Abstract
Analytical techniques encompass a broad spectrum of methods and practices essential for ensuring the quality of medicinal products. These techniques are crucial for the design, development, standardization, and quality control of pharmaceuticals. The quality of drug products is paramount because it directly impacts patient safety and treatment efficacy. Therefore, stringent manufacturing processes and quality control measures are indispensable components of robust primary healthcare systems globally.Pharmaceutical analysis, a specialized area within pharmacy, is pivotal in maintaining the quality of pharmaceuticals. It involves meticulous examination of raw materials and finished products to ensure they meet required standards. Two commonly employed analytical methods in this field are spectroscopic and chromatographic techniques. These methods are highly specific and sensitive, enabling precise detection and quantification of substances within drug formulations. They play a critical role in both quality control and the validation of drugs, ensuring that medicinal products are safe, effective, and of high quality. In this research article we used RPHPLC method for development and validation on Dapagliflozine tablets. Dapagliflozine is primarily used for the treatment of type 2 Diabetes and Heart failure. The purpose of this research is to develop a method for analyzing a drug, which can be optimized according to different parameters and validated according to ICH (Q2 R1) guidelines. All the research work was carried out in a systematic, concise, and serial manner, including a thorough study of the literature available for the analysis of Dapagliflozin.In this research article, we optimized the analysis of Dapagliflozin tablets using the RP-HPLC method. Optimization involved conducting four trials to refine the method. During validation, we assessed various parameters including specificity, linearity, range, accuracy, precision, system suitability, and robustness.Here, we conclude that the developed RP-HPLC method is precise, simple, accurate, sensitive, and reproducible for the quantitative estimation of Dapagliflozin in bulk and its formulation. This RP-HPLC method is also convenient and effective for research studies, quality control, and routine analysis of Dapagliflozin in pharmaceutical industries and in the assay of Dapagliflozin tablets, which are used in the treatment of Type 2 Diabetes
Keywords
RP HPLC, Diabetes mellitus, linearity, accuracy, methanol, calibration.
Introduction
Quality can be defined as the performance is the grade of excellence. A good medicine is one that meets specifications, is safe to purchase, and can be used safely for its intended purpose. Analytical chemistry is a science aimed at continuous improvement in measuring the chemical composition of natural and human resources. Analytical chemistry is a discipline of chemistry whose broad mission is to understand the chemical composition of all substances and to develop tools to describe these elements.
1.Few analytical methods: These analyzes are divided into analytical methods and between them analytical methods, the most commonly used analysis methods include UV-visible spectrophotometry, infrared spectrophotometry, mass spectrometry, nuclear magnetic resonance and other spectroscopic methods. It is based on HPLC, HPTLC, LC-MS, GC-MS and other chromatographic methods.
2.High Performance Liquid Chromatography: HPLC is an analytical technique that uses specialized equipment to separate, measure and analyze the components of chemical mixtures. The sample of interest is entered by the slow flow method, the product is separated by a chromatographic column containing specific information, and the component information is obtained by a combination of detection mechanism and file system. HPLC separation is based on the selective distribution of analytes between a liquid mobile phase and an immiscible stationary phase.
3. Types of High-Performance Liquid Chromatography
- Normal phase HPLC
- Reversed phase HPLC
HPLC method development consists of several simple steps, including sample preparation, test scores, selection of separation conditions, quantification, and validation of the method. The development of high-performance liquid chromatography (HPLC) is complicated by various materials such as columns, eluents, and poor performance.
Drug Profile:
Dapagliflozin is a pharmaceutical drug that belongs to class of medications known as sodium-glucose cotransporter 2 (SGLT2) Inhibitor, it is used for the treatment of type 2 Diabetes and Heart failure.
