiple, but approximately 90% of the causes of liver cirrhosis in Western countries are alcohol abuse, non-alcoholic fatty liver disease (NAFLD) and chronic viral hepatitis (2).
The complications that occur in liver cirrhosis are the same, regardless of the cause. This pathology is one of the main complications of decompensated cirrhosis and is involved in the appearance of ascites and bleeding from esophagogastric varices. In addition, hepatocellular dysfunction causes jaundice, coagulation disorders and hypoalbuminemia and contributes to portosystemic encephalopathy. Patients who develop complications of the hepatopathy they present and who decompensate are candidates for liver transplantation. In addition, liver cirrhosis predisposes to the development of hepatocarcinoma (1).
Currently for the treatment of liver cirrhosis and its complications, there are different drugs of which the main ones are lactulose, L-ornithine, L-aspartate (LOLA) and certain antibiotics, especially rifaximin (RFX); Rifaximin is an antibiotic with a broad spectrum of activity against bacteria, both gram-positive and gram-negative, and especially against anaerobic enteric bacteria. Its action is due to binding to the b subunit of RNA polymerase, which is DNA-dependent and prevents RNA synthesis. The low absorption at intestinal level allows a high concentration in the gastrointestinal tract, thus, modification of the intestinal bacterial flora with blood levels below 1?ter oral administration, making Rifaximin safe in healthy patients. Cirrhotic patients present an altered intestinal microbiota which could influence cognitive ability. Antibiotic administration in hepatic encephalopathy is based on altering the bacterial flora and achieving a reduction of endotoxemia by decreasing the production and absorption of gut-derived neurotoxins. By decreasing ammonium levels and endotoxemia there could be a positive impact on acute and chronic encephalopathy episodes (3,4).
METHODOLOGY:
For this article, a bibliographic search was performed in several databases such as Elsevier, Scielo, Medline, pubmed, ScienceDirect and Ovid, selecting original articles, case reports and bibliographic reviews from 2009 to 2021, in Spanish and English using MeSH terms: rifaximin, hepatic encephalopathy, cirrhosis, hepatic carcinoma and the Boolean operators and and or. Including all documents dealing with rifaximin as an alternative treatment for hepatic encephalopathy, the data found were between 18-32 records, thus 21 articles were used for this document.
RESULT
Rifaximin is a broad spectrum antibiotic used to treat hepatic encephalopathy (HD) secondary to liver cirrhosis and even used also to prevent it, this entity affects approximately 30% to 50% of patients with liver cirrhosis, rifaximin is a non-absorbable antibiotic that has proven to be effective in the treatment of this complication (5). It is an antibiotic with characteristics that give it greater tolerability than others such as lactulose, it is responsible for a marked decrease in mortality in these patients (23.8% vs. 49.1%; p<0>
8.2 +-or 4.6 days) this is supported by the clinical guidelines of the AASLD and EASL where beneficial effects have been recorded in patients with HD and specifically minimal EF, in addition to this several studies such as Shidu and collaborators were able to demonstrate a significant regression of minimal hepatic encephalopathy up to 75% in patients treated with rifaximin, in another study that correlates the performance in a driving simulator in patients with minimal HD and placebo, which showed that those individuals treated with this drug had a decrease in driving errors, speeding tickets and illegal turns, thus demonstrating an improvement in the sickness impact profile or SIP which is a behavioral measure of health status used to assess a person's perception of their health status with respect to the impact of their disease. It is sensitive enough to m o n i t o r changes in health status over time and is used to assess the effect of illness on physical and emotional functioning (6,7).
In a meta-analysis of 2015, a significant comparison was made in the use of rifaximin and some other treatments for HD, where it was evidenced that rifaximin has a greater reduction of plasma ammonium levels (the cause of this complication), likewise, several studies have been conducted comparing rifaximin with placebo, other antibiotics and non- absorbable disaccharide laxatives. These studies have shown that rifaximin is equal to or better than different drugs when compared well tolerated. Long-term studies have also been conducted as maintenance therapy compared to non-absorbable disaccharides or neomycin. A study comparing rifaximin with rifaximin maintenance therapy has recently been published. The placebo group received free nonabsorbable disaccharides during the 2-year follow-up period. It shows a lower rate of HD recurrence in patients treated with rifaximin in the long term with no major side effects observed, as well as a lower rate of HD recurrence in patients treated with rifaximin in the long term with no major side effects observed.
