Abstract
The current pharmaceutical quality assurance approach is no longer enough due to recent changes and limited resources for medication development and manufacturing, which has led to new study in these areas. In 2002, the FDA updated and modernised the regulations controlling pharmaceutical production and product quality in response to these research endeavours, resulting in a paradigm shift in the current Good Manufacturing Practices (cGMP). After the ICH Q8 was approved in 2005, the Quality by Design (QbD) methodology made its way into the pharmaceutical business in the previous ten years. Determining the critical quality ascribed (CQA) and defining the desired product performance profile (Target Product Profile (TPP), Target Product Quality Profile (TPQP)) are crucial steps in the design and development of a product under the QbD concept. We can then design the product formulation and procedure to meet the desired product qualities based on this. This results in the recognition of the influence of key process parameters (CPP) and raw materials [critical material attributes (CMA)] on the CQAs, as well as the identification and management of sources of variability. This paper discusses pharmaceutical QbD and explains how it can be used to develop pharmaceutical products well within the specified period of time. QbD is an emerging idea that offers pharmaceutical manufacturers increased self-regulated flexibility while maintaining tight quality standards and real-time drug product release. When designing and developing pharmaceutical dosage forms, quality-based drug development (QbD) is predicated on an awareness of the target product's quality profile (QTPP) and an evaluation of its hazards. QbD also discusses its tools, which include process analytical technologies, quality risk management, and DoE. The significance of QbD in fostering a science-based methodology in pharmaceutical product development is highlighted by these evaluations.
Keywords
QbD, ICH, analysis, FDA
Introduction
The objective of pharmaceutical development is to create high-quality products and manufacturing processes that reliably produce the desired results. It's critical to understand that products cannot be checked for quality. Rather of being tested into the end analytical process outputs, quality should be ingrained in the process design. The pharmaceutical industry is regarded as one of the most strictly regulated industries, consistently producing high-quality pharmaceuticals for human use that have pharmacotherapeutic effects for treating a wide range of illnesses. The US Food and Drug Administration [FDA] released a document with guidelines for pharmaceutical businesses in 2002. Businesses should incorporate efficacy, safety, and quality into their products. We now refer to this idea as Quality by Design. Pharmaceutical quality is defined by Janet Woodcock, the director of the Centre for Drug Evaluation and Research, as a contaminant-free product in a 2004 paper. They consistently provided the customer with the therapeutic advantage that was advertised on the label. A more recent trend in the development of analytical methods is the QbD technique, which can be quite beneficial when used correctly.The goal of pharmaceutical development is to create high-quality products and manufacturing processes that reliably produce the desired results. Scientific understanding is supported in the formulation of the design space, specifications, and production controls by the data and knowledge gathered from pharmaceutical development research and manufacturing experience. Data from research on pharmaceutical development can serve as a foundation for high-quality risk management. Understanding that products cannot be assessed for quality means that quality should be incorporated into the design from the beginning. It is important to view modifications to the formulation and manufacturing processes that occur during the development and lifecycle management phases as chances to learn more and strengthen the foundation of the design space. In a similar vein, it can be helpful to include pertinent information discovered through studies producing surprising outcomes. The applicant proposes the design space, which is pending regulatory review and approval. Operating inside the design space is not regarded as a modification. Exiting the design space is seen as a change, and doing so would typically start the regulatory post-approval change process.
The International Council for Harmonisation of Technical Requirements for Pharmaceutical Product Registration among the three regions' licencing agencies, pharmaceutical industry experts, and representatives from the USA, Japan, and Europe comprise the ICH forum. Current guidelines must also be published by the ICH. The Q8 guideline (ICH Q8, 2009) was released by the ICH in 2005 and suggested that the pharmaceutical industry adopt the "quality by design (QbD)" concept. While the QbD approach was only recently formalised in the pharmaceutical business, DoE and PAT concepts have been used for decades to improve process understanding, modelling, and control. It is well recognised that creating novel, safe, and effective therapies is a time-consuming, costly process. Product quality is increased at a lower cost by using the new approach (QbD), which guarantees that quality is delivered by the manufacturing of a product rather than by product testing. Despite some early difficulties, QbD has the potential to generate revenue for pharmaceutical companies through QbD studies. Better medications also prioritise patient safety and guarantee that the patient will receive the medication faster. According to ICH Q8, quality-based drug development (QbD) is a methodical approach to drug development that starts with predetermined objectives. This suggests that the comprehension of the product and its processes is grounded in reliable research and quality risk management (ICH Q8, 2009). In addition to traditional dosage forms, sustainable drug manufacturing practices, quantitative risk assessments in pharmaceutical development processes that involve life cycle management, and the downstream processing of biotechnological products, QbD (Gaspar et al., 2012) also includes potentially valuable, patient-specific treatment practices.
