Abstract
Millions of people worldwide suffer from epilepsy, a complicated neurological condition, with many of those patients developing drug-resistant epilepsy.(1)Epilepsy diagnosis and treatment are inextricably linked; proper therapy can only be provided in the absence of an accurate diagnosis. It is uncommon to witness a seizure during the first medical checkup or at an outpatient clinic. As a result, the confirmation and diagnosis of seizure types are typically based mainly on the history obtained from parents or caregivers.(2)The objective of this review is to discuss about the recent developments in diagnostic techniques and therapeutic interventions on epilepsy.
Keywords
Diagnostic Techniques and Therapeutic Interventions, treatment are inextricably, neurological condition.
Introduction
Epilepsy is the most common chronic and dangerous neurological condition, affecting 65 million individuals worldwide. People with epilepsy face discrimination, ignorance, social stigma, and the stress of living with a chronic unpredictable disease that can lead to loss of autonomy in daily tasks. Epilepsy is theoretically defined as "an enduring predisposition of the brain to generate epileptic seizures, with neurobiological, cognitive, psychological, and social consequences.[3] According to the operational definition, epilepsy is thought to be present when any of the following conditions are met: (i) at least two unprovoked (or reflex) seizures occurring more than 24 hours apart; (ii) one unprovoked (or reflex) seizure and a probability of further seizures similar to the general recurrence risk after two unprovoked seizures (at least 60%) occurring over the next ten years; and (iii) diagnosis of an epilepsy syndrome.[3] Diagnosis is problematic since, in practice, the diagnostic electrical characteristic of epilepsy may be absent interictally, particularly in adults or when seizures are infrequent, and interictal epileptiform discharges may infrequently be present in persons without seizures. Furthermore, in rare cases, a "epileptic EEG" may be coupled with an epileptic encephalopathy in which overt seizures are minimal or absent, such as Landau-Kleffner syndrome, and a cognitive impairment dominates the presentation.[4] Among the commonly utilized techniques are computerized tomography (CT), magnetic resonance imaging (MRI), electroencephalography (EEG), positron emission tomography (PET), spectroscopy (SPECT), and magnetoencephalography (MEG). Further advancements in human neuroimaging methods have afforded promising new insights into the biological basis of epilepsy and, impor tantly, our understanding of epilepsy as a network disorder. New techniques and approaches expected to emerge into the mainstream within the next decade will greatly influence our understanding of the biological basis of epilepsies and the mechanisms involved in symptom changes. In particular, investigations increasingly focus on epilepsy as a network disorder and in the context of comorbidities.[5] For epilepsy to be surgically managed, an accurate diagnosis is essential. Therefore, there is an urgent need to improve the accuracy of epilepsy diagnosis as well as the therapeutic results of epileptic drugs. Nanomaterials as drug carriers have been developed in recent years as a result of the biomedical industry's advancements in nanotechnology. These materials' huge specific surface area, ease of surface modification, BBB-crossing capability, and excellent biocompatibility are all intended to enhance medication bioavailability and targeting.[6]
MATERIALS AND METHODS
Role Of Computerized Tomography and Magnetic Resonance Imaging in Epilepsy