Many recently published studies have shown that the role of rifaximin in the treatment of HD is more effective than non-absorbable disaccharides and other antibiotics used for the treatment of HD, as well as it was reported to have noticeable beneficial effects in the prevention of acute episodes, in recurrence and in the management of minimal HD, likewise the different reviews or meta-analyses suggest that rifaximin is at least as effective as the non-absorbable disaccharide and that the latter is better tolerated. However, it has not been found to be statistically superior. Therefore, international guidelines do not yet recommend its use as monotherapy for the treatment and prevention of recurrent episodes of HD (Tables 1-3),
Table 1: Rifaximin in the treatment of HD episodes.
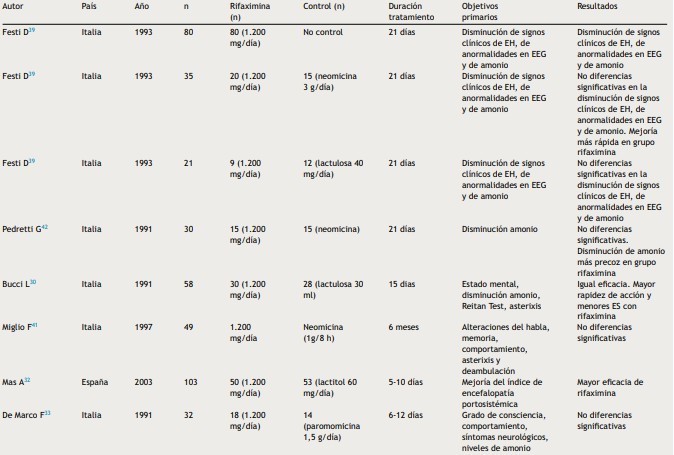
Taken from: Sanchez J, Miquel M, Role of rifaximin in the treatment of hepatic encephalopathy, gastroenterol hepatol, 2016, vol 39 (4).
Table 1: continued
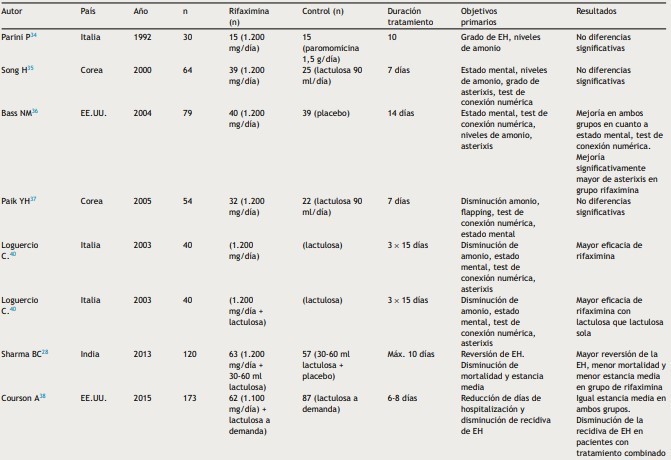
Taken from: Sanchez J, Miquel M, Role of rifaximin in the treatment o f hepatic
encephalopathy, gastroenterol hepatol, 2016, vol 39 (4).
Table 2: Rifaximin at prevention of the recurrence of HD
Taken from: Sanchez J, Miquel M, Role of rifaximin in the treatment of hepatic encephalopathy, gastroenterol hepatol, 2016, vol 39 (4).
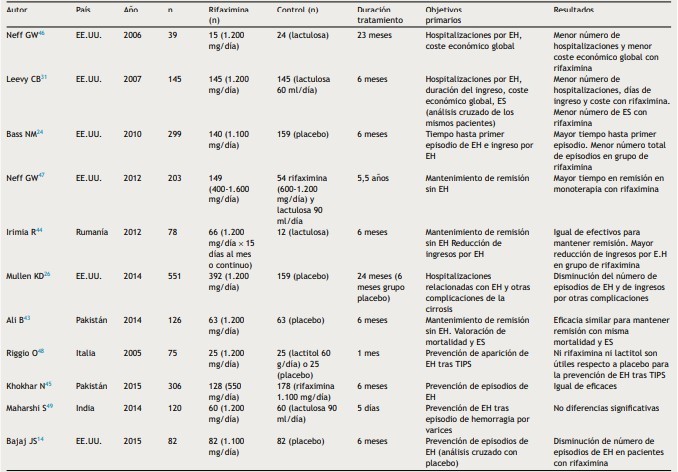
Table 3: Rifaximin at HD minimal

Taken from: Sanchez J, Miquel M, Role of rifaximin in the treatment of hepatic encephalopathy, gastroenterol hepatol, 2016, vol 39 (4).