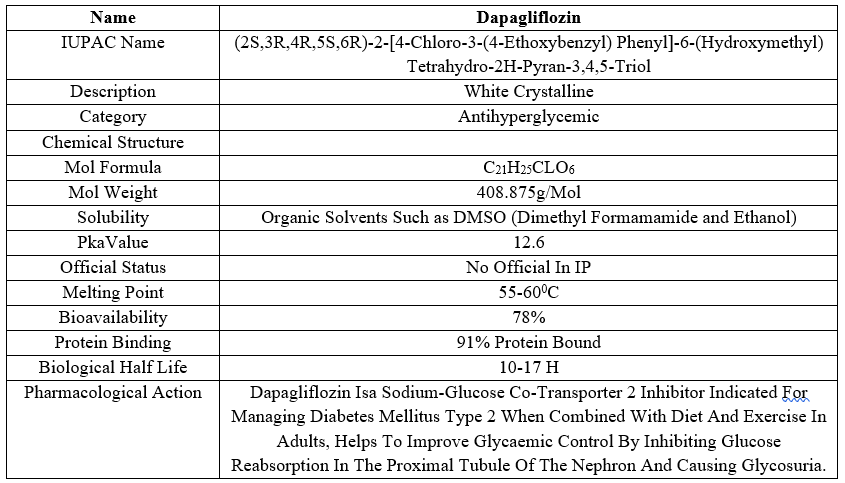
Table 1: Brief Overview Of Dapagliflozin
Research Envisaged:
The aim of this project is to develop and improve the analytical method for dapagliflozin, a drug used in the treatment of type 2 diabetes, and to use the method in accordance with the International Committee for Harmonization (ICH) guidelines, specifically Q2 (R1). The study was carried out in a systematic and sequential manner, starting from a comprehensive review of the available literature for the analysis of Dapagliflozin.
Steps involved in Method development
- Selection of column
- Selection of detection wavelength
- Selection and optimization of mobile phase
- Preparation stock standard solution
- Linearity studies
- Estimation of drug in bulk material
- Analysis of marketed formulation
Validation Of Methods:
- Linearity studies
- Accuracy studies
- PRECISION studies
- Limit of Detection (LOD) and Limit of Quantification (LOQ)
- Robustness
- Ruggedness studies
- Repeatability
- Recovery studies
- System suitability studies
- Specificity
- Range
- Materials And Equipments:
Materials utilized/procured for Analytical Method development and Validation are Dapagliflozin drug supplied by
Dr. Reddy’s laboratory Hyderabad and its potency strength is 100%.
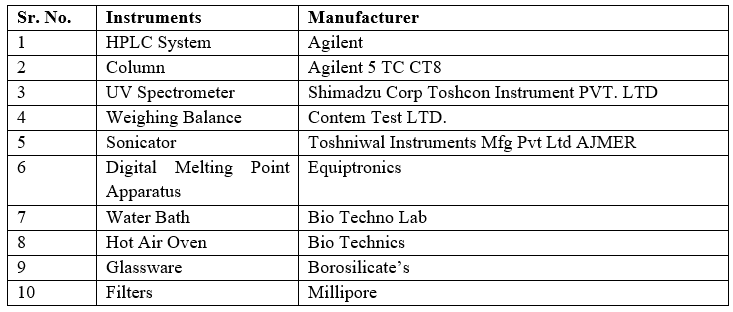
Table 2: List of Instruments
Reagents And Chemicals:
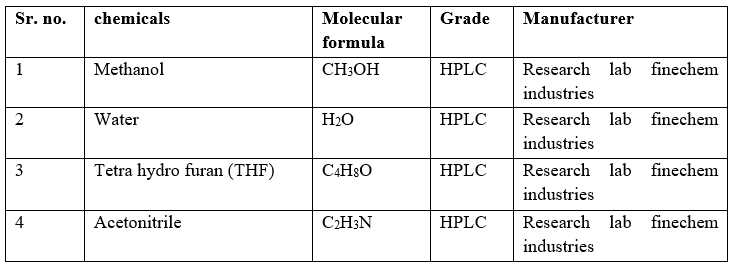
Table 3: List Of Chemicals
Experimental Work:
The spectrum of dapagliflozin was examined using a UV spectrophotometer. Use a UV spectrophotometer to record the UV spectrum of dapagliflozin API and determine the maximum wavelength. Predictions for medicine.
Selection of chromatographic method:
The correct choice depends on the nature of the sample (ionic/neutral molecules, their molecular weight and solubility). The chemicals selected in this study are polar and reverse phase chromatography can be used. Here, the reversed-phase HPLC method was chosen for the initial separation because it is simple, practical, robust and versatile.