Epileptic seizures are uncommon in moderate trauma but occur in 12% of cases of severe head trauma. About five to twelve hours after trauma, a CT scan shows a hypo-dense lesion in the brain. As soon as they become hyperdense, extra and intracerebral hemorrhages are observed. On the other hand, right after trauma, MRI may reveal a brain contusion as a hyper-intense region in T2-weighted images. After two to three days, the appearance of hemorrhages changes. They are therefore clearly visible as hypointense regions in T2-weighted images and hyper-intense signals in TI. Epilepsy is extremely unlikely to develop if the CT scan does not show a focal lesion shortly after the injury.[7] Patients with tumors that cause epileptic seizures often range in age from 25 to 60. The frontal, temporal, or parietal lobe brain is primarily affected. Compared to highly malignant gliomas, slow-growing tumors such as meningiomas and oligodendrogliomas cause more frequent epileptic episodes. When it comes to these malignancies, CT and MRI have almost comparable sensitivity. Since CT clearly demonstrates calcifications and enhances differential diagnosis, the specificity is different. Ischemic and degenerative illnesses are the main causes of generalized epileptic seizures in people aged 35 to 60, particularly after the age of 55. MRI displays smaller infarcts, particularly in the basal ganglia, whereas CT is more sensitive in detecting even tiny hemorrhages.[7]
Pet And Meg Studies In MRI-Negative Focal Epilepsy
Up to 25% of patients with drug-resistant focal epilepsy who appear for presurgical evaluation do not show any lesion on MRI, despite significant advancements in neuroimaging. Compared to patients with MRI-visible lesions, these patients have worse surgical results. In MRI-negative patients, accurate localization of the seizure onset zone (SOZ) is more challenging and frequently necessitates invasive EEG recordings. Prior to intracranial EEG recordings, the SOZ can be located clinically using Positron Emission Tomography (PET) and Magnesium Electroencephalography (MEG). Nevertheless, there is disagreement about the best gold standard to apply for evaluating the effectiveness of these presurgical examinations.[8] It is crucial to identify any structural abnormalities on brain MRIs since these abnormalities typically form the basis of the preoperative and surgical strategy. Up to 25% of patients presenting for presurgical evaluation still have negative MRI results, despite significant advancements in neuroimaging.The inability to precisely locate and extend the SOZ is one factor contributing to the poorer surgical outcomes in individuals with MRI-negative partial epilepsy. Intracranial EEG recording is typically required for these patients in order to guarantee the SOZ's delineation.[8] Safety concerns continue to restrict brain sampling regardless of the invasive recording method, subdural electrodes, or stereoelectruencephalography (SEEG). Therefore, the most accurate and reliable theory regarding the location of the SOZ should be used to customize the placement of subdural or depth electrodes. In general, the quality and interpretation of the non-invasive data used to determine which brain regions to target determines the outcome of invasive EEG monitoring.[8]
A modern epilepsy surgery treatment algorithm
Recent developments in epilepsy surgery have made it necessary to continuously improve the algorithms used to treat this condition. Both diagnostic and therapeutic methods have seen technological advancements in this discipline, and previously unattainable treatment choices have been made available. The fundamental approach to diagnosing drug-resistant epilepsy is still largely the same: a therapeutic intervention is followed by a noninvasive presurgical examination, either with or without invasive intracranial monitoring.However, we now have more surgical choices than just subdural electrodes (SDE) and resection or disconnection, and many of our diagnostic capabilities have increased. The advancement or invention of minimally invasive diagnostic and ablative methods, along with the advent of nondestructive neurostimulation techniques, are the main drivers of these innovations in the new era of epilepsy surgery.[9]
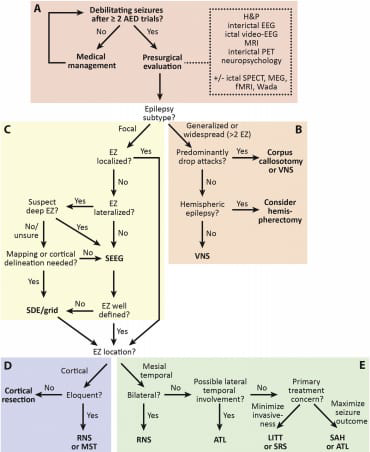
Fig. 1.
