Abstract
Objective: The present study focused to the acute and subacute toxicity of methanolic extracts of Ctenolepis garcinii burm.f in albino rats. Materials and Methods: In the acute study, a single dose of 2000 mg/kg was administered to albino rat which were observed for 72 h. In sub-acute toxicity study repeated doses of the MECG was administered to albino rats of both genders, separately. The animals received two doses of MECG (200 and 400 mg/kg p.o. respectively) for a period of 28 days. On the 28th day of the experiment, blood sample was collected by cervical decapitation after anaesthetizing them with anaesthetic ether and it was used to identify the hematological and biochemical analysis. Throughout the study, behavior changes, mortality, body weight, food and water consumption were observed and then sacrificed to remove the organ like liver and kidney for histopathological examination. Results and Discussion: There were no morbidity and mortality were reported. In sub-acute toxicity study, there is no any significant changes were observed in haematological and biochemical parameters at 200 and 400 mg/kg/p.o. respectively. Conclusion: It was concluded that no toxicity was observed in methanolic extracts of Ctenolepis garcinii (MECG).: The present study focused to the acute and subacute toxicity of methanolic extracts of Ctenolepis garcinii burm.f in albino rats. Materials and Methods: In the acute study, a single dose of 2000 mg/kg was administered to albino rat which were observed for 72 h. In sub-acute toxicity study repeated doses of the MECG was administered to albino rats of both genders, separately. The animals received two doses of MECG (200 and 400 mg/kg p.o. respectively) for a period of 28 days. On the 28th day of the experiment, blood sample was collected by cervical decapitation after anaesthetizing them with anaesthetic ether and it was used to identify the hematological and biochemical analysis. Throughout the study, behavior changes, mortality, body weight, food and water consumption were observed and then sacrificed to remove the organ like liver and kidney for histopathological examination. Results and Discussion: There were no morbidity and mortality were reported. In sub-acute toxicity study, there is no any significant changes were observed in haematological and biochemical parameters at 200 and 400 mg/kg/p.o. respectively. Conclusion: It was concluded that no toxicity was observed in methanolic extracts of Ctenolepis garcinii (MECG).
Keywords
Ctenolepis garcinii, Methanolic, Acute and Subacute
Introduction
The pharmacopoeias of many countries of the world include even today a large number of drugs of plant origin [1]. Medicinal plants still play an important role in emerging and developing countries of Asia, both in preventive and curative treatments, despite advances in modern western medicine [2]. Over the years, the use of herbal products globally either as a food supplement or curative medicine has witnessed a phenomenal growth [3]. In the last few years there has been an exponential growth in the field of herbal medicine and these drugs are gaining popularity both in developing and developed countries because of their natural origin and less side effects. A number of medicinal plants, traditionally used for over 1000 years named rasayana are present in herbal preparations of Indian traditional health care systems [4]. The WHO has listed 21,000 plants, which are used for medicinal purposes around the world. Among these 2500 species are in India, out of which 150 species are used commercially on a fairly large scale [5]. People in India and China are known to have used plants in organized health care regime for over 5000 years. European herbal medicine blossomed in the Graeco-Roman era and remained the mainstream until six decades ago. Toxicology can be defined as that branch of science that deals with poisons, and a poison can be defined as any substance that causes a harmful effect when administered either by accident or design to a living organism. Toxicity itself can rarely, if ever, be defined as a single molecular event, but is, rather, a cascade of events starting with exposure, proceeding through distribution and metabolism, and ending with interaction with cellular macromolecules and the expression of a toxic end point [6]. The herbal drugs from the traditional system of medicine have contributed very useful form of drugs over the years and it was aimed to bring the treasure of these traditional systems of medicine. The aim of present study to identify the toxicological risk of selected plant (Ctenolepis garcinii burm.f).
MATERIALS AND METHODS
Collection of specimens
The species for the proposed study is Ctenolepis garcinii (burm.f) leaves and were collected in the month of January to February from the areas near the town of Sivakasi, Virudhunagar district, Tamilnadu, India.
Taxonomic identification
The species for the proposed study Ctenolepis garcinii (burm.f) was positively identified and authenticated by Dr.V.Ganesan, Dept. of Botany, Ayyanadar Janakiammal College of Arts and Science Sivakasi, Virudhunagar District, Tamilnadu, India. The plant specimen was certified as Ctenolepis garcinii (burm.f) of family Cucurbitaceae.
The leaves of the plant Ctenolepis garcinii (Burm.f) were collected and it was size reduced into small pieces and shadow dried. The dried materials were coarsely powdered before extraction, which was used for further detailed studies [7].
Preparation of the extracts
