Abstract
A rare consequence of poorly managed type 1 diabetes mellitus that typically affects adolescents is called Mauriac syndrome, also known as glycogen hepatopathy (GH). Although it is still present, it has become even less common since breakthroughs in diabetes therapy have emerged. Growth failure, hepatomegaly, increased liver enzymes, and glycogen buildup in hepatocytes associated with poorly managed diabetes and a glucose trap in the liver are some of its symptoms. With proper glucose management, GH has a favourable prognosis and quickly resolves, without advancing to end-stage liver disease. A male patient, age 18, who had been suffering from type 1 diabetes for 15 years, visual impairment, and dyspnoea, presented with worsening symptoms. Examining revealed hepatomegaly, high blood creatinine, anaemia, and increased HbA1c. After the diagnosis of Mauriac syndrome, patients needed to get intense insulin therapy, nutritional assistance, antibiotics (Ceftriaxone), and other supportive medications to address metabolic imbalances, manage comorbidities, and enhance overall health outcomes. After receiving intensive therapy for a week to address metabolic imbalances and issues, the patient's diabetes was under control and their symptoms got better.
Keywords
Mauriac syndrome, massive hepatomegaly, type1diabetes mellitus, Premix insulin, glycogen hepatopathy.
Introduction
A rare side effect of type 1 Diabetes Mellitus (DM1), Mauriac Syndrome (MS) is characterized by hepatomegaly (hepatic glycogenosis), dyslipidaemia, delayed puberty and growth, elevated transaminase, and decreased IGF1 (insulin-like growth factor 1).[1] Cushingoid characteristics might also exist. The Mauriac syndrome may have several manifestations and etiologies. Nonetheless, these individuals share some characteristics, and growth failure and hepatomegaly, if present, can be reversed with proper insulin therapy. MS is the most prevalent cause of hepatic dysfunction in children and adolescents with DM1, and it is more common in those with poor glycemic control and a greater vulnerability to consequences. Short-acting insulin was used to treat children with T1DM who had growth failure, maturational delay, hepatomegaly, and abdominal distension, according to Mauriac in 1930.[2] According to whether obesity is present or not, there are two distinct types of Mauriac syndrome that have been identified. According to traditional descriptions, in the first type, therapy with normal insulin alone is linked to Cushingoid obesity and a recorded wide range of hyper- and hypoglycaemia, which suggests a pattern of over- and under insulinization with secondary hypoadrenalism. It seems that periods of over insulinization are necessary for the onset of hypoadrenalism and the development of obesity. Mauriac syndrome has been observed in individuals recently who do not have a history of alternating hypoglycaemia and ketoacidosis, nor are they obese. Patients who get regular, underdosed insulin experience this.[3]
PATHOPHYSIOLOGY
Mauriac syndrome is thought to be a consequence of inadequate glycemic control, leading to persistent hyperinsulinemia and dysregulation of glucose metabolism. - Prolonged exposure to elevated insulin levels contributes to abnormal glycogen storage in the liver and results in hepatomegaly.
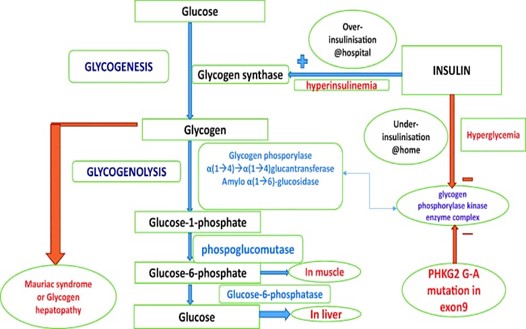
Fig-1 pathophysiology of Mauriac syndrome
CLINICAL FEATURES
- Hepatomegaly: Enlargement of the liver is a prominent feature, resulting from chronic exposure to elevated insulin levels and subsequent increased glycogen deposition.
- Growth Retardation: Children with Mauriac syndrome often exhibit delayed growth and development, which can be attributed to metabolic disturbances associated with uncontrolled diabetes.