A modern epilepsy surgery treatment algorithm. The algorithm begins with (A)noninvasive surgical evaluation, (B) includes treatment of generalized or multifocal epilepsy, (C)invasive monitoring decisions, (D)treatment of neocortical or (E) mesial temporal lobe epilepsy. [9] AED: antiepileptic drug; ATL: anterior temporal lobectomy; EEG: electroencephalography; EZ: epileptogenic zone; fMRI: functional magnetic resonance imaging; LITT: laser interstitial thermal therapy; MEG: magnetoencephalography; MRI: magnetic resonance imaging; MST: multiple subpial transections; PET: position emission tomography; RNS: responsive neurostimulation; SAH: selective amygdalohippocampectomy; SDE: subdural electrodes; SEEG: stereotactic electroencephalography; SPECT: single-photon emission computed tomography; SRS: stereotactic radiosurgery.[9]
Non-Conventional Therapeutic Strategies
About 60% of people respond well to the antiepileptic medications that are already on the market, which reduce the propensity for seizures. Furthermore, the majority of currently available drugs have problems with hypersensitivity and adverse drug responses. The rising prevalence of mental health issues, anxiety, sadness, and suicide thoughts exacerbates it even more.Managing the condition through patient care and developing various therapy strategies is one of the key coping mechanisms in such complex situations.[10]
Combination Therapy: Numerous investigations and research findings indicate that a combination of medications aids in disease control. It has been observed that different patients respond differently to the same medications at different dosages. This could be because different pathophysiological mechanisms can happen in the same person at any time. For improved outcomes, such a scenario would call for the application of several therapeutic approaches at various levels. In animal models of epilepsy, a number of natural or herbal medications, such as Cicuta virosa and Nux vomica, have been demonstrated to be successful in lowering seizure activity and other physiological parameters. A combination of lamotrigine and sodium valproate has been shown to help treat partial-onset and generalized seizures in a number of animal models.Other frequently suggested combinations include lamotrigine and topiramate for the treatment of a variety of seizures and valproate and ethosuximide for the treatment of absence seizures.[10]
Antiepileptic medications that are rarely used in adult medicine but are utilized in pediatrics
The examples below demonstrate how a few medications have been created and registered for specific pediatric disorders. Children who get these AEDs will eventually move to adult care, at which point there may be little data on efficacy and pharmacokinetics.[11]
•Stiripentol is currently only approved in several countries for children with Dravet syndrome, as an adjuvant therapy with valproate and clobazam.This AED was initially designed for focal seizures, but failed to show efficacy in phase 3 trials in adults.[11]
•Vigabatrin was widely used in the 1990s by people suffering from focal onset seizures, however its usage has been severely limited due to retinal damage. Vigabatrin is now a "third line" AED in adults with pharmacoresistant focal epilepsy, as this toxicity rises with the dose and duration of medication exposure.[11]
•Cannabidiol has only recently been used to treat epilepsy. Several small open studies suggested that it could be useful in treating pharmacoresistant pediatric epilepsy and epileptic encephalopathies.Cannabidiol has been demonstrated to be useful in the adult treatment of pain and multiple sclerosis; however, the ideal dose and drug interaction profile need to be further investigated. A probable interaction with clobazam (increased serum levels). CYP450 inhibition has been documented but needs more research.[11]
AEG treatment prior to first seizure Presymptomatic
As shown in Figure 2, only a small proportion of patients who present with their first seizures have an identifiable etiology (for example, trauma, stroke, CNS infection, or tumor) that could have been reasonably predicted to increase the risk of developing epilepsy, allowing for presymptomatic treatment. Unfortunately, clinical trials of ASDs as AEGs have been consistently negative. Although disheartening, it appears that these trials failed because to flaws in the trial designs and the fact that ASDs were not developed or selected for AEG activity.