Reverse Phase High Performance Liquid Chromatography Method Development
1. Optimization of Chromatographic Methods:
To obtain the best chromatographic conditions for the separation, elution, and quantification of OLAPARIB, adjust one to two parameters in each experiment and collect chromatograms under all specified chromatographic conditions.

Table 4: Optimization of method
Conclusion: The elution peak is negative. The next experiment was done by changing the phase of the cell, column and volume, and the elution peak was good and satisfactory. Storage time is also satisfactory. Analysis was performed and chromatographic conditions were carried out.
2. Methodology Followed in Analytical Method Validation
Reagents- HPLC grade water, Methanol (HPLC grade), Distilled water
Preparation of Mobile phase- The mobile phase was prepared by mixing of HPLC grade methanol: water (70:30v/v).
Preparation of diluents- mixed with 90 ml 100% distilled water and 10 ml HPLC grade methanol. Shake well till homogeneity obtained, later used as mobile phase.
Preparation of standard solution: 20 mg of standard (API) dapagliflozin was weighed and transferred into 100 ml volumetric flask, and the volume was made up to 100 ml mark with diluents. (stock -1) 10 ml of from above solution is pipette out and transferred into another 100ml volumetric flask and volume make up with 100 ml .(stock solution II) concentration 20 ppm.
Preparation of sample solution: The sample solution was prepared by dapagliflozin equivalent to 20 mg of dapagliflozin standard dissolve in 90 ml 100% distilled water & 10ml hplc grade methanol this mixture prepared in 100 ml volumetric flask .(stock I) Pipette out 10 ml and transferred into another 100 ml volumetric flask. Volume make up with 100 % distilled water, solution sonicate for 10 – 15 min with ultra-sonic Sonicator. This solution is filtered through 0.45u whattman filter paper. (Concentration 20ppm).