- Cushingoid Features: The syndrome is characterized by round face and central obesity, resembling features seen in Cushing's syndrome, likely due to prolonged exposure to high insulin levels.
CASE REPORT: -
An 18-year-old male patient presented with complaint of blurring of vision since 1.5 year. He also complained about facial puffiness since 8days, frequent urination since 8days, abdominal pain since 1 day and breathlessness on exertion since 7days. The patient is a known case of type 1 diabetic since 15years and on regular medication inj. insulin 1U before breakfast and 1U before dinner and inj. glargine 5U alternate day. There was no family history of diabetes mellitus, hypertension, etc. On examination, it was revealed that the patient had pallor and icterus. His blood pressure was 110/70mmHg, pulse was 112/min, respiratory rate was 18/min, random blood glucose was 525mg/dl and SPO2 was 99%RA.
LABORATORY FINDINGS: -
Table 1: Hemogram
Table 2: Biochemistry
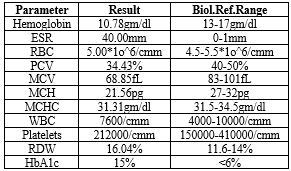
Table 3: sonography of abdomen
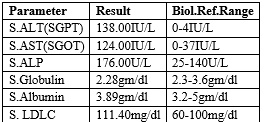
Serum creatinine was 49.47 mg/dl (normal: 0.5-1.2 mg/dl) and other tests like HBsAg rapid, HIV and HCV Rapid and sickling test all were negative. Therefore, a final diagnosis of Mauriac syndrome (MS) was made.
TREATMENT GIVEN: -

DISCHARGE MEDICATION: -
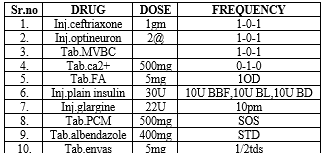
DISCUSSION: -
Before long-acting insulins were developed for the treatment of type 1 diabetes, Mauritius syndrome was a more common condition. Our patient exhibited moon facies, hepatomegaly, small height, and delayed sexual maturity—all classic signs of Mauriac syndrome. Based on the existence or lack of obesity, two distinct forms of Mauriac syndrome have been identified in the literature. A pattern of excessive or insufficient glucose utilisation is suggested by the broad fluctuations in hyper- and hypoglycaemia associated with poor glycaemic control in the obese subtype. Individuals with an insufficient insulin dosage and no prior history of fluctuating hypoglycaemia and ketoacidosis comprise the non-obese subtype. [4,5] Our case falls under the former phenotype, which includes episodes of uncontrolled hyperglycaemia at home treated with pre-mix insulins and quick glucose correction achieved in the hospital via intravenous insulin infusion. This causes glucose to enter the hepatocyte through stimulation of glycogen synthase, which is then converted into glycogen and stored in the liver. Following his release, under insulinization prevents glycogenolysis (inhibition of glycogen phosphorylase), which causes the hepatocyte's glycogen content to gradually rise and eventually cause glycogen hepatopathy (Image. 1). Hyperinsulinemia causes poor glycaemic control, which causes lipolysis and ketone liberation. Ketosis stimulates the production of cortisol, which causes fatty acid release and hyperglycaemia.[6] In 2016, a patient with Mauriac syndrome was the first to have a genetic link documented.[7] One patient with Mauriac syndrome experienced significant hepatomegaly and growth failure due to a mutation in exon 9 of the catalytic subunit of the liver glycogen phosphorylase kinase enzyme complex (PHKG2G-A mutation).
The initial stage of glycogen degradation is catalysed by glycogen phosphorylase, which is activated by this enzyme complex.[4] It was demonstrated that the mutant subunit functions dominantly to totally block the action of the glycogen phosphorylase kinase enzyme, and that this interferes with glycogenolysis to raise the amounts of glycogen in human liver cells.[4] The degree of liver enzyme rise in our patient was an intriguing feature. Children with Mauriac syndrome and poorly controlled diabetes may have transaminase elevations up to the 1000s. [8] The other causes of abnormally elevated transaminases, such as autoimmune hepatitis and acute viral hepatitis in a child with diabetes mellitus, should also be considered as differentials when considering Mauriac syndrome.
