Abstract
Herbal Medicinal plants are rich in phytochemical which have been used as source of raw material in medicine since ancient time. An herb is a plant or plant part. They are sold as tablet, capsule, powder, and fresh or dried plants. About 80% of the population use traditional medicine (medicinal plants) as the sole means of therapy against diseases. Crisium arvense is a perennial species of flowering arable growing crop. It is belonging to family Asteraceae.It is dioecious plant. The other names have been used in past are Canadian thistle, lettuce from hell thistle, California thistle, corn thistle, cursed thistle, field thistle, green thistle, hard thistle, perennial thistle, prickly thistle, setose thistle, small-flowered thistle, way thistle, and stinger-needles. Cirsium arvense contain many phytochemical constituents, it contains flavonoids, phenolic acid, polyacetylenes, Acetylenes, Hydrocarbons, Fatty acids, Aldehydes, Ketones Essential oils, and triterpenoids and Sterols. The different kind of pharmacological activities could be performed for pharmaceutical product development. This article provides an overview of medicinal property of the Cirsium arvense. This plant has different medicinal properties like diuretics, astringent, hepatoprotective and antiphlogistic. The biological potential of this plant is contributed by these secondary metabolites. Mainly we can use roots of the Cirsium arvense. Root decoction of Cirsium arvense has been traditionally use for treating warm infection in children. Root paste of Cirsium arvense in combination with Amaranthus spinosus is used in the treatment of indigestion.
Keywords
Antimicrobial Activity Antioxidant Activity Antiproliferative Activity Analgesic and Anti-Inflammatory Activity Hepatoprotective Activity Immunomodulatory Activity Anticancer Activity Anti-Anxiety Effect Nephroprotective Other Therapeutic Effects
Introduction
Cirsium word derived from the Greek word “thistle” and occidental from the Latin word meaning “Western” or more accurately for the Western sky(1). In recent year, Cirsium arvense used for the prevention and cure of many disease such as cancer, arithritis(2), cardiovascular disorder(3), Nephroprotective(4), Anti-Anxiety(5), Immunomodulatory Activity(6), Hepatoprotective(2), Analgesic and Anti-Inflammatory Activity(2), Antiproliferative Activity(6), Antioxident Activity(7), Antifungal Activity, Antimicrobial Activity and diabetes(8) and it is also known also used as cold blood hemostatic medicins. And reservoir of phytoconstituents that have anti-microbial, antioxidant, anticancer, antifungal and anti-diabetic properties.according to the plant of the world online collection at the Royal Botinical Gurdens of kew there are around 450-480 recognised species in this genas. Crisium arvense found in Norhtan hemisphere including Eurasia, Asia, North America and North Africa. Around 120 and 50 crisium specirs are found in Japan and China.Around 16 species found in indian deep green forest some species are found in region of boarding of Nepal It grow relatively dry habitates includings sandy fields as well as on the edges of wet habitate and edge of wood but is rarely found in forest optimal growth at 77Dgree fernaheiht 59 degree Fahrenheit plant grow west where rainfall average 50-75cm per year .species spread by seed and vertical and horizontal roots, giving rise to aerial shoot(9). In early development stages, C. arvense is rather susceptible to shading and competition for light from neighbouring plants in crop stands. The Crisium Arvense plants produce fewer shoots with less flowers as compared to plants growing in an unshaded environment Well-established and fast-growing cereal crops may, therefore, suppress the growth and development of C. arvense substantially(10). The species spread by seed and by vertical and horizontal roots give rise to ariel shoot and vegetative propagation. Seeds are produced by females but some cases seed are produced by males. Crisium Arvense shoot reached a height of 10cm to 20cm and the plant grow 180cm above the ground level. The crissum arvence has two types of root horizontal and vertical . Horizontal roots produce numerous shoot while vertical root store water and nutrients un there many small branches Its roots are yellowish in colour and deep vertical located below the top 20cm of the soil that have been found as deep as 6.75m and have a hollow slenders stem and the leaves are yellowish to dark in colour(11). Phytochemical investigation show that the crisium species contain flavonodies,polyacetylenes, acetylenes , phenolic acid phenyl propanoids sterols and terpenoids among them flavinoides phenylpropanoids and terpinoids considered to the main phyto compounds that are capable for large number of biological properties(12).
Plant Profile:
Botanical Name:
Crisium Arvense
Genus:
Cirsium
Species:
arvense
Family:
Asteraceae
Life Cycle:
Summer

Some Other Details: -
- Recommended Propagation Strategy: Seed
- Country Or Region Of Origin: Eurasia, Northwestern,Africa , India
- Distribution: Most of the US and southern Canada
- Wildlife Value: The seeds are rich in oil, an important food source for seed-eating birds.
- Whole Plant Traits: Plant Type: Herbaceous, Weed
- Habit/Form: Erect
- Growth Rate: Rapid
- Maintenance: High
- Texture: Coarse
- Appendage: Prickles, Spines
Cultural Conditions:
- Light: Full sun (6 or more hours of direct sunlight a day)
- Partial Shade (Direct sunlight only part of the day, 2-6 hours)
- Soil Texture: Clay, High Organic Matter, Loam (Silt), Sand
- Soil pH: Alkaline (>8.0), Neutral (6.0-8.0)
- Soil Drainage: Moist, Occasionally Dry, Occasionally Wet, Very Dry
- Available Space to Plant: 12 inches-3 feet
Fruit:
- Fruit Color: Cream/Tan
- Display/Harvest Time: Fall
- Fruit Type: Achene
- Fruit Length: < 1>
- Fruit Description: Tiny dry 1 inch, 0.1" long with feathery white to light brown pappus
Flowers:
- Flower Color: Pink, Purple/Lavender, White
- Flower Inflorescence: Head
- Flower Value to Gardener: Fragrant
- Flower Bloom Time: Summer
- Flower Shape: Dome
- Flower Petals: Bracts
- Flower Size: < 1>

Leaves:
- Leaf Color: Green
- Leaf Feel: Prickly
- Leaf Type: Simple
- Leaf Arrangement: Alternate, Rosulate
- Leaf Shape: Elliptical, Lanceolate, Oblong
- Leaf Margin: Lobed
- Hairs Present: Yes
- Leaf Length: 3-6 inches
- Leaf Width: 1-3 inches
Stem:
- Stem Color: Green
- Stem Is Aromatic: No
- Stem Surface: Hairy (pubescent)
- Common name
- English: Canada thistle, Californian thistle, creeping thistle, field thistle
- Hindi: Kandai
- Urdu: Leh, Bhurbhur
- Pakistan: Aghzikai, Kandehiara
- Bangladesh: Shial-kanta
- Jammu: Jhashkantu, Boban
- Ladakh: Biangtser, Jangchar
- Himachal: Dudhli
Flower Description:
It is a dioecious with male and female plants. Female flowers have ascent and male flowers do not. Pink, deep lavender, or white flowers; bracts without spines. Flowering occurs in August, the blooming period is longer in northern locales than in more southerly areas, In Canada blooming period is mid-June to early July and continues into September and an average flower produces 2000 seeds per plant. The small flower clustered in head that are typically 1- 1.5 cm in diameter and 1.3-1.5 cm tall.
Leaf Description:
Dark green, deeply lobed, elliptic to oblong-lanceolate leaves 2"-6"long and basal leaves are 12-20cm long. Its leaves are hairy and contain spines.