Assay procedure: Separately injected the equal volume of blank (diluent), five replicates injection of standard and two injection of sample solution was injected into the HPLC system, chromatogram was recorded.
Evaluation of system suitability: Standard solution was injected five replicates into chromatogram and the responses of dapagliflozin peak were measured. The relative standard deviation of five replicate injections should not be more than 2%. the number of theoretical plates should not be less than 2000 and The tailing of the dapagliflozin peak should not be more than 2.0.
Estimation of dapagliflozin tablet dosage form: Standard solution and sample solution were prepared. Peak area was measured and Assay %, label claim calculated.
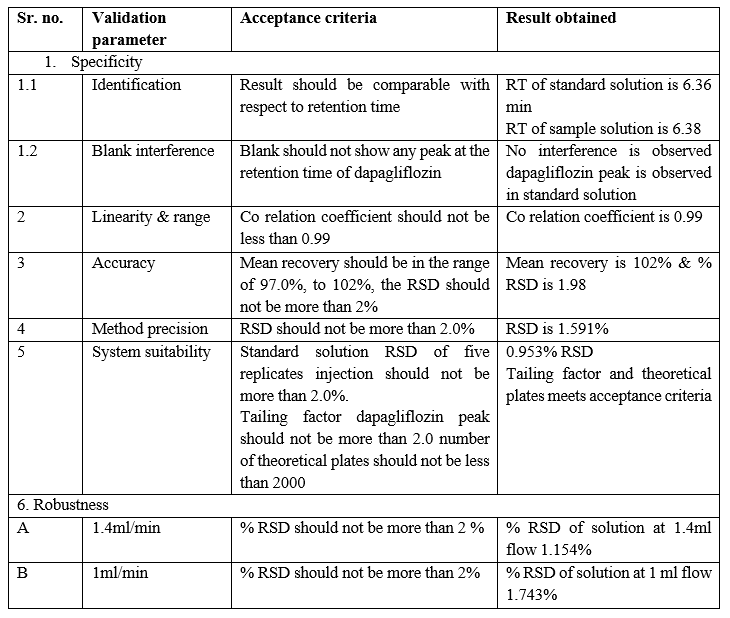
Parameters to be evaluated under validation:
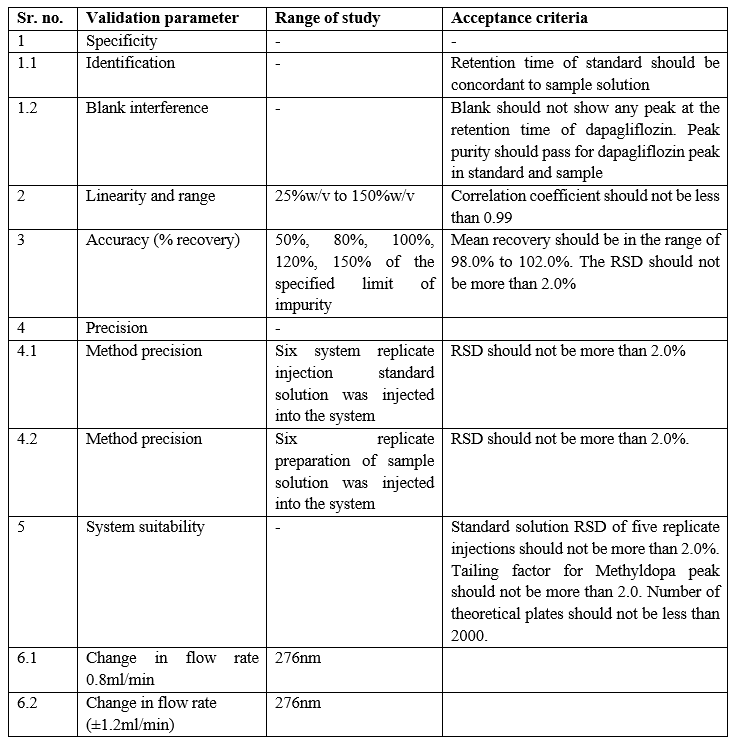
Table 5: Parameters to be evaluated under Validation
RESULT AND DISCUSSION:
The UV spectrum was obtained after preparing dapagliflozin stock solution in diluents methanol: water (10:90), spectra of the dapagliflozin were scanned in diluents. LaFig 1: UV spectra of dapagliflozin
Determination of functional group by FTIR
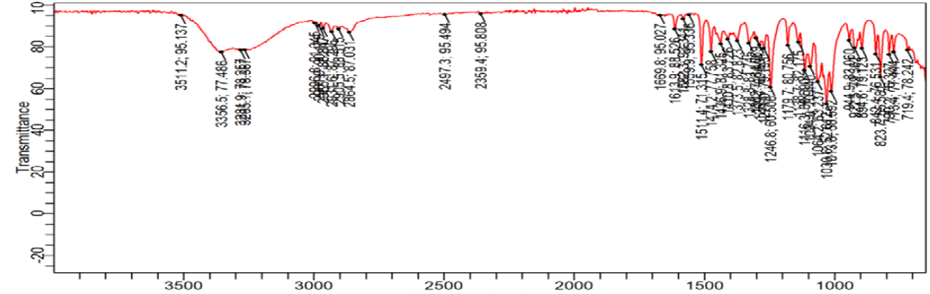
Fig 2: IR spectra of dapagliflozin
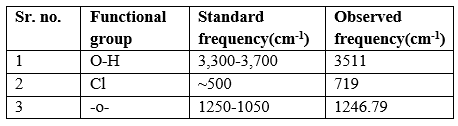
Table 6: Interpretation of Dapagliflozin
Interpretation of Dapagliflozin
Optimized chromatographic conditions for HPLC method
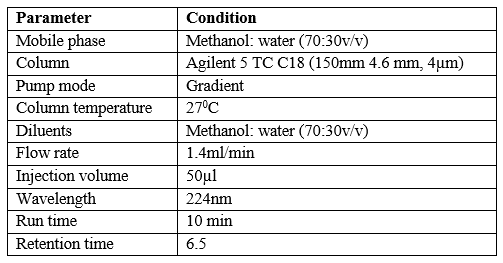
Table 7: chromatographic conditions
1. Estimation of dapagliflozin in tablet dosage form:
% Assay
Pour equal amounts of blank (diluent), sample solution 5 times and sample solution 2 times into the HPLC system and record the chromatogram. Prepare the sample and sample solution as described in the example above, measure the peak area and calculate the % content.