About 66 g of Ctenolepis garcinii air dried, powdered material was taken in 1000 ml Soxhlet apparatus and extracted with methanol for 72 hours. At the end of the 3rd day the powder was taken out and it was dried The methanolic extracts were concentrated by distillation and solvent was recovered. After completion of extraction it was filtered and the solvent was removed by distillation under reduced pressure. The extract was dried and stored in desiccators. A reddish brown residue was obtained. The percentage yield was calculated [8].
Identification of Phytoconstituents
The MECG were subjected to routine Phytochemicall analysis for the presence of various phytoconstituents [9][10].
Selection of suitable animal model
Healthy young Wistar albino rats weighing 100-150 gm (8 to 12 weeks old) were selected for toxicity studies. The rats were kept in properly numbered large polypropylene cages with stainless steel top grill having the necessary facilities and given a standard diet and water ad libitum throughout the experimental period [11]. The animals were maintained with 12 hr light and dark cycle at 220 C (± 30C) in a well-ventilated animal house under natural conditions [12]. The experimental protocol has been approved by institutional animal ethics committee, Ref no. SBCP/2012-13/CPCSEA/IAEC-III/06.
Acute oral toxicity study
The acute oral toxicity was conducted as per the OECD guidelines 423. Twelve Wistar albino rat 100-150g were used for the acute toxicity study. They were randomly distributed into five groups, containing three animals per group and were on a normal diet provided with water ad libitum. The treated group received orally varying doses (5, 50, 300, 2000 mg/kg/body weight) for 14 days. Animals treated with normal saline 10ml/ kg served as control. They were continually observed for 4h, detect any changes in skin and fur, eyes and mucous membranes, and also autonomic and central nervous system and somatomotor activity and Behavioural patterns, signs of tremors, convulsions, salivation, diarrhoea, lethargy, sleep and coma also to be noted. The animals were kept under observation up to 14 days after administration methanolic extract of Ctenolepis garcinii (MECG) to find out the delayed mortality [13],[14].
Sub-acute toxicity study
The sub-acute toxicity procedure was followed by using OECD guidelines 407. The albino rats of both sexes were randomly selected and put into three groups with six rats in each group. Food was withheld overnight, but water made available. The animals were weighed prior to administration of the extract. First group served as solvent control and was given normal saline 10ml/kg p.o. and other groups were administered methanolic extract of Ctenolepis garcinii (MECG) at 200 and 400 mg/kg p.o. respectively. The test compounds were given once daily orally for 28 days. All the rats were observed for any physiological and behavioural changes and mortality if any. Food and water consumption were checked at an interval of 7 days. All the animals were taken weight once a week upto the end of the day [15].
At the end of the period, the animals in all the groups were sacrificed by cervical decapitation after anaesthetizing them with anaesthetic ether. Blood was collected in heparinized tubes for the analysis of hematological parameters. The collected blood sample was centrifuged for 10 minutes at 4000 rpm at 4ºC to obtain the serum for biochemical estimations [16].
Relative organ weights and Histopathology
After sacrifice, organ weights (liver, kidney, brain, stomach and heart) were recorded and relative organ weights (ROW) were calculated as follows.
ROW= Absolute organ weight (g)/Body weight of rat on sacrificed day (g) x 100
Then the liver and kidney were preserved in 10% formalin solution for histological examination [17].
STATISTICAL ANALYSIS
Values are expressed as mean SEM. The mean differences in body weight and plasma biochemical analysis were analyzed using one-way ANOVA followed by Dunnest ‘t’ test. The difference between each group were considered statistically significant at P<0>
RESULTS AND DISCUSSION
Percentage yield
The dried powder of leaves of Ctenolepis garcinii was extracted with methanol by using a Soxhlet apparatus. The extract was dried and finally percentage yield was calculated. The percentage yield of methanolic extract of Ctenolepis garcinii (MECG) was found to be 9.84 (%w/w).
Identification of Phytoconstituents
In this present investigation, the phytochemical analysis revealed the Alkaloids, carbohydrates, glycosides, tannins, amino acids, flavonoids were present in the methanolic extract of Ctenolepis garcinii (MECG).
Acute toxicity study
The results of acute toxicity studies in albino rats were administered with various extracts of Ctenolepis garcinii at the doses of 5, 50, 300 and 2000 mg/kg by oral route. The test extracts were dissolved in distilled water and administered orally. No acute mortality was observed even at 2g/kg for methanolic extracts of Ctenolepis garcinii. All the animals were found to be normal and there were no gross behavioural changes at the end of the period (14 days). The results were shown in Table-1. From these results, LD50 or the maximum tolerated dose was found to be 2000mg/kg. From this 1/5th and 1/10th of the maximum tolerated dose was selected for the screening toxicological studies. Hence the biological dose was identified as 1/5th and 1/10th of MTD and it was fixed as 200 and 400 mg/kg body weight. The results are shown in Table 2.