Seed description:
Seed range in size from 2.5-3.2 mm long and average 1mm in diameter. Ripe seed have a tawny color. Seed weight is 1.08mg and the seed weight is highest in seeds produced early in summer. Seed formation has been documented when male and female plants are 50- 90m apart 50-100m apart,180m apart and 390m apart. Flower must be open 8-10 days before seed are matured to germinate. Single plant produced average of 1500 seeds and up to 5300seeds. Multiple plants produced an average of 100-64,300 viable seeds/m2 in Australia.

Seed germination description:
Germination is affected by genotype, planting depth, substrate, temperature, day length, and seed freshness. Seed germination best at shallow depth but seed longevity increases with increased depth of planting. Seed germinates best at temperature of 25-30degree but can germinate at lower temperature in high light condition. Young seeds germinate well in high light and old seeds in low light- condition. Seed germination in April and may fresh. Seed may have low or high germination rate.

Root description:
Cirsium arvense spreads primarily by vegetative growth of its roots. The root system can be extensive, growing horizontally as much as 6 m in one season. Cirsium arvense has two types of roots; horizontal and vertical. Horizontal roots produce numerous shoots, while vertical roots store water and nutrients in their many small branches. Most Cirsium arvense roots can be found directly below the above-ground shoots, with little extension beyond the border of a patch. Horizontal roots grow within 15-30 cm of the soil surface, and typically grow in a straight line for 60-90 cm, then bend down and grow vertically.Vertical roots can grow as deep as 6.8 m but most roots are in the upper 60 cm of soil. Root growth and survival are affected by environmental factors, especially soil moisture, soil temperature, and substrate. Under high soil moisture, Canada thistle roots are susceptible to damping off drought tolerance in established plants. In northern locales (Sweden) mild winters are linked to spread of Cirsium arvense, as growth begins earlier in the spring when more roots survive the winter. Cirsium arvense roots are cold-sensitive, injured when directly exposed to cold temperatures for 8 hours at -2°C, and dying after 8 hours at -6°C.
GROWTH
Plants grow rapidly from seed, developing roots 1.5 m deep at the end of the first growing season, and flowering the second year. Seedlings first develop a branched primary root 5-10 cm deep, and then produce their first true leaves. Roots grow rapidly in young plants, up to 1 cm/day in the first 13 weeks. Growth is strongly influenced by environmental factors. Seedlings require high light and low competition to survive. Seedlings grow rapidly under high humidity (90-100%), with a 50% increase in stem height and both shoot and root weight compared to seedlings growing at 50% humidity. Established plants develop drought tolerance by increasing root length in the top 30 cm of soil. However, shoot density decreases the year following a growing season drought.
Habitat
Throughout its distribution, Cirsium arvense is found in almost all upland herbaceous communities. It poses a special danger to riparian habitats and prairie communities. It is unable to thrive in tropical climates, even higher altitude places like East Africa. In addition, it spreads from the western plains through the northern portion of the intermountain west to the Sierra Nevada and Cascade ranges, invading riparian zones and irrigation ditches. Cirsium arvense grows on tussocks raised above the typical high water line in deteriorated sedge meadows in the upper Midwest (Wisconsin and Illinois). Cirsium Arvense is commonly found in sedge meadows and prairie marshes in Canada. It is common along roadsides, in pastures, old fields, croplands, and deep, well-aerated, mesic soils throughout its range. It can occasionally be found in relatively dry environments in eastern North America, such as sand dunes and sandy fields, as well as on the margins of wet environments, such as lakeshores, stream banks, cleared swamps, muskegs, and ditches. The Canada thistle is sensitive of shade. It is rarely seen in actual forests, preferring to grow on the margins of both deciduous and coniferous forests. All soil types, including clay, clay loam, silt loam, sandy loam, sandy clay, sand dunes, gravel, limestone, and chalk, except for wet, poorly aerated soils, are suitable for the growth of Cirsium arvense.
Phytochemical Composition
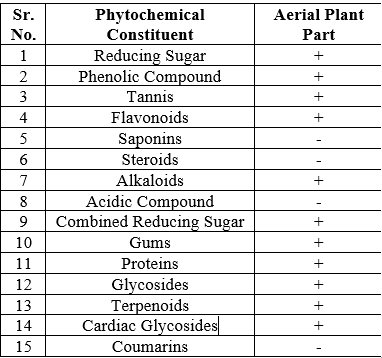
It was discovered that the plant was a good source of secondary metabolites. Alkaloids are among the phytochemicals found in Crisium Arvence.
Flavonoids
Flavonoids, a type of polyphenol present in medicinal plants, have been shown to possess anti-inflammatory and anticancer activities. All three forms of flavonoids—flavones, flavonoids, and flavanols—are found in the various Cirsium species. C. rivulare, C. japonicum, and C. arvense contain the bulk of flavonoids that have been identified. The chemical analysis identified several significant chemicals, including pectolinarin, luteolin, luteolin 7-O-?-D-glucoside, apigenin, apigenin 7-O-glucoside, and hispidulin, that were detected in several Cirsium species(13).
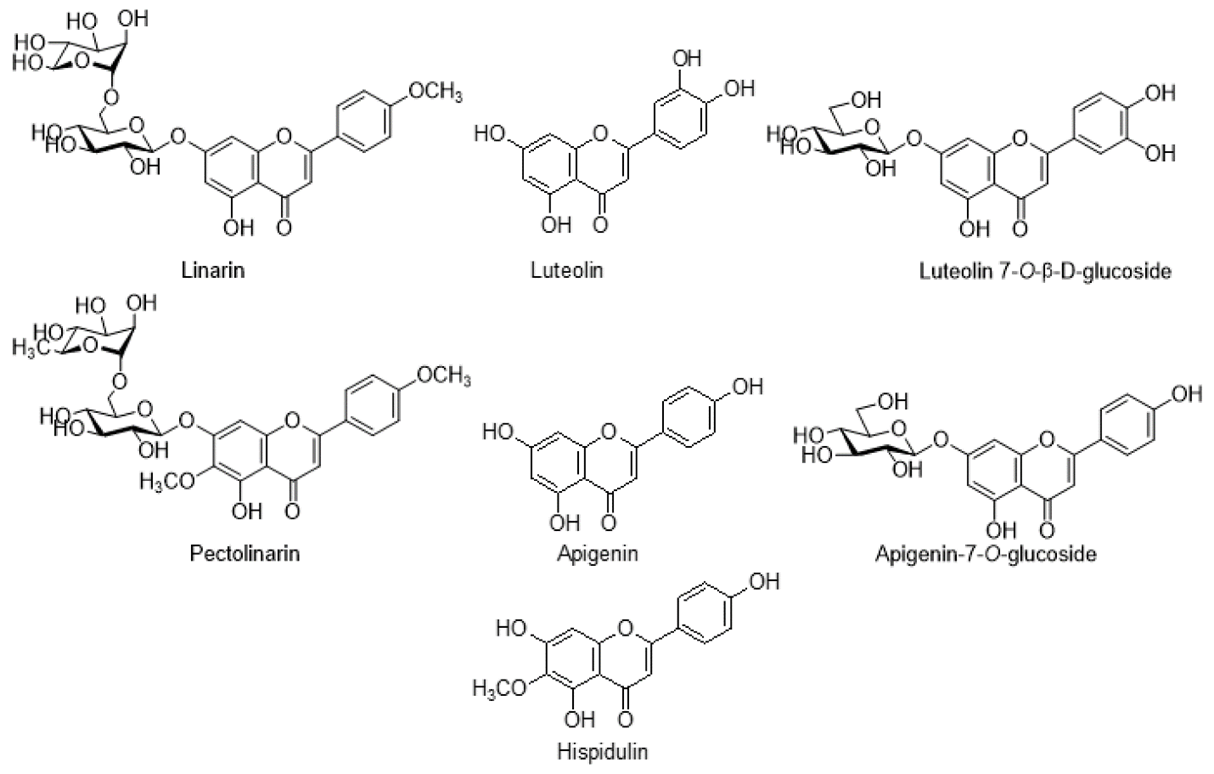
Figure 1 Commonly found flavonoids in different species of Cirsium.