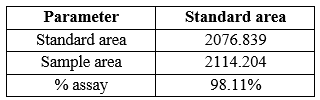
Table 8: % Assay
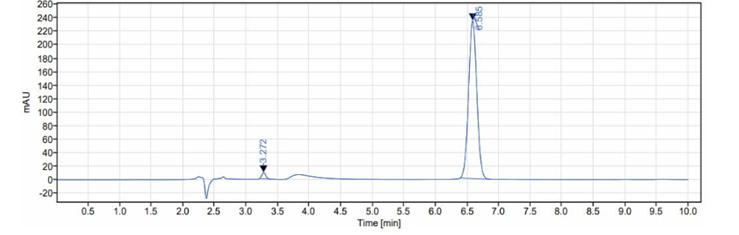
Fig 3: Chromatogram of assay of sample dapagliflozin solution
System suitability:
The dapagliflozin standard was reinjected into the HPLC system five times to measure the reaction of the dapagliflozin peak. The RSD percentage of five repeated injections should be no more than 2%, the tail factor of the dapagliflozin peak should be no more than 2.0, and the theoretical plaques should be no less than 2,000.
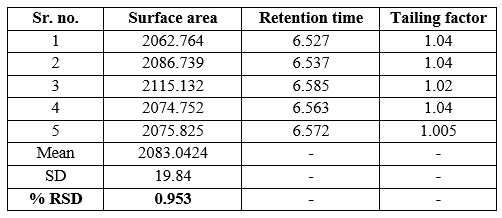
Table 9: system suitability parameters
Acceptance criteria: The %RSD of peak area for 6 repeated injections of the standard dose of dapagliflozin should not be more than 2.0%. The number of theoretical plots for the dapagliflozin peak in the sample solution should not be less than 2,000.
Fig 4: chromatogram of specificity of blank injection
Fig 5: chromatogram of specificity Standard solution of dapagliflozin
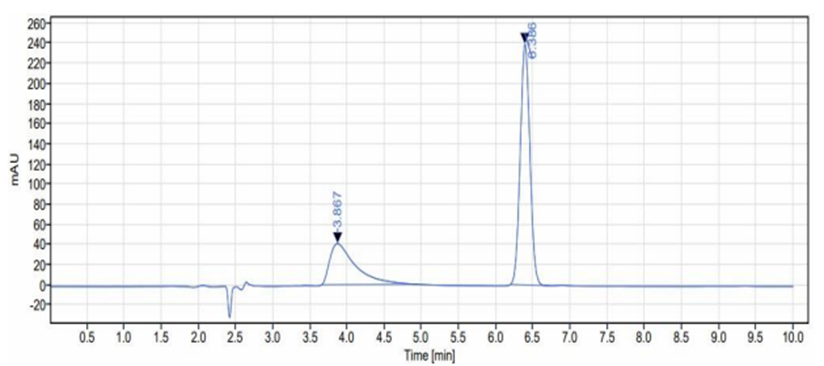
Fig 6: chromatogram of Sample solution of dapagliflozin
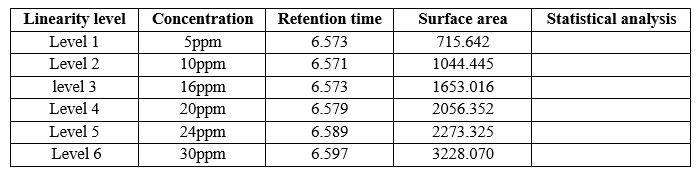
Prepare necessary dilutions of dapagliflozin (e.g. Solution Method-I). The concentration of Solution-I is 20 ppm i.e. 100% and different concentrations are pipetted to prepare more dilute concentrations. This is 5 ppm, 10 ppm, 16 ppm, 24 ppm, 30 ppm. Six different concentrations were prepared and injected into the HPLC system. Draw a graph showing height on the x-axis and area on the y-axis. Regression coefficients, linear range and results are as follows.