Table: 1 Effect of Methanolic extract Ctenolepis garcinii on its behavioural signs of acute toxicity in albino rats.

Table: 2 Acute toxicity study of Methanolic extract of Ctenolepis garcinii for dose selection
MTD - Maximum Tolerated dose
Subacute toxicity study
In subacute toxicity study of methanolic extracts of Ctenolepis garcinia (MECG) at the doses of 200 mg/kg p.o, and 400 mg/kg p.o respectively, there were no deaths during the treatment period either in the control or in the treated groups. Food and water consumption also did not significantly differ which is shown in the Table- 4 & 5. There was no change in general behaviour or other physiological activities of the animals. Normally, the control and the drug treated groups showed an increase in body weight. The results are shown in Table 3.
Haemoglobin (Hb)
There were no significant changes bserved the levels of Hb, RBC, WBC and Platelet on the methanolic extract treated groups at 200 and 400 mg/kg p.o. respectively, when compared to Group-I.The results are shown in Table 6.
Erythrocyte Sedimentation Rate (ESR)
The MECG (200 & 400 mg/kg p.o respectively) treated groups were compared with the control animals. The slight elevation and significant changes were appeared when compared to Group- I. The results are shown in Table 6.
Biochemical evaluation of sub-acute toxicity
The MECG (200 and 400 mg/kg p.o. respectively) treated groups were compared to Group-I. There was a slight elevation in serum glucose levels, urea, total protein and albumin but no significant changes were appeared. The results were shown in Table-7 The MECG treated groups at 200 and 400 mg/kg p.o. did not show any difference in creatinine, globulin, total bilirubin, Direct and Indirect bilirubin when compared to Group-I. The results are shown in Table 7 & 8. The MECG (200 & 400 mg/kg p.o respectively) treated groups were compared with Group-I. No significant changes were reported in SGPT, SGOT, ALP Cholesterol and Triglycerides. The results are shown in Table 9 & 10.