Rifaximin was more effective in reducing blood ammonia levels, resulting in fewer hospitalizations and length of stay, and savings in hospital costs. Treatment with nonabsorbable disaccharide and salvage therapy with rifaximin has been shown to be cost-effective for those patients who initially did not respond to lactulose/lactitol, however, further studies are needed for this (8).
On the other hand, according to several European and American clinical practice guidelines on the management of HD, the best therapeutic options for the management of HD secondary to liver cirrhosis are the management with lactulose as first line of treatment, rifaximin and the salt of amino acids aspartate and ornithine (L-aspartate, L- ornithine or also called LOLA), also supported by the Mexican Association of Gastroenterology, considering lactulose as first line followed by rifaximin and LOLA, however, some guidelines such as the clinical practice guide of the Mexican Ministry of Health since 2013 continue to recommend the use of neomycin and metronidazole in patients who do not respond correctly to lactulose or lactitol, which is why the study conducted by Coronel and colleagues reviewed the evidence regarding the effectiveness of rifaximin compared to lactulose and others, in aspects such as efficacy in different degrees of HD, cost reduction, improvement of cognitive status, efficacy in reducing and preventing patient falls and improvement in their quality of life (Table 4) (9).
Table 4: comparison of rifaximin efficacy on different variables in persistent HD
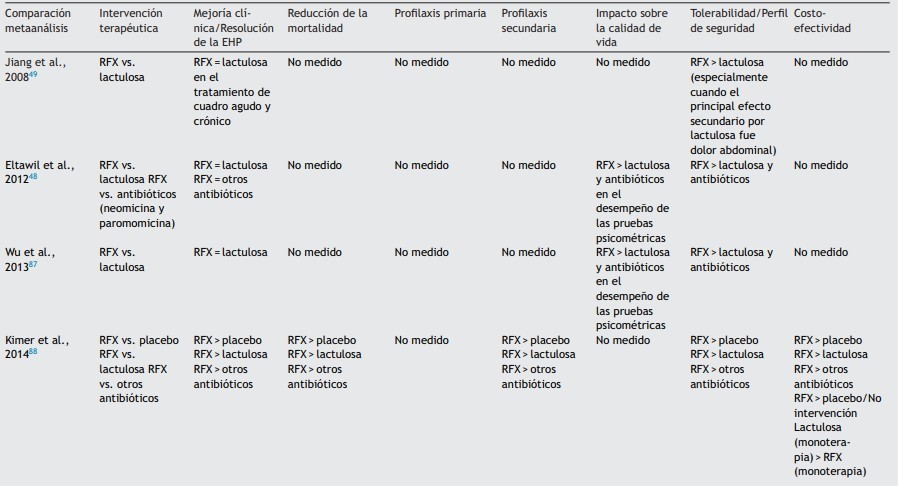
Taken from: Coronel C, Contreras J, Frati A, Uribe M, Mendez N, Efficacy of rifaximin in different clinical scenarios of hepatic encephalopathy, revista de gastroenterologia de mexico, 2020, vol 85 (1).
Table 4: Comparison of rifaximin efficacy on different variables in persistent HD (continued)

Taken from: Coronel C, Contreras J, Frati A, Uribe M, Mendez N, Efficacy of rifaximin in different clinical scenarios of hepatic encephalopathy, revista de gastroenterologia de mexico, 2020, vol 85.