Figure 2
Opportunities for AEG therapy are represented by a schematic diagram. Epilepsy etiologies are illustrated by curves of varying thickness that indicate relative incidences (not to scale). Opportunities to intervene are depicted by vertical bars, showing that certain treatments may be exclusive to a single etiology, while others are likely to act further down a common path, altering the development of epilepsy of various etiologies. Blue vertical bars indicate presymptomatic treatment, which could be antiseizure or AEG. The red vertical dashed line depicts the appearance of epilepsy. The x-axis represents conceptual elements of the chronological path of development.[12] This emphasizes the value of targeted AEG strategies. No well-controlled trials of AEG therapy have been done in situations known to increase the likelihood of developing seizures, such as infarction, hemorrhage, or malignancy.[12] Although these patient categories have typically been believed to be the best candidates for AEG treatment, a broader perspective implies that alternative presymptomatic opportunities exist. For example, new options to prevent epilepsy may arise in circumstances where genetic testing might identify at-risk patients, such as childhood absence epilepsy. Similarly, there are new chances to avoid symptomatic, immune-mediated epilepsy with anti-inflammatory or immunologic interventions.[12]
Treatment targets for mild to severe epilepsy:
The first approach and treatment aim varies significantly between the most common forms of epilepsy (such as absence epilepsy or a mild form of focal epilepsy) and the complicated and severe forms of epilepsy with resistant seizures and, in many cases, numerous seizure types. In the first group, seizure management without adverse events, in the most convenient and least expensive way, is a legitimate goal that, if met, will allow the kid to live a normal life with few or no restrictions. Treatment strategies for these reasons differ slightly depending on the specific preferences of the treating doctor. In complex and severe epilepsy, the primary goal may not be total seizure control.[13] In many situations, reducing the frequency of seizures, the impact of severe electrographic abnormalities, and the adverse effects of the medication or medications is a very fair goal. Sophisticated parents of children with severe, chronic epilepsy would be well aware of this delicate balance for their own child and should always pay close attention to what they have to say.[13]
Gene Therapy In Epilepsy
In the nervous system, gene therapy entails expressing and transferring genes into brain tissue, usually neurons. Simian virus (SV), lentivirus, adeno-associated virus (AAV), and herpes simplex virus (HSV)-based vectors are among the few viral vectors that have been effectively employed for this purpose; all of these vectors, with the exception of the SV vector, have been employed in clinical trials.These vectors have the capacity to transduce nondividing cells and enable long-term gene expression without causing noticeable harm. These vectors are guaranteed to function as delivery vehicles for genetic material without the possibility of replication or expression of genes that cause toxicity because they also employ helper virus-free systems.[14] The promoter, viral tropism, and injection site(s) can all be used to target the expression of therapeutic genes to particular cell types or locations. These viral vectors' advantageous characteristics make them potentially appropriate for therapeutic usage; if they are employed to treat epilepsy, they can target prospective targets by working through a number of mechanisms:
1. Inhibitory modulator expression and endogenous cell transduction reduce hyperexcitability.
2.Trophic support promotes the survival of impacted neurons and the restoration of partially destroyed circuits by transducing endogenous cells and expressing neurotrophic factors.
3.Reducing hyperexcitability by expressing opsins and transducing endogenous cells to either stimulate firing in inhibitory cells or silence neuronal firing in excitatory cells.[14]
Potential Therapeutic Strategies Of Gene Therapy
In theory, neuropeptides may limit the progression of seizures or even stop ictogenesis or epileptogenesis by blocking glutamate release from presynaptic terminals. They might even effect targets far from the discharge point by acting through volume transmission. They are regarded as endogenous anticonvulsants in this sense.[15]
Gene Therapy Interventions