The flavones Pectolinarigenin, Acacetin, 5,7-Dihydroxy-6,4?-dimethoxyflavone, Cirsimaritin, Cirsimarin, Rutoside, and Tricin were among the other compounds that were poorly distributed in Cirsium. In addition, flavones glucoside were also discovered in Cirsium species: 6-Hydroxyluteolin 7-O-glucoside, Pectolinarigenin-7-O-glucopyranoside, Hispidulin-7-neohesperidoside, Hispidulin-7-glucoside, Luteolin 7-O-?-D-glucuronide, Isokaempferide-7-glucuronide, Isokaempferide-7-O-?-D-(6?-methylglucuronide), Apigenin 7-(6?-methylglucuronide), and Isokaempferide-7-O-glucuronide. These polyphenolic phytoconstituents include Scopoletin, 6,7-Dimethoxycoumarin, 4-Vinyl guaiacol, and 4-Ethyl guaiacol, found in some species of Cirsium. These compounds confer a natural fragrance on plants and have fungicidal properties. They can prevent conidium germination and the extension of the germ tube in a variety of plant-pathogenic fungus. The flavones Pectolinarigenin, Acacetin, 5,7-Dihydroxy-6,4?-dimethoxyflavone, Cirsimaritin, Cirsimarin, Rutoside, and Tricin were among the other compounds that were poorly distributed in Cirsium. In addition, flavones glucoside were also discovered in Cirsium species: 6-Hydroxyluteolin 7-O-glucoside, Pectolinarigenin-7-O-glucopyranoside, Hispidulin-7-neohesperidoside, Hispidulin-7-glucoside, Luteolin 7-O-?-D-glucuronide, Isokaempferide-7-glucuronide, Isokaempferide-7-O-?-D-(6?-methylglucuronide), Apigenin 7-(6?-methylglucuronide), and Isokaempferide-7-O-glucuronide. These polyphenolic phytoconstituents include Scopoletin, 6,7-Dimethoxycoumarin, 4-Vinyl guaiacol, and 4-Ethyl guaiacol, found in some species of Cirsium. These compounds confer a natural fragrance on plants and have fungicidal properties. They can prevent conidium germination and the extension of the germ tube in a variety of plant-pathogenic fungus.
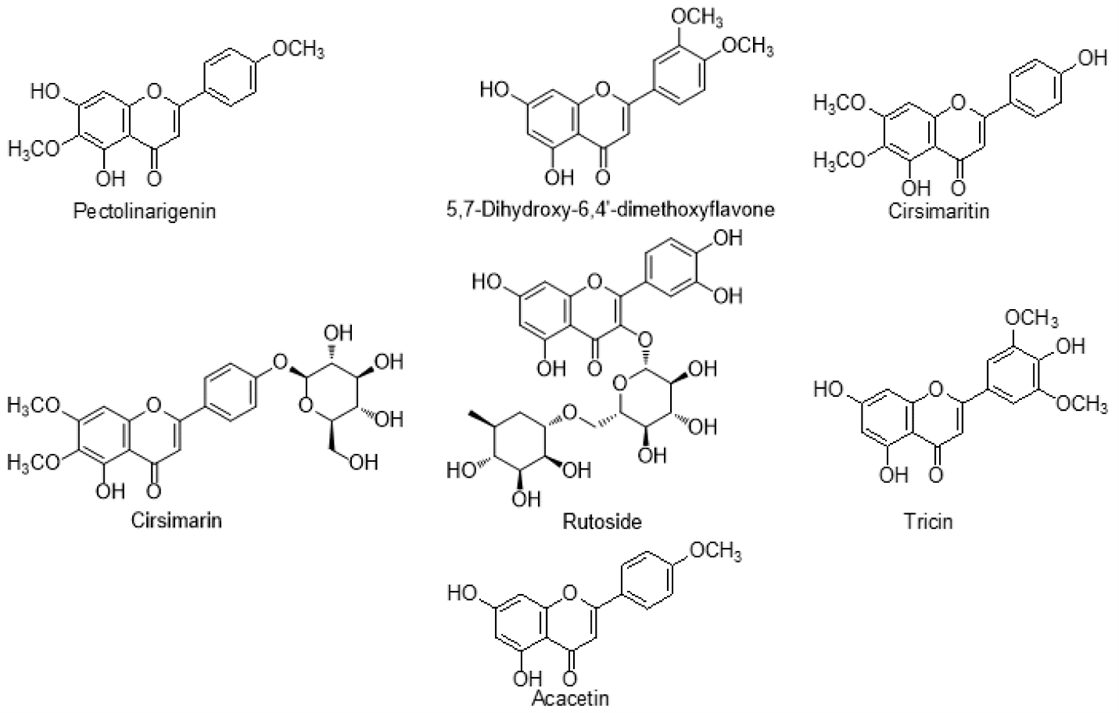
Infrequently distributed flavonoids in Cirsium species.
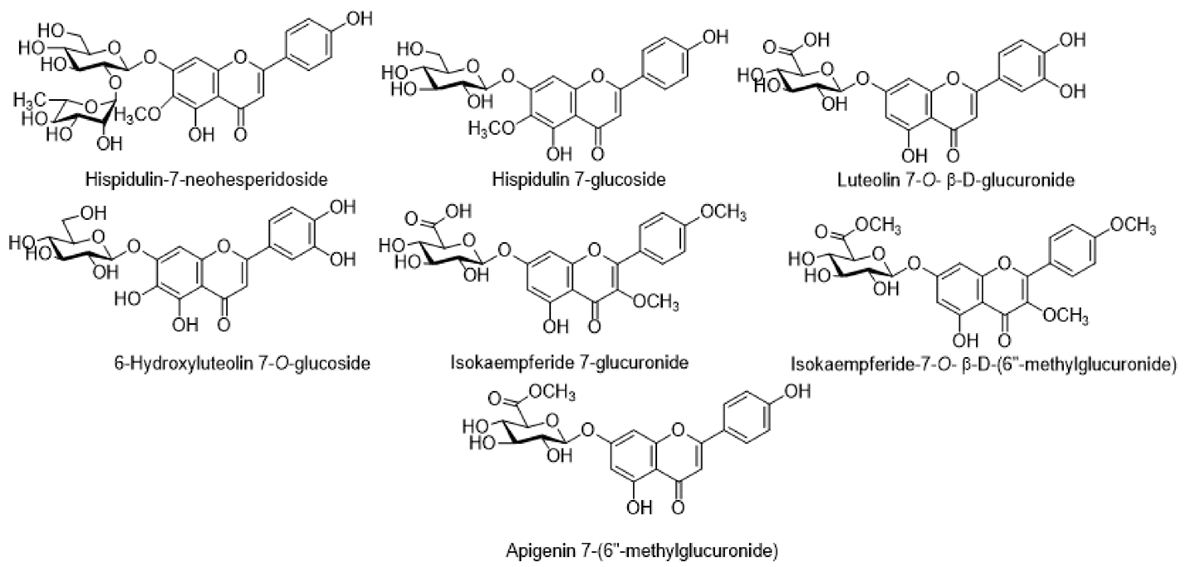
Major flavone glucoside detected in different species of Cirsium.