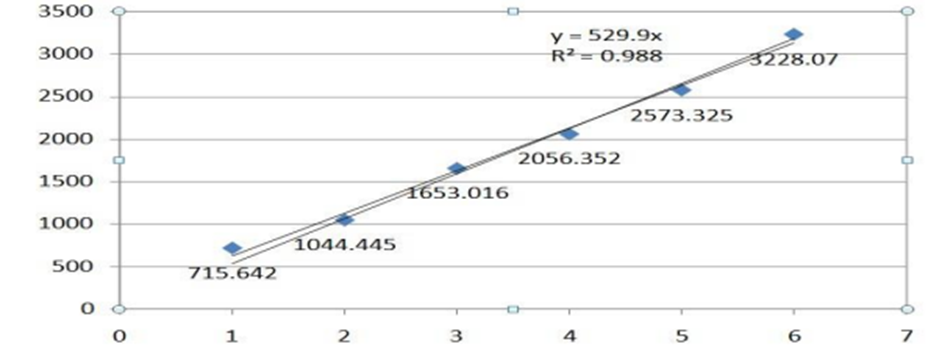
Fig 7: Calibration Graph of Dapagliflozin
Acceptance criteria:
Correlation coefficient should not be greater than 0.99. Response should be linear.
Conclusion- The results showed that the data for power lines did not meet their requirements. Express the linear response. The response has been shown to be linear; See calibration chart. The correlation coefficient meets the acceptance criteria. The correlation coefficient is 0.98 and the method is linear on the given test.
Accuracy: The accuracy was evaluated by spiking at 5 levels from 50 %, 80 %, 100%, 120%, and 150 %. Levels of the corresponding stock concentration level for sample in triplicate and prepared as described under methodology.
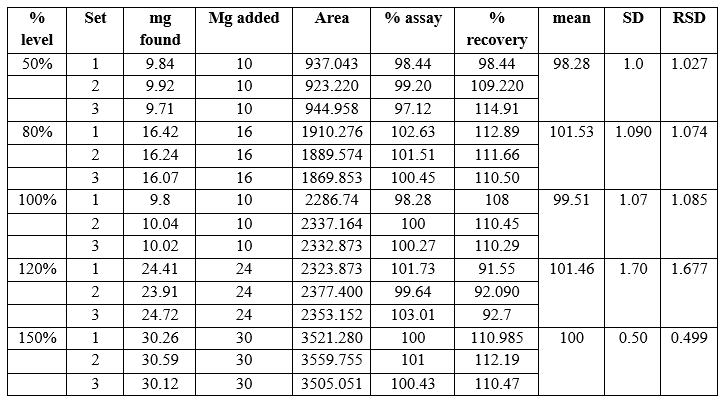
Fig 8: Data sheet of Accuracy
Acceptance criteria – mean recovery for assay precision of dapagliflozin should be in the range of 97% -103 % The RSD for % recovery of all obtained result should not be more than 2.0 %
Conclusion – result shows that all resulting value meets its acceptance criteria, HPLC method is suitable from above data we conclude all the result of% recovery data is meets acceptance criteria. And all % RSD value meets acceptance criteria.
Precision –
The degree of consistency of test values ??obtained under specified conditions from consecutive samples of the same homogeneous sample is indicated by the accuracy of the analytical method.
Method precision: Six sample solution of dapagliflozin drug were injected into HPLC system, record the area and calculate % assay and % RSD for resulting data.
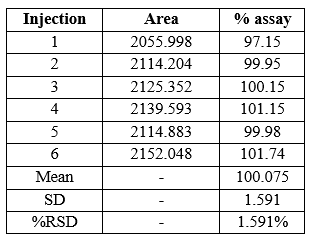
Acceptance criteria - % RSD should not be more than 1% and assay precision of dapagliflozin should not be less than 97 % and should not be more than 103 %.
Conclusion – from above data we conclude that all result meets its acceptance criteria; the developed precision method of dapagliflozin is reproducible. % RSD of all 5 assay is 1.591 which is in the range of 2.0
Robustness: It is the capacity to remain unchanged by small but deliberate variations, in method parameters. Robustness of method was verified by deliberately varying instrumental condition by flow rate (±) 0.4.
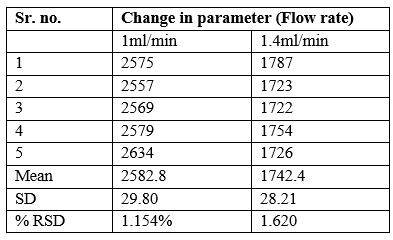
Acceptance criteria – the difference for each modified condition and original condition should not be more than 2.0%.