DISCUSSION
In cirrhosis, Rifaximin treatment in overt hepatic encephalopathy is effective and well tolerated to reduce mortality and hospitalization to prevent recurrence of hepatic encephalopathy and reduce subsequent hospitalizations; it cures minimal hepatic encephalopathy and is cost-effective both therapeutically and in reducing hospital costs, with positive economic results because it treats the same number of patients compared to other treatment alternatives, in a pharmaco-economic evaluation showed that rifaximin combined with lactulose is more effective and more cost-effective than other treatments against acute episodes of hepatic encephalopathy and for the prevention of its relapses. Therefore, it is a highly recommended alternative for the treatment of this complication secondary to liver cirrhosis (9,10).
Recently, metronidazole treatment has been compared with RFX in hepatic encephalopathy, based on patients with mild hepatic encephalopathy (grade I or II), evaluating the improvement after three days of treatment, there was no difference between rifaximin and metronidazole, but all of them also received lactulose, L-ornithine L-aspartate and enemas.
(11). In one study, the efficacy of rifaximin in improving survival in patients with cirrhosis was also demonstrated. There was evidence of a significant decrease in short-term mortality for 10 days after treatment in patients who received lactulose plus rifaximin compared to patients who received lactulose alone (12).
There are additional advantages of rifaximin treatment in patients with liver disease. Rifaximin treatment has been observed to prevent episodes of spontaneous bacterial peritonitis (13,14), as bacterial overgrowth in the small intestine in cirrhotic patients is common and is associated with systemic endotoxemia, leading to further worsening of portal hypertension.
A recent meta-analysis found that rifaximin prevents episodes of spontaneous bacterial peritonitis compared to placebo and quinolone therapy.
and also reduces the occurrence of hepatorenal syndrome (16). A study carried out in patients who had had hepatic encephalopathy without hepatocarcinoma, the rifaximin- lactulose combination in comparison with lactulose alone was related to greater survival at 48 months, in addition to a decrease in complications, such as recurrent encephalopathy, spontaneous bacterial peritonitis and hemorrhage due to esophageal varices; in individuals with hepatocarcinoma, on the other hand, there was only a decrease in peritonitis (17).
A prospective study also showed that rifaximin reduces the levels of endotoxins, such as lipopolysaccharides, and improves systemic inflammation in cirrhotic patients. These data suggest that rifaximin may contribute to the control of gut microbiota imbalance and act as an important therapeutic agent to control bacterial overgrowth in the small intestine. These modulatory activities on the bacterial composition of the intestinal microbiota may underline the efficacy of rifaximin.
rifaximin for intestinal-derived toxins that contribute to the development of complications of cirrhosis (18).
There are cost-effectiveness analyses of rifaximin-? for the reduction of episodes of overt hepatic encephalopathy in the United Kingdom, France and Italy. In all of them, treatment with RFX was considered cost-effective with variable savings with respect to standard treatment with lactulose, derived mainly from the decrease in hospitalization expenses; this treatment increases quality-adjusted life years (QALY) with an accessible cost (19,20,21).
CONCLUSION:
It shows a lower rate of recurrence of eh in patients treated with rifaximin in the long term without major side effects and in different patients there was a significant decrease in short-term mortality during 10 days after treatment in patients who received lactulose plus rifaximin compared to patients who received lactulose, thus demonstrating that rifaximin is a dominant alternative in t h e treatment of acute episodes of hepatic encephalopathy and to prevent relapses.
REFERENCES
- G buey, F Gonzalez, R Moreno. Liver cirrhosis. Digestive System Service. Hepatology Unit. La Princesa University Hospital. Madrid. Spain. Medicine. 2012;11(11):625-33.
- Lefton HB, Rosa A, Cohen M. Diagnosis and epidemiology of cirrhosis. Med Clin North Am. 2009;93:787-99.
- Cajamarca K. Effectiveness of treatment with rifaximin and/or lactulose aimed at reducing recurrence, mortality and hospitalizations in patients with hepatic encephalopathy. [Undergraduate Thesis]. Cuenca: Catholic University of Cuenca; 2020.
- Sanchez J, Miquel M. Role of rifaximin in the treatment of hepatic encephalopathy. CIBERehd, Gastroenterology and Hepatology (English Edition) 2016, vol. 39, no 4, p. 282- 292.
- Torre A, Garcia S, Juarez F, Rodriguez H, guidelines for diagnosis and treatment of hepatic encephalopathy, treatment and future prospects, rev gastroenterol mex, 2009, vol 74.