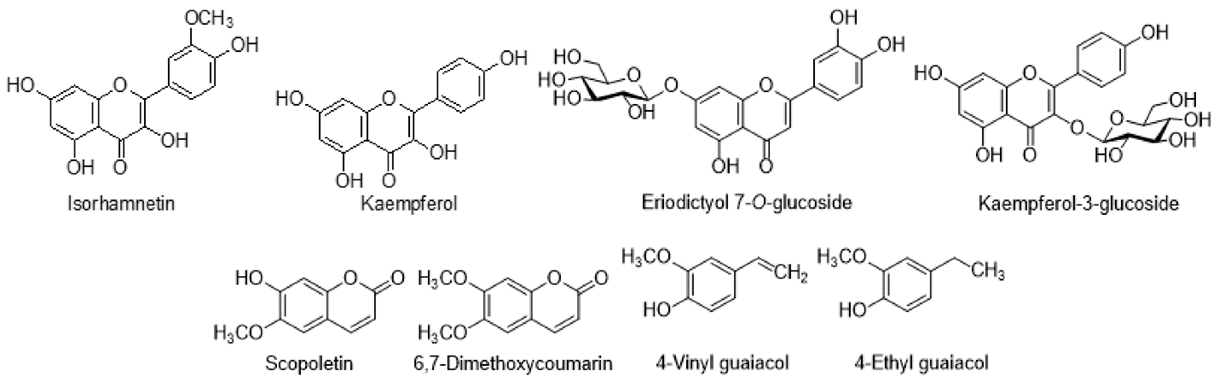
Other polyphenolic compounds isolated from Cirsium species.
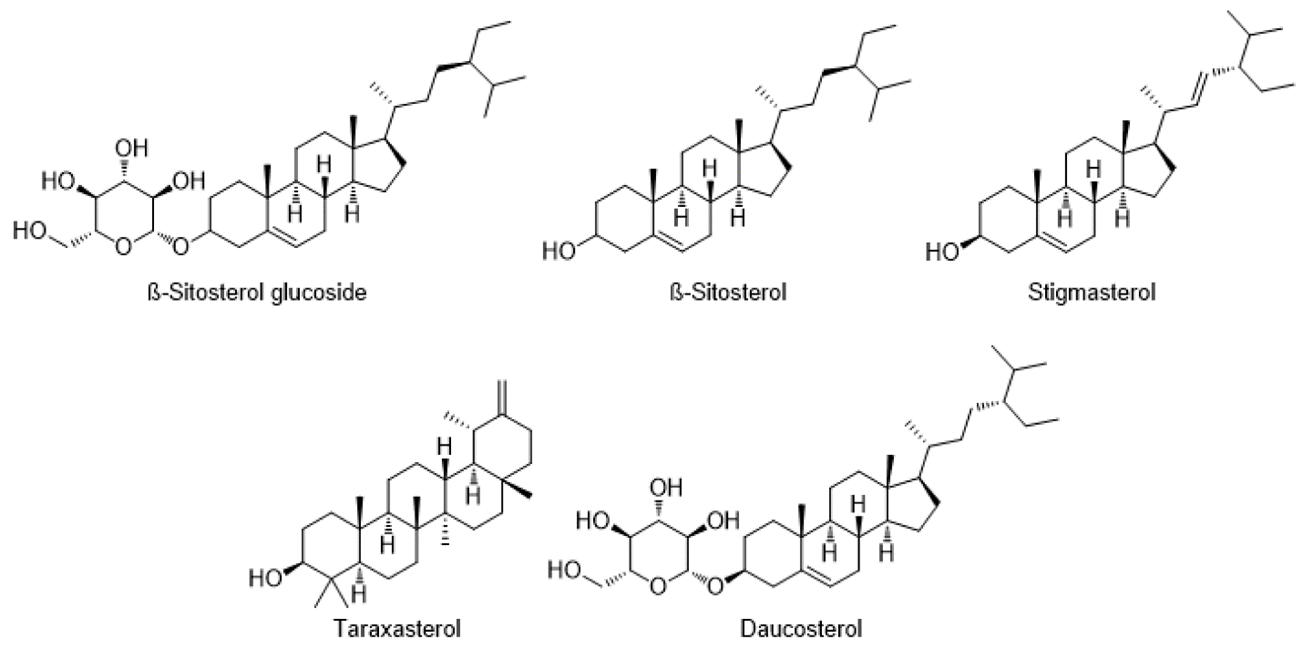
Terpenoids and Sterols:
Terpenes, also known as terpenoids, are the most abundant secondary products. They are synthesized by the Mevalonic acid pathway in plant chloroplasts and have been shown to have immunomodulatory properties. Terpenes include lupeol, luteol acetate, taraxasterol acetate, 25-Hydroperoxycycloart-23-en-3?-ol, 24-Hydroperoxycycloart-25-en-3?-ol, ?-Amyrin acetate, ?-Amyrin acetate, and ?-Amyrin(5).
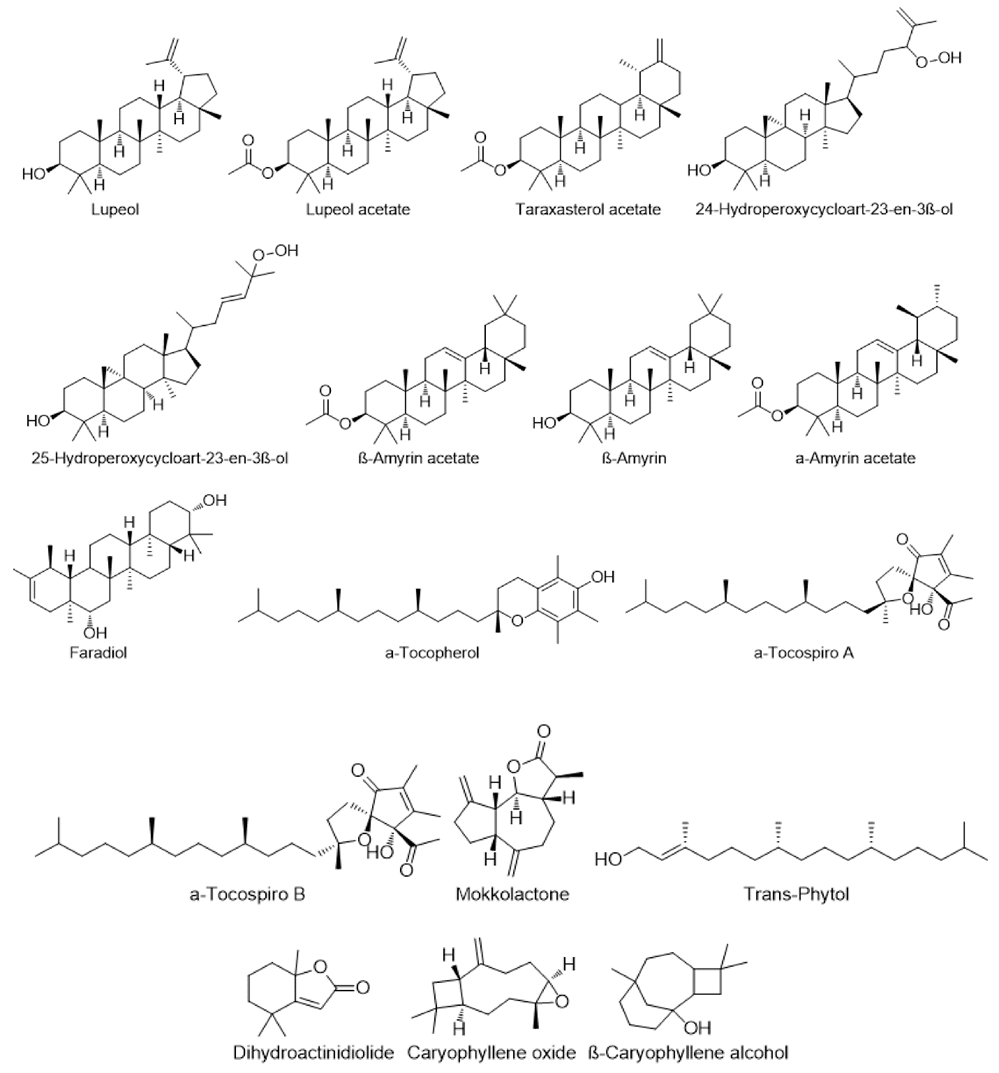
Terpenoids found in different species of Cirsium.