At least 30% of epilepsy cases are thought to be hereditary in origin. At first look, these disorders may appear to be ideal candidates for gene therapy, but this is not the case. Epilepsy is typically caused by the inheritance of two or more susceptibility genes, rather than a single defective gene.[16] Furthermore, the pathology in these situations frequently affects a significant area of the brain, necessitating widespread gene transfer, whereas current gene therapy approaches only deliver localized results. Efforts are being undertaken to create techniques for worldwide delivering genes to the brain via the blood-brain barrier (BBB) following the delivery of vectors in the peripheral blood. One such technique is to follow the same pathway that many circulating endogenous chemicals, such as transferrin or insulin, take to reach neurons and glia.After these ligands bind to the luminal side of the capillary endothelial cell membrane, a caveolar vesicle forms, enveloping both the receptor and the bound conjugate.An internal transport mechanism (transcytosis) transports the caveola and its cargo across the cytoplasm of endothelial cells from the luminal to the abluminal side.[16]
Non-Pharmacological Approaches to Epilepsy
Epilepsy can be treated non-pharmacologically by surgery, vagal nerve stimulation, a ketogenic diet, and other alternative/complementary therapy.Alternative therapies include yoga, acupuncture, chiropractic, massage therapy, EEG biofeedback, aromatherapy, homeopathy, herbal medicines (traditional Chinese medicine), and so on. Most persons with epilepsy require antiepileptic medication to control their seizures, and alternative therapies are frequently complementary. Yoga meditation helps people avoid stress-induced seizures and improves their quality of life. Acupuncture employs needles to stimulate nerve endings in order to improve a person's mental, physical, and emotional well-being.[17] In epilepsy, biofeedback techniques use EEG devices to assist patients in identifying and altering their own seizure-related brain activity. Over time, the person with epilepsy learns to employ relaxation or other biofeedback techniques to establish a more regulated brain wave pattern, which may help minimize seizures. A ketogenic diet is frequently employed as a last resort in the treatment of catastrophic epilepsy in children, and it is seen to be both safe and successful.[17]
Ketogenic Diet
Classic ketogenic diet KD (CKD) has a high proportion of lipids, a sufficient amount of protein, and a low carbohydrate level. The majority of CKD is available with a 4:1 ratio of lipids to protein and carbs.Fat provides 85-90% of total energy, with carbs and protein accounting for the remaining 10-15%. Other ketogenic diet modifications include modified Atkin's diet (MAD), medium-chain triglyceride diet (MCT), and low glycemic index treatment. The primary goal of these variations is to improve palatability and adherence to the diet program.[18] Fasting has been known since Hippocrates to help reduce the frequency of seizures. In the 1920s, Wilder at the Mayo Clinic (USA) discovered that fasting has an antiepileptic effect through ketosis. So, to replicate the body's metabolism during fasting, Dr. Wilder created a ketogenic diet (KD) for epileptic patients. However, after the discovery of phenytoin, interest in this dietary regimen has diminished.[18] KD is a high-fat diet with a low carbohydrate composition. The human central nervous system (CNS) does not use fat as a primary fuel source; but, if we do not ingest carbs for three to four days, our CNS is forced to discover alternative energy sources. The ketogenic diet stimulates the generation of ketone bodies while shifting metabolism from glycolysis to betaoxidation. Muscles and other tissues use fatty acids as a key energy source. Acetyl-CoA is produced by the oxidation of these fatty acids, which is then processed by mitochondria into ketone bodies. Ketone bodies can penetrate the blood-brain barrier (BBB), therefore they can be utilized by the brain as an alternative energy source.[18]
Vagus Nerve Stimulation
Surgery is not recommended for all resistant patients because to potential post-surgical complications. Alternative therapy for managing seizures in intractable epilepsy patients with seizure-free periods have been clinically demonstrated to be effective. Neuromodulation treatments include vagus nerve stimulation (VNS), responsive neurostimulation therapy (RNS), and transcranial magnetic stimulation (TMS).[18]