Table: 3 Effects of methanolic extract of Ctenolepis garcinii burm.f. on body weight of sub-acute toxicity studies in albino rats
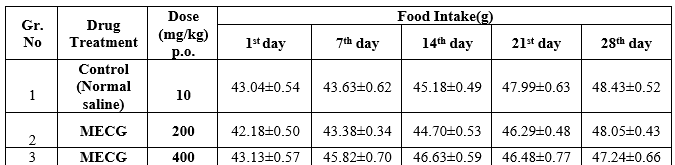
Table: 4 Effects of methanolic extract of Ctenolepis garcinii burm.f. on Food intake of sub-acute toxicity studies in albino rats
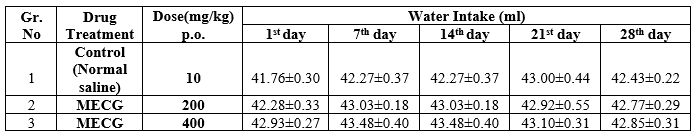
Table: 5 Effects of methanolic extract of Ctenolepis garcinii burm.f. on water intake of sub-acute toxicity studies in albino rats

Table 6: Effects of MECG on haematological parameters of sub-acute toxicity studies in albino rats.
Values were expressed as Mean ± Standard error mean (SEM) of six animals in each group. Statistical analysis was performed using one-way ANOVA followed by Dunnet’s t-test using GraphPad prism 5.0. **p<0>

Table:7 Effects of MECG on biochemical parameters of sub-acute toxicity studies in albino rats
Values were expressed as Mean ± Standard error mean (SEM) of six animals in each group. Statistical analysis was performed using one-way ANOVA followed by Dunnet’s t-test using GraphPad prism 5.0. **p<0>

Table:8 Effects of MECG on biochemical parameters of sub-acute toxicity studies in albino rats.
Values were expressed as Mean ± Standard error mean (SEM) of six animals in each group. Statistical analysis was performed using one-way ANOVA followed by Dunnet’s t-test using GraphPad prism 5.0. **p<0>
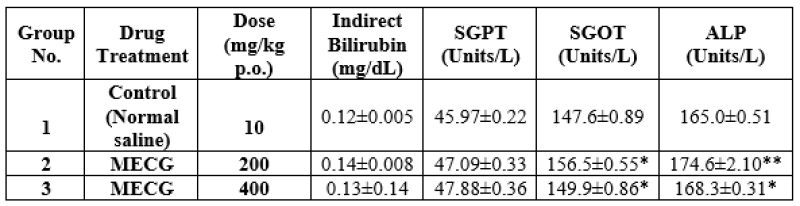
Table:9 Effects of MECG on biochemical parameters of sub-acute toxicity studies in albino rats.
Values were expressed as Mean ± Standard error mean (SEM) of six animals in each group. Statistical analysis was performed using one-way ANOVA followed by Dunnet’s t-test using GraphPad prism 5.0. **p<0>

Table:10 Effects of MECG on biochemical parameters of sub-acute toxicity studies in albino rats.
Statistical analysis was performed using one-way ANOVA followed by Dunnet’s t-test using GraphPad prism 5.0. **p<0>

Table:11 Effect of MECG on organ weight of albino rats during sub-acute toxicity studies.
Values were expressed as Mean ± Standard error mean (SEM) of six animals in each group. Statistical analysis was performed using one-way ANOVA followed by Dunnet’s t-test using GraphPad prism 5.0. **p<0>
The Histopathology studies of various organ for drug treated groups

Fig:1 The Histology of Kidney for control and drug treated groups of sub-acute toxicity study