Major sterols identified in different species of Cirsium.
Phenolic Acids
Trans-Cinnamic acid, p-Coumaric acid, p-Hydroxybenzoic acid, Vanillic acid, Chlorogenic acid, Protocatechuic acid, and Caffeic acid were identified to be phenolic compounds in Cirsium species.
Major phenolic acids found in different species of Cirsium.
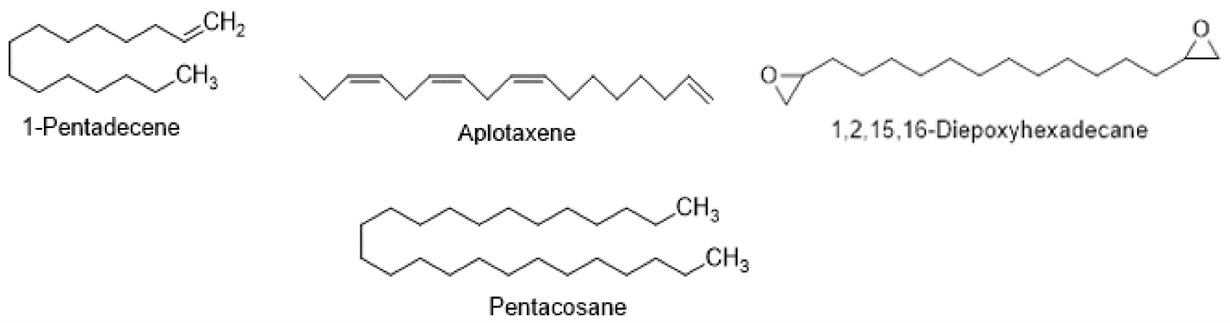
Polyacetylenes, Acetylenes, and Hydrocarbons
Aplotaxene, Dihydroaplotaxene, Pentacosane, Tetrahydroaplotaxene, and 1-Pentadecene are examples of Acetylenes and Polyacetylenes, which have been found in the Cirsium. Despite this, studies have shown that Acetylenes and Polyacetylenes are distinct members of the Asteraceae family. It was discovered that 1,2,15,16-Diepoxyhexadecane and ciryneol were the two hydrocarbons present in the Cirsium. All of these acetylenes and polyacetylenes were extracted from the non-aerial section of the Cirsiumse.
Fatty acids, Aldehydes, and Ketones
Hexadecanoic acid, 9,12,15-Octadecatrienoic acid, 9,12-Octadecadienoic acid, and palmitic acid were discovered in the Cirsium. Sinapaldehyde and 6,10,14-Trimethyl-2-pentadecanone were also identified.
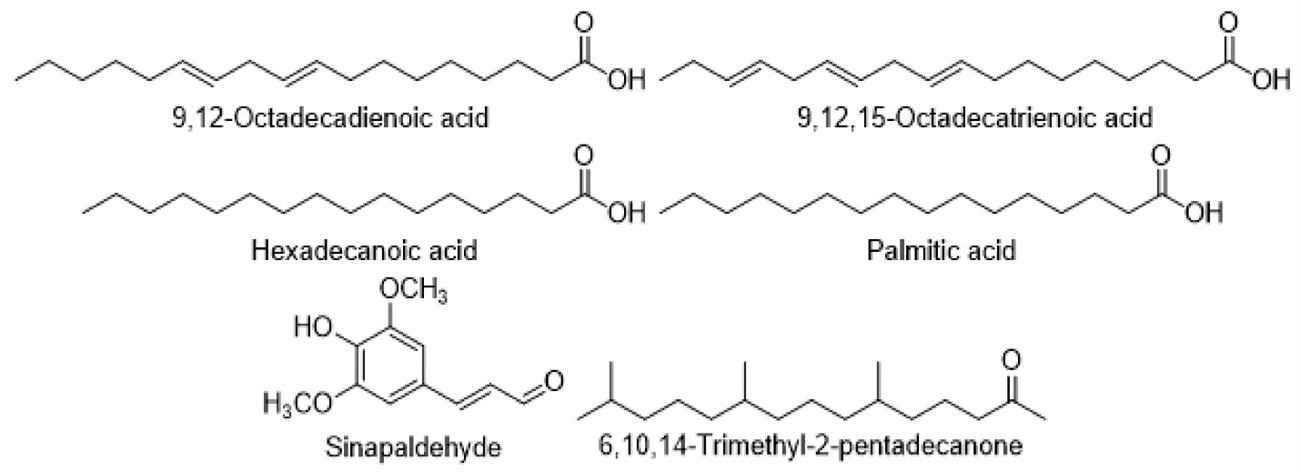
Polyacetylenes, acetylenes, and hydrocarbons found in Cirsium species. The fatty acids found in the Cirsium were 9,12,15-Octadecatrienoic acid, 9,12-Octadecadienoic acid, Hexadecanoic acid, and Sinapaldehyde. Palmitic acids found in the Cirsium were 6,10,14-Trimethyl-2-pentadecanone.
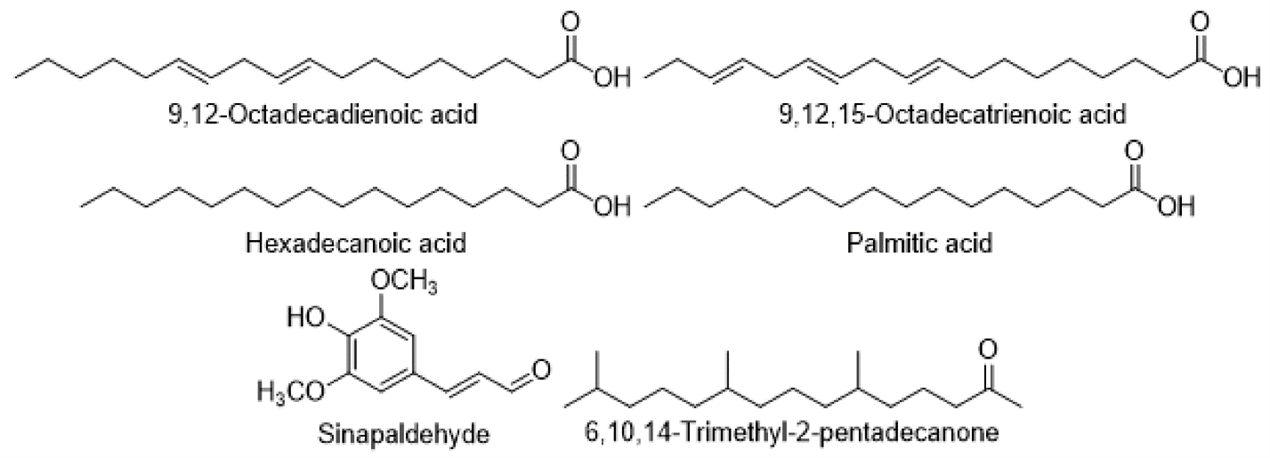
Fatty acids, aldehydes, and ketones found in Cirsium species.