Currently, VNS is only used on the left cervical vagal trunk, which includes fibers from the recurrent laryngeal, cardiopulmonary, and subdiaphragmatic vagal branches. Because many branches perform multiple functions, not all of them necessarily contribute to VNS-induced seizure suppression. In fact, data suggests that stimulating the smaller vagal branches can help to reduce seizures.[19] In one investigation, rats were implanted with a cuff electrode on their ventral (left) subdiaphragmatic vagal branch.After two days, VNS or sham stimulation was used, and seizures were induced using PTZ. The reciprocal therapy was administered 48 hours later, such that each rat served as its own control. Subdiaphragmatic VNS significantly reduced seizure severity relative to the baseline. This reduction was equivalent to that observed with cervical VNS in prior studies, implying that stimulation of subdiaphragmatic vagal branches alone is effective in suppressing seizures. While targeted stimulation of the subdiaphragmatic branch effectively suppresses seizures, other branches may also play a role.[19]
Herbal Remedies
Herbal remedies are often regarded as alternative and supplementary forms of medicine by patients and physicians. There is, however, a tiny body of research supporting the efficacy of several herbs in managing seizures. Cannabinoids have the most evidence to support their usage as anticonvulsants of any natural therapy. Although laboratory studies show the efficacy of kava (Piper methysticum) and mistletoe (Viscum sp), there is no clinical evidence to support their usage. A variety of additional herbal therapies often used to treat seizures have been demonstrated to either have no effect on seizure intensity or to have proconvulsant qualities, exacerbating the disease.[20] Furthermore, much data is anecdotal, and when clinical trials are conducted, they frequently demonstrate inadequate methodology and lack sufficient statistical power to draw definite conclusions. Additionally, high-quality data in the form of controlled trials is required to support the use of herbal medicines in clinical settings.[20]
CONCLUSION
Epilepsy is a complicated and multifaceted neurological illness that necessitates ongoing improvements in diagnostic tools and therapeutic strategies. Recent developments have greatly improved our understanding and treatment of epilepsy. Advanced neuroimaging techniques, such as CT, MRI, PET, and MEG, increase seizure focus localization. Genetic testing discovers underlying problems and guides personalized therapies. EEG monitoring improves seizure classification and diagnosis. Antiepileptic medicines (AEDs) have shown better efficacy and tolerance. Surgical procedures, including epilepsy surgery and neurostimulation, provide new hope. Alternative therapy include ketogenic diets and cannabinoids, as well as the development of novel AEDs with higher efficacy and fewer adverse effects. Advances in neurostimulation and brain-computer interfaces. An increased emphasis on epilepsy prevention and comorbidity treatment. Improved diagnostic and therapeutic outcomes. Increased quality of life and decreased stigma. Continued research and collaboration amongst healthcare professionals. Integrated diagnostic approaches that include clinical examination, imaging, and EEG. Personalized medication based on genetics and seizure type. Multidisciplinary care teams ensure thorough management. Continuous monitoring and revision of treatment strategies. Healthcare providers can improve epilepsy management by adopting cutting-edge diagnostic techniques and pharmacological approaches, altering the lives of affected individuals and families worldwide.
ACKNOWLEDGEMENT
With great pleasure, we express our profound gratitude to faculties of Department of Pharmacy Practice, Pulla Reddy Institute of Pharmacy.Dundigal. Hyderabad.
Conflict Of Interest
The authors declare that there is no conflict of interest.
Abbrevations
CT:Computerized tomography;MRI:magnetic resonance imaging;EEG:Electroencephalography;PET:Positron emission tomography;SPECT:Spectroscopy; MEG:Magnetoencephalography;BBB:Blood Brain Barrier;SOZ: Seizure Onset Zone;SEEG:Stereoelectruencephalography;SDE:Subdural electrodes;AED: antiepileptic drug; ATL: anterior temporal lobectomy; EZ: epileptogenic zone; fMRI: functional magnetic resonance imaging; LITT: laser interstitial thermal therapy; MST: multiple subpial transections;RNS: responsive neurostimulation; SAH: selective amygdalohippocampectomy;SDE: subdural electrodes; SRS: stereotactic radiosurgery;SV:Simian virus; AAV:Adeno-associated virus;HSV:Herpes simplex virus;CKD:Classic Ketogenic Diet;MAD:Modified Atkin's diet;MCT:Medium-chain triglyceride diet;VNS:Vagus nerve stimulation;RNS:Responsive neurostimulation therapy and TMS: Transcranial magnetic stimulation