Fig:2 The Histology of Liver for control and drug treated groups of sub-acute toxicity study
CONCLUSION
Ctenolepis garcinii belonging to the family Cucurbitaceae is a useful herbal plant to pharmacological and therapeutic point of view. In the current study, an attempt was made to analyze the acute, subacute studies of methanolic extracts of Ctenolepis garcinii in albino rats. The preliminary phytochemical investigation showed the presence of alkaloids, carbohydrates, glycosides, steroids, tannins and flavonoids in the extract of Ctenolepis garcinii. In acute toxicity studies, there was no mortality or morbidity observed in methanolic extracts of Ctenolepis garcinii in the dose level upto 2g/kg. It represent that the selected plant Ctenolepis garcinii was maximum safe. The results suggest that the haematological, biochemical and histopathological parameters revealed that the plant of Ctenolepis garcinii was not possess any type of toxicity.So, concluded that the above details, the plant of Ctenolepis garcinii is used for in future studies. The plant of Ctenolepis garcinii was used in traditionally for anticancer, analgesic ad anti inflammatory [18] but still not isolate the compound. Finally we conclude that the above plant have not found the toxicity so in future, we isolate the compound to further conform the above activity.
REFERENCE
- Benl P.N., Srirastara G., Herbs useful in terminological therapy, 2nd edition, pp: 1.
- Ansari. S.N, Essential of Pharmacognosy, 3rd edition, pp:719.
- Raskin, I., D.M. Ribnicky, S. Komarnytsky, N. Ilic and A. Poulev. Plants and human health in the twenty-first century. Trends Biotechnol. 2002; 20: 522-531.
- Kalia A.N., Textbook of Industrial Pharmacognosy, 1st edition, CBS Publishers, New Delhi; 2005; 1-9
- Gopal V, (2012),Yesterday Today and Tomorrow of Industrical Pharmacognosy, International Journal of Pharma and Bio Sciences, 16th edition; 3-5.
- Ernest Hodgson. A Textbook of Modern Toxicology, 3rd edition, John Wiley, New York, 2004; 3-10
- Annes, Ahmed siddiqui, Mohammed Ali, Practical Pharmaceutical Chemistry,1st edition.CBS publishers and distribution, New Delhi, 1997; 126-132.
- Khadabadi S.S. Deore S.L., Baviskar B.A. Experimental Phytopharmacognosy,1st edition, Nirali Prakashan, Pune, 2011; 1.2-1.8.
- Kokate C.K, (1984), Pratical pharmacognsy, Vallabh Prakashan, New Delhi, 1984; 4-108.
- Kokate C.K. Practical pharmacology, 4th edition; Vallabh Prakashan, NewDelhi, 1996; 22- 56
- Khandelwal K.R. Practical pharmacognosy techniques and experiments,13th edition; Nirali Prakashan, Pune, India, 2006; 30-76.
- Thanabhorn S, Jaijoy K, Thamaree S, Ingkaninan K, Panthong A, Acute and subacute toxicities of the ethanol extract from Alternanthera philoxeroides Griseb, Mahidol University Journal of Pharmaceutical Sciences, 2005; 32(2); 7-14.
- OECD guidelines 423,2001
- Velpandian V, Ashwini Anjana, Anbu J, Prema S, Acute and subacute toxicity studies of Kodi pavala chunnam in rodents, Asian Journal of Pharmaceutical and Clinical Research, 2012; 5 (4); 36-41.
- OECD guidelines, 407
- Siharat Chunlaratthanaphorn, Nirush Lertprasertsuke, Umarat Srisawat, Amornnat Thuppia, Anongnad Ngamjariyawat, Nadthaganya Suwanlikhid, Kanjana Jaijoy, Acute and subchronic toxicity of the water extract from root of Imperata cylindrical (Linn.) Raeusch. in rats, Songklanakarin Journal of Science and Technology, 2007; 29(1); 141-155.
- Stanley O. Aniagu , Florence C. Nwinyi, David D. Akumka (2005), Toxicity studies in rats fed nature cure bitters, African Journal of Biotechnology, 2007; 4 (1): 72-78
- Natarajan P, Thanga Thirupathi A, Analgesic and Anti inflammatory Activities of Ctenolepis garcinii (burm. f), Research J. Pharmacology and Pharmacodynamics. 4(2), 2012, pp; 119-12


 Natarajan P.* 1
Natarajan P.* 1
 Melba Y. 1
Melba Y. 1













 10.5281/zenodo.10908282
10.5281/zenodo.10908282