Conclusion - % RSD meets acceptance criteria.
Stability Analytical Solution:
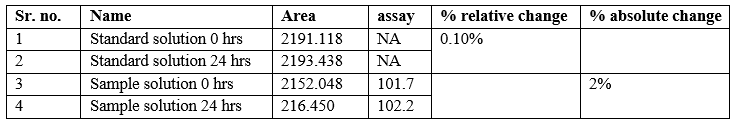
Acceptance criteria:
1. For standard solution: % Relative difference in area of the active ingredients in standard solution should not be more than 2.0% with respect to initial value.
2. For Sample Solution: Absolute difference in assay of active ingredients in sample solution should not be more than 2.0% with respect to initial assay value.
Conclusion: Standard and sample solutions are stable for 24 hrs at room temperature.
CONCLUSION:
The aim of this study is to develop a convenient, sensitive and economical analytical method to estimate the content of dapagliflozin propylene glycol monohydrate in bulk and dosage forms. , robustness verification and found that the method meets the predetermined acceptance. The parameters considered for acceptance and the results obtained are summarized in the Table;
DISCUSSION:
High-performance liquid chromatography plays an important role in analysis due to its simplicity, high specification, sensitivity and ability to analyze the structure of complex drugs. Agilent HPLC CDS software using Agilent 55 TC × CT8 (2) 250 × 4.6 mm column in open laboratory. DAD detector for this study. Prepare dapagliflozin standard solution and diluent sample. Try using different pure solvents with different polarities as mobile phases to create chromatograms. A suitable time was found to be methanol: water (70%: 30% v/v). The selected wavelength is 224 nm. This system has good resolution and good storage time with reasonable queuing factor. Once the chromatographic system was established, test mixtures were prepared and analyzed according to the procedures described in Materials and Methods. It provides accurate, reliable and consistent results in drug prediction in capsules. The measurement signal is accurate and precise in the measurement range (10-50ug/ml), the correlation coefficient is better than 0.99. Additionally, the use of low solvents results in an efficient and environmentally friendly chromatography process. The actual average return is 102% and the RSD percentage is in the range of 1.98%. In fact, RSD comes in many forms. The above data clearly show that the RPPHPLC method can be used to determine dapagliflozin in samples. Here, we conclude that the RP HPLC method is cost-effective, simple, specific, linear, accurate, precise and robust for the quantitative analysis of dapagliflozin (API).
REFERENCE
- Mendham J., Denny R. C., Thomas M.; Vogel’s text book of Quantitative Chemical Analysis; Pearson Education Limited; 6th Edition, 2008; 29-39.
- Chatwal G. R., Anand S. K.; Instrumental methods of Chemical Analysis; Himalaya Publishing House, Mumbai, 2005; 11: 1.1-1.2, 2.108-2.109, 2.151-2.153.
- Kasture A. V., Wadodkar S. G., Mahadik K.R., More H.N.; Pharmaceutical analysis instrumental methods; Nirali prakashan, 2005; 12: 148-156.
- Jayapal P, Basha VS, Vali SKK, Rao VS, Venkateswarlu B, Suresh D, Ramesh G (2012) Determination of assay for Ethambutol in Ethambutol Hydrochloride Tablets by RP-HPLC. International Journal of Science and Technology 3: 182-186.
- Anjani QK, Sabri AHB, Donnelly RF (2022) Development and validation of simple and sensitive HPLC-UV method for ethambutol hydrochloride detection following transdermal application. Royal Society of Chemistry 14: 125-134.
- P. D. Sethi. Quantitative Analysis of Drugs in Pharmaceutical Formulations. 3rd edition, CBS P publishers& Distributors, New Delhi, India, 1997.
- T Schaberg, K. Rebhan, and H. Lode. Risk factors for side effects of isoniazid, rifampin and pyrazinamide in patients hospitalized for pulmonary tuberculosis. Eur Respir J. 1996; 9(2): 2026–2030
- Prasanthi B, Vijaya ratnar. S, Phani Ch. Development and validation of RP-HPLC method for simultaneous estimation of rifampicin, isoniazid and pyrazinamide in human plasma. J of Anal Chem.2015; 70(8):1015-1022.