- Romero M, Sendra C, Ampuero J, the impact of minimal hepatic encephalopathy treatment on long-term prognosis, health (i) science, 2018, vol 20.
- Sidhu SS, Goyal O, Parker R, Rifaximin vs. lactulo- se in treatment of minimal hepatic encephalopathy. Liver Int, 2016, 36:378-385
- Sanchez J, Miquel M, Role of rifaximin in the treatment of hepatic encephalopathy, gastroenterol hepatol, 2016, vol 39.
- Coronel C, Contreras J, Frati A, Uribe M, Mendez N, Efficacy of rifaximin in different clinical scenarios of hepatic encephalopathy, revista de gastroenterologia de mexico, 2020, vol 8.
- Frati A, Galindo R, Rifaximin-? in hepatic encephalopathy. Pharmacoeconomic considerations for Mexico Med Int Mex. 2020; 36 (5): 621-632.
- Mekky MA, Riad AR, Gaber MA, Abdel-Malek MO, Swifee YM. Rifaximin versus metronidazole in management of acute episode of hepatic encephalopathy: an open labeled randomized clinical trial. Arab J Gastroenterol 2018; 19: 76-9. doi: 10.1016/j.ajg.2018.06.001.
- Sharma BC, Sharma P, Lunia MK, Srivastava S, Goyal R, Sarin SK. A randomized, double-blind, controlled trial comparing rifaximin plus lactulose whit lactulose alone in treatment of overt hepatic encephalopathy. Am J Gastroenterol. 2013;108:1458- 1463.
- Hanouneh MA, Hanouneh IA, Hashash IG, Law R, Modaresi Esfeh J, Lopez R, et al. The role of rifaximin in the primary prophylaxis of spontaneous bacterial peritonitis in patients with liver cirrhosis. J Clin Gastroenterol 2012; 46: 709-715.
- Shokoohi S, Zivony A, Le TD, Zaman A, Jou J. Rifaximin is associated with decreased incidence of spontaneous bacterial peritonitis in cirrhotic with ascites. Hepatology 2013; 58 (suppl): 858A Abst 1337.
- Kamal F, Kahn MA, Kahn Z, Cholankeril G, Hammad TA, Lee WM et al. Rifaximin for the prevention of spontaneous bacterial peritonitis and hepatorenal syndrome in cirrhosis: a systematic review and meta-analysis. Eur J Gastroenterol Hepatol 2017; 29: 1109-17.
- Ibrahim ES, Alsebaey A, Zaghla H, Abdelmageed SM, Gameel K, Abdelsameea E. Long-term rifaximin therapy as a primary prevention of hepatorenal syndrome. Eur J Gastroenterol Hepatol 2017; 29: 1247-50.
- Kang SH, Lee YB, Lee JH, Nam JY, Chang Y, Cho H et al. Rifaximin treatment is associated with reduced risk of cirrhotic complications and prolonged overall survival in patients experiencing hepatic encephalopathy. Aliment Pharmacol Ther 2017; 46: 845-55.
- Vlachogiannakos J, Saveriadis AS, Viazis N, et al. Intestinal decontamination improves liver haemodynamics in patients with alcohol-related decompensated cirrhosis. Aliment Pharmacol Ther. 2009;29:992-999.
- Berni E, Poole CD, Conway P, Radwan A, Currie CJ. Costeffectiveness of rifaximin-A. 550 mg (Xifaxan/Tagaxan) in the reduction of recurrence of overt hepatic encephalopathy. UEG journal 2015; 3 (5S): A510.
- Berni E, Murphy D, Whitehouse J, Conway P, Di Maggio P, Currie CJ, et al. Evaluation of the cost-effectiveness of rifaximin-? for the management of patients with hepatic encephalopathy in the United Kingdom. Curr Med Res Opin 2018; 34: 2001- 2008.
- Roggeri DP, Roggeri A. Economic impact of the use of rifaximin 550 mg twice daily for the treatment of overt hepatic encephalopathy. Hepat Med 2017; 9: 37-43.


 Luis Fernando Puello Meza* 1
Luis Fernando Puello Meza* 1
 Melissa Sanabria Diaz 1
Melissa Sanabria Diaz 1






 10.5281/zenodo.14043484
10.5281/zenodo.14043484