CONCLUSION: -
A 15-year history of type 1 diabetes, impaired vision, face puffiness, frequent urination, stomach pain, and dyspnoea were among the deteriorating symptoms that the 18-year-old male patient was presenting with. During examination, pallor, icterus, and noticeably high blood sugar levels were found. Hepatomegaly, increased HbA1c (15%), anaemia with microcytic anaemia (low MCV and MCH), and renal impairment with elevated blood creatinine were all found in the laboratory. Renal cortical echogenicity on both sides and hepatomegaly were confirmed by sonography. The combination of hepatomegaly, poorly managed diabetes, and renal impairment was identified as the diagnosis of Mauriac syndrome.
Treatment for Mauriac syndrome included a multifaceted strategy that included:
- Intense insulin therapy to enhance glycaemic control, utilising both insulin glargine and plain insulin.
- Ceftriaxone antibiotic medication to treat any possible infection.
- Nutritional and supportive care that includes folic acid, calcium, and vitamin supplements.
- Enalapril may help to enhance renal function.
- Other supportive drugs, such as albendazole and optineuron, for further requirements.
The treatment plan aimed to correct metabolic imbalances, manage complications, and improve overall health outcomes. A week of in-depth care was given to the patient to address metabolic imbalances and handle problems while they were hospitalised. The patient was allowed to go home once their condition improved after a week. After a week, they were told to come back for another visit. Following up with the patient, it was determined that their diabetes was under control and that their prior symptoms had completely subsided.
REFERENCE
- Haller MJ, Silverstein JH, Rosenbloom AL. Type 1 diabetes in the child and adolescent. In: Lifshitz F, editor. Pediatric Endocrinology. 5th ed. Vol. 1. Switzerland: Informa Healthcare; 2007. p. 70
- Dias J, Martins S, Carvalho S, Marques O, Antunes A. Mauriac syndrome still exists. Endocrinología y Nutrición (English Edition). 2013 May 1;60(5):245-8.
- Mauriac P. Gros ventre, hepatomegalie, troubles de croissance chez les enfants diabetiques traites depuis plusiers annee par l’insuline. Gaz Hebd Med Bordeaux. 1930;26:402–10
- Singh M, Mani B, Mago C, Nawaz HH, Bhardwaj AK. Mauriacc syndrome, a rare complication of insulin dependent diabetes mellitus. J Obes Metab Res. 2015;2:234–6.
- GutchM,PhilipR,Saran S,TyagiR,Gupta KK. Re-emergence of a rare syndrome: A case ofmauriacsyndrome. Indian J Endocr Metab. 2013;17:S283–5.
- Madhu SV, Jain R, Kant S, Prakash V. Mauriac syndrome: a rare complication of type 1 diabetes mellitus. Indian J Endocrinol Metab. 2013;17(4):764–5.
- MacDonald MJ, Hasan NM, Ansari IU, Longacre MJ, Kendrick MA, Stoker SW. Discovery of a genetic metabolic cause for Mauriac syndrome in type 1 diabetes. Diabetes. 2016;65(7):2051–9.
- Patita M, Nunes G, Alves de Matos A, Coelho H, Fonseca C, Fonseca J. Mauriac syndrome: a rare hepatic glycogenosis in poorly controlled type 1 diabetes. GE Port J Gastroenterol. 2019;26:370–4.


 S P Srinivas Nayak*
S P Srinivas Nayak*
 Chauhan Sneha Arvind
Chauhan Sneha Arvind
 Tanishka Bairagi
Tanishka Bairagi
 Riddhi Patel
Riddhi Patel






 10.5281/zenodo.13834811
10.5281/zenodo.13834811