- S.K. Dhal and R. Sharma. Development and Validation of RP-HPLC Method for Simultaneous Determination of Pyridoxine Hydrochloride, Isoniazid, Pyrazinamide and Rifampicin in pharmaceutical Formulation. Chem. Anal. (Warsaw).2009; 54:1487
- G. V. Mante, A. T.et .al. RP-HPLC Method for Estimation of Dapagliflozin from its Tablet International Journal of ChemTech Research CODEN (USA): IJCRGG, ISSN: 0974-4290, ISSN(Online):2455-9555 Vol.11 No.01, pp. 242-248, 2018
- MITALI V. VERMA et .al. DEVELOPMENT AND STABILITY INDICATING HPLC METHOD FOR DAPAGLIFLOZIN IN API AND PHARMACEUTICAL DOSAGE FORM International Journal of Applied Pharmaceutics ISSN- 0975-7058 Vol 9, Issue 5, 2017
- Jitendra Debata et .al. A New RP-HPLC Method Development and Validation of Dapagliflozin in Bulk and Tablet Dosage Form. International Journal of Drug Development and Research Debata et al., Int J Drug Dev & Res 2017
- Manasa Sanagapati et.al Development and Validation of stability-Indicating RP-HPLC method for determination of Dapagliflozin Journal of Advanced Pharmacy Education & Research Jul-Sep 2014 Vol 4 Issue 3
- Vaghela Yavantika V.et .al “DEVELOPMENT AND VALIDATION OF STABILITY INDICATING ESTIMATION METHOD OF DAPAGLIFLOZIN IN IT’S TABLET DOSAGE FORM” [VOLUME 6 I ISSUE 2 I APRIL– JUNE 2019] E ISSN 2348 –1269, PRINT ISSN 2349-5138
- Subrata Sarkar et al Method Development and Validation of Dapagliflozin Drug in Bulk and Tablet Dosage form by RP-HPLC Int J Pharma Res Health Sci. 2017; 5 (4): 1755-59 1755 © International Journal of Pharma Research and Health Sciences. All rights reserved DOI:10.21276/ijprhs.2017.04.07 S Sarkar and Vipul D Patel CODEN (USA)-IJPRUR, e-ISSN: 2348-6465.
- Vaishali B. Bhamareet.al. ANALYTICAL METHOD DEVELOPMENT, VALIDATION AND FORCED DEGRADATION OF DAPAGLIPFLOZIN BY RP-HPLC World Journal of Pharmaceutical Research SJIF Impact Factor 8.084 Volume 9, Issue 12, 1006-1076
- Landge A K et.al. RP-HPLCAssay Method Development and Validation of Dapagliflozin in Tablet Dosage Form International Journal of Pharmaceutical Research and Applications Volume 7, Issue 2 Mar-Apr 2022, and pp.: 112-120
- Gunasekar Manoharan et.al. Stability-Indicating RP-HPLC Method Development for Simultaneous Determination and Estimation of Dapagliflozin in Raw and Tablet Formulation Chemistry Research Journal, 2018, 3(2):159-164
- Deepan .et.al. European Journal of Applied Sciences 9 (4): 189-199, 2017 ISSN 2079- 2077 © IDOSI PThiyagarajan Deepan et. al Stability indicating HPLC method for the simultaneous determination of dapagliflozin and saxagliptin in bulk and tablet dosage form Curr. Issues Pharm. Med. Sci., Vol. 31, No. 1, Pages 39-43ublications, 2017 DOI: 10.5829/idosi.ejas.2017.189.199
- Monika Waksmundzka- hajnosjoseph Sharma High performance liquid chromatography in phytochemical analysis .chapter 1. dynamics of chromatography: principles and theory .j. Calvin Giddings
- Dr saurab arora et.al introduction to high performance liquid chromatography bt Lab training. Com


 Mr. Lohiya G. V.*
Mr. Lohiya G. V.*
 Kulkarni Y. P
Kulkarni Y. P
 Mahima Murlidhar Jadhav
Mahima Murlidhar Jadhav





















 10.5281/zenodo.12806716
10.5281/zenodo.12806716