REFRENCES
- Ghosh, S., Sinha, J. K., Ghosh, S., Sharma, H., Bhaskar, R., & Narayanan, K. B. (2023). A comprehensive review of emerging trends and innovative therapies in epilepsy management. Brain Sciences, 13(9), 1305. https://doi.org/10.3390/brainsci13091305
- Oguni, H. (2004). Diagnosis and Treatment of Epilepsy. Epilepsia, 45(s8), 13–16. https://doi.org/10.1111/j.0013-9580.2004.458003.x
- Moshé, S. L., Perucca, E., Ryvlin, P., & Tomson, T. (2014). Epilepsy: new advances. The Lancet, 385(9971), 884–898. https://doi.org/10.1016/s0140-6736(14)60456-6
- Manford, M. (2017). Recent advances in epilepsy. Journal of Neurology, 264(8), 1811–1824. https://doi.org/10.1007/s00415-017-8394-2
- Goodman, A. M., & Szaflarski, J. P. (2021). Recent Advances in Neuroimaging of Epilepsy. Neurotherapeutics, 18(2), 811–826. https://doi.org/10.1007/s13311-021-01049-y
- He, S., Zheng, L., Li, J., & Liu, S. (2024). Epilepsy Treatment and Diagnosis Enhanced by Current Nanomaterial Innovations: A Comprehensive Review. Molecular Neurobiology. https://doi.org/10.1007/s12035-024-04328-9
- Radue, E., & Scollo-Lavizzari, G. (1994). Computed Tomography and Magnetic Resonance Imaging in Epileptic Seizures. European Neurology, 34(1), 55–57. https://doi.org/10.1159/000119510
- Rheims, S., Jung, J., & Ryvlin, P. (2013). Combination of PET and Magnetoencephalography in the Presurgical Assessment of MRI-Negative Epilepsy. Frontiers in Neurology, 4. https://doi.org/10.3389/fneur.2013.00188
- Englot, D. J. (2018). A modern epilepsy surgery treatment algorithm: Incorporating traditional and emerging technologies. Epilepsy & Behavior, 80, 68–74. https://doi.org/10.1016/j.yebeh.2017.12.041
- Ghosh, S., Sinha, J. K., Khan, T., Devaraju, K. S., Singh, P., Vaibhav, K., & Gaur, P. (2021). Pharmacological and Therapeutic Approaches in the Treatment of Epilepsy. Biomedicines, 9(5), 470. https://doi.org/10.3390/biomedicines9050470
- Nabbout, R., Camfield, C. S., Andrade, D. M., Arzimanoglou, A., Chiron, C., Cramer, J. A., French, J. A., Kossoff, E., Mula, M., & Camfield, P. R. (2017). Treatment issues for children with epilepsy transitioning to adult care. Epilepsy & Behavior, 69, 153–160. https://doi.org/10.1016/j.yebeh.2016.11.008
- French, J. A., White, H. S., Klitgaard, H., Holmes, G. L., Privitera, M. D., Cole, A. J., Quay, E., Wiebe, S., Schmidt, D., Porter, R. J., Arzimanoglou, A., Trinka, E., & Perucca, E. (2013). Development of new treatment approaches for epilepsy: Unmet needs and opportunities. Epilepsia, 54(s4), 3–12. https://doi.org/10.1111/epi.12294
- Guerrini, R., & Parmeggiani, L. (2005). Practitioner Review: Use of antiepileptic drugs in children. Journal of Child Psychology and Psychiatry, 47(2), 115–126. https://doi.org/10.1111/j.1469-7610.2005.01458.x
- Sørensen, A. T., & Kokaia, M. (2012). Novel approaches to epilepsy treatment. Epilepsia, 54(1), 1–10. https://doi.org/10.1111/epi.12000
- Zhang, L., & Wang, Y. (2021). Gene therapy in epilepsy. Biomedicine & Pharmacotherapy, 143, 112075. https://doi.org/10.1016/j.biopha.2021.112075
- Simonato, M. (2013). Gene therapy for epilepsy. Epilepsy & Behavior, 38, 125–130. https://doi.org/10.1016/j.yebeh.2013.09.013
- Nadkarni, V., & Saxena, V. (2011). Nonpharmacological treatment of epilepsy. Annals of Indian Academy of Neurology, 14(3), 148. https://doi.org/10.4103/0972-2327.85870
- Alqahtani, F., Imran, I., Pervaiz, H., Ashraf, W., Perveen, N., Rasool, M. F., Alasmari, A. F., Alharbi, M., Samad, N., Alqarni, S. A., Al-Rejaie, S. S., & Alanazi, M. M. (2020). Non-pharmacological Interventions for Intractable Epilepsy. Saudi Pharmaceutical Journal, 28(8), 951–962. https://doi.org/10.1016/j.jsps.2020.06.016
- Krahl, S. (2012). Vagus nerve stimulation for epilepsy: A review of the peripheral mechanisms. Surgical Neurology International, 3(2), 47. https://doi.org/10.4103/2152-7806.91610
- Mitchell, J. W., Seri, S., & Cavanna, A. E. (2012). Pharmacotherapeutic and Non-Pharmacological Options for Refractory and Difficult-to-Treat Seizures. Journal of Central Nervous System Disease, 4, JCNSD.S8315.https://doi.org/10.4137/jcnsd.s8315


 Dr. V. Saidurga Shravya*
Dr. V. Saidurga Shravya*
 Boddupelli Madhusudhan
Boddupelli Madhusudhan


 10.5281/zenodo.14899507
10.5281/zenodo.14899507