Essential Oil Composition
Cerebella acaule, Cerebella arvense, Cereticum, Cerressatum, Ceres dissectum, Cerephorum eriophorum, Cerephyllum heterophyllum, Cerebella japonicum, Cerebella ligulare, Cereraceum, Cerebella palustre, Cerebella pannonicum, Cerebella rivulare, and Ceres eriophorum species Hexadecanoic acid and aplotaxene were found to be abundant in Cirsium essential oil. C. japonicum, C. palustre, and C. rivulare had the highest quantities of aplotaxene.
However, hexadecanoic acid concentrations were found to be higher in C. japonicum, C. creticum, and C. arvense. Additional components found in C. palustre and C. rivulare were (Z)-8,9-Epoxyheptadeca-1,11,14-triene [Pentadecanoic acid, Heptacosane, Heptadecanoic acid, Tetradecanoic acid, Palmitic acid, Caryophyllene oxide, and Myristic acid in C. japonicum rhizomes]; ?-Bisabolol, ?-Cadinene, Hexacosane, ?-Selinene, ?-Humulene, Docosane, Octadecane, Eicosane, Germacrene-D, and Nonacosane in C. palustre and C. rivulare. Thymol was detected in C. decussatum and C. pannonicum inflorescences(14).
Pharmacological Studies
Cirsium has a diverse array of phytochemicals that contribute to its rich pharmacological activity. The several species of Cirsium have been found to possess a wide range of biological qualities to date, including antimicrobial, anti- analgesic, anticancer, hepatoprotective, and anti-inflammatory effects.
Antimicrobial Activity
Nazaruk and Jakoniuk evaluated the bacteria strains Escherichia coli, Bacillus subtilis, Pseudomonas aeruginosa, Micrococcus, and Staphylococcus aureus as well as the fungus Candida albicans in order to determine the microbicidal capability of Cirsium rivulare's flowers and leaves. All of the extracts from Cirsium rivulare were found to have antiproliferative and bactericidal effects. Conversely, research demonstrated that a water extract derived from Cirsium leaves shown more efficacy in combating Gram-positive bacteria. Cirsium arvense extracts, including Tracin, 9,12,15-Octadecatrienoic acid, Luteolin, Hispidulin, and ?-Tocopherol, were tested for antibacterial effectiveness against diverse bacterial strains. Tracin, Luteolin, and Hispidulin demonstrated significant protection against bacterial strains, while ?-tocopherol had moderate antibacterial action. But the bactericidal action of 9,12,15-Octadecatrienoic acid was minimal.It has been demonstrated that C. arvense, C. oleraceum, or C. palustre possess strong antibacterial properties. Compared to the leaf extract, the flower extract of the Cirsium species exhibited a stronger antibacterial action. With MIC and MBC values ranging from 25 to 200 ?g/mL for six strains of S. aureus, methanol extracts from Cirsium roots exhibit a high bactericidal action against both Gram-positive and Gram-negative bacteria in a range of fungi(8).
Antioxidant Activity
Numerous studies conducted in vitro revealed that C. arvense's roots, leaves, and flowers exhibited a strong antioxidant effect. Hossain et al. (2016) investigated the antioxidant activity of C. arvense ethanolic extract. The ethanolic extract of C. arvense was found to exhibit strong antioxidant properties. In a different study, the ABTS method was used to track the total antioxidant potential of crude aqueous extracts of the Cirsium species. The highest levels of antioxidant activity were found in C. vulgare (2.31 m/mL), C. rivulare (2.78 m/mL), C. palustre (2.78 m/mL), C. oleraceum (2.76 m/mL), and C. arvense (2.74 m/mL).In a different investigation, Lee et al. assessed the antioxidant potential of C. setidens leaf and root extracts using the DPPH free radical test. The butanolic portion of leaves and roots was found to have IC50 values of 33.53 g/mL and 9.75 g/mL, respectively. It was discovered that C. setidens has a greater capacity for scavenging free radicals than both tocopherol and ascorbic acid. Furthermore, a comparison between the hexane extract and the methanolic extract of C. rivulare roots revealed greater DPPH scavenging activity. Kaempferol-3-O-L-rhamnopyranoside, vanillic acid, and balanophonin that were extracted from C. sipyleum, C. eriophorum, and C. leucopsis demonstrated significantly higher levels of antioxidant activity(15).Chlorogenic acid was shown to be the most powerful antioxidant ingredient among the several species of Cirsium in Poland. Another study discovered that hexane extracts of Cirsium species contain the active component Linoleic acid, which demonstrated a significant antioxidant activity. According to a different study, luteolin and silibinin are the main antioxidant components of Cirsium japonicum. Additionally, certain species of Cirsium included luteolin, apigenin, and their glucosides, which had antioxidant properties.
Analgesic and Anti-Inflammatory Activity
Analgesic and anti-inflammatory properties of aqueous extracts from the aerial part of C. subcoriaceum and its active ingredient pectolinarin were observed. It was found that pectolinarin and crude extract of C. subcoriaceum inhibited mice's writhing in response to acid in a concentration-dependent way. It was found that the crude extract of C. subcoriaceum (ED50 83.18 mg/kg) was less effective as an analgesic than pectolinarin (ED50 28.44 mg/kg). At comparable concentrations, Pectolinarin was found to exhibit comparable protective efficacy to Acetylsalicylic, a common analgesic chemical.Pectolinarin and C. subcoriaceum water extract prevented carrageenan-induced edema. C. subcoriaceum and pectolinarin were found to have ED30 values of 25 mg/kg and 3.7 mg/kg, respectively.Similarly, in LPS-treated macrophages, pectolinarigenin and pectolinarin isolated from the aerial portions of C. cinerea inhibited cyclooxygenase-2 mediated prostaglandin E2 synthesis and leukotrienes, resulting in reduced eicosanoid production(16).
Hepatoprotective Activity
The hepatoprotective efficacy of C. arisanense aqueous extract of roots and leaves was observed in mice and mammalian cell lines with hepatocellular cancer. The hepatocellular carcinoma cells were protected against tacrine-stimulated hepatotoxicity by the roots and leaves of C. arisanense, which also reduced the expression of the hepatitis B surface antigen. Rats' CCl4-induced liver damage was lessened when 500 mg/kg of C. setidens butanol extract was administered. The levels of the antioxidant indicators glutathione peroxidase, superoxide dismutase (SOD), and glutathione peroxidase increased in the rats' livers after C. setidens treatment. The biochemical analysis was supported by histological investigations, which showed that the C. setidens extract significantly reduced the amount of hepatic ballooning degeneration. Furthermore, the hepatic derangement in rats caused by galactosamine toxicity was lessened by the active ingredients pectolinarin and pectolinarigenin found in methanolic extracts of C. setidens leaves. Following treatment with pectolinarin and pectolinarigenin, there was a decrease in the concentration of SGOT, SGPT, alkaline phosphatase (ALP), and lactate dehydrogenase (LDH), suggesting the hepatoprotective potential of both components(17).
Immunomodulatory Activity
Mice tumor growth was inhibited by C. japonicum and its phytoconstituent pectolinarin, which also improved cellular and humoral immune responses. It also improved spleen cell transformation and increased natural killer cell activity in tumor-bearing animals by stimulating the complement system(18).
Antifungal Activity
Methanol was used to extract the asteraceous weed Circium arvense's leaves, stems, roots, and inflorescence during a two-week period. We used a rotary evaporator to evaporate methanol. Using malt extract broth as a growth medium, an investigation was conducted on the antifungal properties of methanolic extracts at varying doses (1, 2, 3, 4, and 5%) against Macrophomina phaseolina. Extraction of every plant part generally exhibited varying degrees of antifungal activity. The methanolic extract of leaves exhibited the best antifungal activity, with the biggest reduction in fungal biomass over control being observed in the cases of stem and root extracts (10-74%, 6-57%, and 11-39%, respectively). With a 2-30?crease in fungal biomass over control, inflorescence extract exhibited the least amount of antifungal efficacy.The results of this study indicate that methanolic leaf extracts of C. arvense can be used for the management of M. phaseolina. There was a linear and inverse relationship found between extract concentrations and fungal biomass for extracts of all four parts. GC-MS analysis revealed that the most effective methanolic leaf extract contained ten compounds: 10-octadecanoic acid, methyl ester (26.442%), 2H-1-benzopyran, 6,7-dimethoxy-2-2-dimethyl (20.195%), hexadecanoic acid, methyl ester (15.752%), and 9,12-octadecadienoic acid (Z,Z)-, methyl ester (12.628%)(19).
Anticancer Activity
Numerous studies demonstrate the anticancer properties of ceria and its prospective therapeutic potential in preventing cancer. The anticancer action of pectolinarin and 5,7-Dihydroxy-6,4-dimethoxyflavone from C. japonicum was observed in mice. It was shown that in a concentration-dependent way, both chemicals demonstrated a notable decrease in the proliferation of tumor cells. Similarly, in MDA-MB-231 cells, the extracts of Cirsium japonicum and Cirsimaritin reduced the number of breast cancer cells as shown by the inhibition of Akt, VEGF, and ERK expression. Moreover, C. japonicum extract inhibited the growth of MCF-7 cancer cells during the G1 phase of the cell cycle and stimulated apoptosis in the cells via affecting the pathways leading to mitochondrial death. Cirsium setosum contains triterpenes that have been shown to have modest cytotoxicity against human colon and ovarian cancer cells. On the ovarian tumor cell line A2780, however, 3?-Hydroxy-22-oxo-20-dandelion-30-oleic acid showed a strong selective suppression. Silybin, which comes from Cirsium japonicum, inhibited the growth of gastric cancer cells by lowering the synthesis of proteins involved in the cell cycle and preventing the growth of gastric tumor cells during the G2/M phase, which led to the death of the cells(20).
Oviposition Stimulatory Activity
Handling of roots Ostrinia zealis underwent oviposition in response to the essential oil of C. japonicum. It has been noted that the primary component of plant root essential oil, Aplotaxene, may act as an oviposition stimulant. In a different study, ovariectomized rats treated with C. japonicum extract showed a significant decrease in body weight, triglycerides, and cholesterol along with a notable increase in bone mineral density and estradiol. Furthermore, the active phytoconstituents of C. japonicum have a binding affinity with the ligand-binding sites of the estrogen receptor, according to molecular docking studies. It suggested that C. japonicum extract may be useful in easing pre- and post-menopausal symptoms(18).
Anti-Anxiety Effect
The anti-anxiety effect of Cirsium japonicum ethanolic extract in mice was tested. In the open-field test, the C. japonicum extract had no influence on the mice's mobility. It did, however, result in more exploration of the open middle zone. Plant extract administration also lengthened the mice's stay in the elevated plus-maze test, indicating that Cirsium japonicum has anti-anxiety qualities. The anti-anxiety effects of C. japonicum were shown to be comparable to those of benzodiazepines. Additionally, the anti-anxiety properties of C. japonicum were verified through testing on human neuroblastoma cells. In neuroblastoma cells, treatment with C. japonicum increased the influx of chloride ions in a concentration-dependent manner; this was lowered by co-administering bicuculline.In another study, C. japonicum significantly reduced immobility in mice during the forced-swimming test, which had an effect akin to that of an antidepressant. In the open field test, it was found that C. japonicum treatment did not increase locomotor activity. Furthermore, it increased Cl- ion inflow while having little effect on monoamine uptake in mammalian neuroblastoma cells. The only active ingredient in the C. japonicum extract that demonstrated comparable antidepressant-like effects was luteolin. Thus, it was thought that the luteolin present in C. japonicum extract was responsible for its antidepressant-like effects(21).
Nephroprotective
Circium protects the kidney from a variety of poisons, which makes it nephroprotective. In diabetic rats produced by streptozotocin and fed a high-carbohydrate/high-fat diet, the nephroprotective effects of pectolinarin and flavones isolated from Cirsium japonicum were investigated. In rats, both flavones showed signs of antidiabetic action. In diabetic rats, however, a combination of pectolinarin and 5,7-Dihydroxy-6,4'-dimethoxy flavone was found to be more effective in lowering triglycerides, glucose, and cholesterol. When diabetic rats were treated with flavones, aberrant levels of enzymes linked to glucose metabolism were reversed. The flavones did not appear to have any effect on the aberrant concentration of insulin, leptin, or glucose transporter 4, but it did increase the concentration of adiponectin in diabetic rats. Furthermore, Cirsium japonicum flavones increased PPAR? levels, which in turn enhanced adipocyte growth. Because adipocytes have higher amounts of GLUT4 and adiponectin, it increased their uptake of glucose caused by insulin(21)(22).
Other Therapeutic Effects
In rats treated with amyloid ?-peptide, treatment with C. japonicum (50–100 mg/kg/day) improved cognitive abilities by reducing oxidative stress; this herb has the potential to be a powerful treatment for Alzheimer's disease. Furthermore, numerous researchers have looked into the mechanism and hemostatic effect of various Cirsium species. When rats were given Cirsium setosum extract, it significantly affected blood coagulation, hemostasis, and bleeding. The hemostatic effect of the nanoscale components in Cirsium charcoal was previously observed by Wang (2018). Moreover, the in vitro enzyme inhibitory activities of Shikokiol A, which was extracted from the roots of C. nipponicum, were examined. It was found to be a strong inhibitor of lipoxygenase, an enzyme that does not contain heme iron, during tracheal contraction in guinea pigs. Anti-acne formulation: As effective acne remedies, a mixture of Quercus robur, Sesamum indicum, Houttuynia cordata, Cirsium japonicum, and Thuja orientalis was created. Maximum antibacterial activity against C. acnes was found in this formulation.
moisturizer and booster of ceramide synthesis: Cirsium japonicum alone or in combination with Melia toosendan, Indigofera tinctoria, Catalpa ovata, Tagetes erecta, and Chenopodium hybridum demonstrated a remarkable ability to increase ceramide in the composition.
Pharmacokinetics
Pharmacokinetics plays a crucial role in preclinical drug development, toxicity assessment, and drug concentration optimization. It can be quantified as a useful method of identifying putative active ingredients and elucidating the mechanism of action of medicinal formulations or botanicals. The pharmacokinetic behavior of Cirsium has not been the subject of many scientific studies, according to the literature. The various flavonoids of C. setosum in rat plasma were observed using a liquid chromatography–mass spectrometry (LC-MS) method. Rutin, Acacetin, Naringin, Wogonin, and Quercetin were found to be the long-acting constituents of C. setosum, with greater bioavailability and elimination time. In a similar vein, Zhang et al. used UPLC-MS to identify 27 flavonoids in the blood, bile, and urine of rats following the administration of Cirsium japonicum. The maximum concentrations of Linarin, Pectolinarigenin, Hispidulin, Pectolinarin, Diosmetin, Acacetin, and Apigenin in plasma were found to be 86 ng/mL, 6 ng/mL, 32 ng/mL, 876 ng/mL, 37 ng/mL, 19 ng/mL, and 148 ng/mL, respectively, in another study that involved rats treated with the Cirsium japonicum extract.
Pectolinarin, Linarin, Pectolinarigenin, Hispidulin, Diosmetin, and Acacetin were quickly absorbed and within five minutes of reaching their peak plasma concentration. However, after 360 minutes of administration, the maximum concentration of apigenin was reached despite its slow absorption.
Toxicology
The expansion of novel medications or drugs necessitates a systemic safety investigation of Cirsium extract. Rats treated orally with 1.25, 2.5, and 5 g/kg body weight of C. setidens extract for 28 days did not show any appreciable changes in toxicological parameters including hematological, biochemical parameters, or mortality. The liver, heart, spleen, and kidney all showed normal histological architecture after treatment with C. setidens extract, indicating the plant extract's broad safety index. Likewise, no discernible toxic effects or mutagenicity were observed after 15 days of treatment with C. japonicum extract at a dose of 2 g/kg body weight.
CONCLUSION
The crisium arvence is a small herb which possess various pharmacological activities such as anticancer, hepatoprotective, anti-inflammatory, antifungal and nephroprotective. The surprice and extensive work done on the herb exhibit many numbers of phytoconstituents such as terpenoids, flavonoids, sterols and alkaloids which are responsible for pharmacological profile. The herb is ignored by scientific community and are kept in the category of weeds. Somewhere which is not justified in our point of view seen the potential of plant for many other activities in the treatment of various disease. The herbs may show extraordinary result and it is abundantly available for any type of scientific regions throughout the world. The medicinal herbs categorized in weeds but exhibit very significant reported by reference 19. The review in this view about study is worth explaining the potential of crisium arvence.
REFERENCE:
- Donald WW. The biology of Canada thistle (Cirsium arvense). Rev Weed Sci. 1994;6:77–101.
- Shahrajabian MH. Medicinal herbs with anti-inflammatory activities for natural and organic healing. Curr Org Chem. 2021;25(23):2885–901.
- Nisar Z, Shah SMA, Ayaz S, Rashid A, Mustafa I. TARAXACUM OFFICINALE AND CIRSIUM ARVENSE METHANOLIC EXTRACTS AMELIORATE OXIDATIVE STRESS AND LIPID PROFILE IN HIGH FAT DIET-INDUCED HYPERCHOLESTEROLEMIC RATS. J Popul Ther Clin Pharmacol. 2023;30(19):472–82.
- Tienda-Vázquez MA, Morreeuw ZP, Sosa-Hernández JE, Cardador-Martínez A, Sabath E, Melchor-Martínez EM, et al. Nephroprotective plants: a review on the use in pre-renal and post-renal diseases. Plants. 2022;11(6):818.
- Sultana S, Hossain ML. Assessment of Neuropharmacological Activity of Methanol Extract of Leaves of Nerium oleander (Family: Apocynaceae). Saudi J Med Pharm Sci. 2020;149–54.
- Refaat J, Desoukey SY, Ramadan MA, Kamel MS. Rhoifolin: A review of sources and biological activities. Int J Pharmacogn. 2015;2:102–9.
- Demirtas I, Tufekci AR, Yaglioglu AS, Elmastas M. Studies on the antioxidant and antiproliferative potentials of Cirsium arvense subsp. vestitum. J Food Biochem. 2017;41(1):e12299.
- Khan ZUH, Khan S, Chen Y, Wan P. In vitro antimicrobial activity of the chemical constituents of Cirsium arvense (L). Scop. J Med Plants Res. 2013;7(25):1894–8.
- Tiley GED. Biological flora of the British Isles: Cirsium arvense (L.) scop. J Ecol. 2010;98(4):938–83.
- Donald WW. Management and control of Canada thistle (Cirsium arvense). Rev weed Sci. 1990;5:193–249.
- Heimann B, Cussans GW. The importance of seeds and sexual reproduction in the population biology of Cirsium arvense?a literature review. Weed Res. 1996;36(6):493–503.
- Khan ZUH, Ali F, Khan SU, Ali I. Phytochemical study on the constituents from Cirsium arvense. Mediterr J Chem. 2011;1(2):64–9.
- U.G. MihiriEkanayakeabcdHelapiyumiWeerathungaabJanithWeerasingheabcdEric R.WaclawikabZiqiSunabJennifer M.MacLeodabAnthony P.O’MullaneabKostya (Ken)Ostrikovabcd. scholar. 2022. p. 45–9.
- De Bruyn A, De Taeye L, Simonds R, Verzele M, De Pauw C. Alkaloids from Catharanthus Roseus. Isolation and Identification of 17?Desacetoxyvinblastine and 17?Desacetoxyleurosine. Bull des Sociétés Chim Belges. 1982;91(1):75–85.
- Demirtas I, Tufekci AR, Yaglioglu AS, Elmastas M. Studies on the Antioxidant and Antiproliferative Potentials of Cirsium arvense subsp. vestitum. J Food Biochem. 2017;41(1).
- Vijayabhaskar K, Chaitanyaprasad K, Srisailam K, Arunadevi NM, Swathi S, Subhashini P. Analgesic and Anti-Inflammatory Activities of the Extract of C Assia Occidentalis Linn Animal Model. Int J Res Pharm Chem. 2013;3(4):759–62.
- Mag P, Martínez EF, Santana MJ, Álvarez MC, Martín J, Valencia T, et al. Hepatoprotective Effects of Nonpolar Extracts from Inflorescences of Thistles Cirsium vulgare and Cirsium ehrenbergii on Acute Liver Damage in Rat. 2018;
- Aggarwal G, Kaur G, Bhardwaj G, Mutreja V, Sohal HS, Nayik GA, et al. Traditional Uses, Phytochemical Composition, Pharmacological Properties, and the Biodiscovery Potential of the Genus Cirsium. Vol. 4, Chemistry. 2022. p. 1161–92.
- Banaras S, Javaid A, Shoaib A, Ahmed E. Atividade antifúngica dos extratos de cirsium arvense contra o fungo fitopatogênico macrophomina phaseolina. Planta Daninha. 2017;35(April).
- Sahli R, Rivière C, Dufloer C, Beaufay C, Neut C, Bero J, et al. Antiproliferative and Antibacterial Activities of Cirsium scabrum from Tunisia. Evidence-based Complement Altern Med. 2017;2017.
- Sultana S, Hossain L. Evaluation of Anxiolytic and Antibacterial Activity of Methanol Extract of Leaves of Mimosa pudica. 2019;7(3):44–9.
- 22. Suliska N, Praviska M, Kurniati NF, Sukandar EY. Nephroprotective effect of ethanol extract of sonchus arvensis L. Leaves in gentamicin-piroxicam induced rat renal failure. J Res Pharm. 2021;25(4):4


 Shivani*
Shivani*
 Dev Prakash
Dev Prakash















 10.5281/zenodo.11402556
10.5281/zenodo.11402556