Definition [FDA PAT Guidelines, Sept. 2004]
An approach for planning, evaluating, and managing production by promptly measuring important quality and performance characteristics of new and in-process materials and processes (i.e., during processing), with the aim of guaranteeing the safety of the finished product. "Quality by Design" (QbD) is a concept that encompasses improved scientific understanding of critical process and product qualities, designing controls and tests during the development phase based on the limits of scientific understanding, and using the knowledge gained during the product's life-cycle to work on an environment of continuous improvement.
In order to maintain the required level of product quality, manufacturing procedures and formulation design and development are included in the Quality-by-Design (QbD) approach to pharmaceutical development. To guarantee that the information on the subject is established and used in an independent and integrated manner, guidelines and mathematical models are employed.
Purpose and Objectives
To enhance innovation and efficiency in product development, Quality by Design (QbD) promotes process and product understanding. Additionally, using a QbD method aids with FDA compliance. Product development can be accelerated and expenses and waste can be decreased with the help of QbD. When it comes to custom 3D printed prostheses, the quality by design (QbD) method can assist guarantee that the goods are built and designed accurately from the start.
The FDA publication defined QbD as:
- Developing a product to meet predefined product quality, safety, and efficacy.
- Designing a manufacturing process to meet predefined product quality, safety and efficacy.
Benefits of QBD:
- QbD is good Business.
- Eliminate batch failures.
- Minimize deviations and costly investigations.
- Avoid regulatory compliance problems.
- Organizational learning is an investment in the future.
- QbD is good Science.
- Better development decisions.
- Empowerment of technical staff.
- Increase Manufacturing efficiency, reduce cost, and project rejections and waste.
- Implementation of a Question-based review (QbD) process has occurred in CDER’s office of generic drugs.
- Implementation of QbD for a biological license application (BLA) is progressing.
- Optimization Design performed using Quality by Design as 2?3; full factorial design 24
- Organization learning is an investment in the future
Benefits to Industry:
- Ensure better design of the product with less problems in manufacturing.
- Improve interaction with the FDA deal on a science level instead of a process level.
- Nanocellulose prepared by the QbD approach had better flow property and compatibility.
Opportunities
- Efficient, agile, flexible system
- Increase manufacturing efficiency, reduce costs and
- project rejections and waste
- Build scientific knowledge base for all products
- Better interact with industry on science issues
- Ensure consistent information
- Incorporate risk management
ICH Q8:
Quality by design (QbD), a novel pharmaceutical regulation concept introduced by the US FDA a decade ago, has forced the pharmaceutical industry to design the end product's quality rather than relying solely on product testing. "A systematic approach to development that begins with predefined objectives and emphasises product and process understanding and process control, based on sound science and quality risk management 14," is how the ICH guideline Q8 defines QbD.
Components of Drug Product Given by ICH Q8:
- Drug Substances: The drug products physiochemical and biological characteristics, which can affect how well they work and how easily they can be produced.
- Example of physicochemical and biological properties includes:
- Solubility
- Water content
- Particle size
- Excipients
It is important to assess how well the medicinal ingredients and excipients work together. The degree to which different pharmacological substances are compatible with one another should also be assessed in products that contain several drug substances.
- Formulation Development:
As part of formulation development, the qualities of the drug products that are essential to its quality and identified, and the progression of the formulation design from its original concept to its final design is highlighted. Clinical formulations are linked to the suggested formulation by comparative in-vitro studies, such as dissolution or in-vivo investigations such as BE.
- Container and Closure System:
- The choice of materials for primary packaging and secondary packaging should be justified.
- A possible interaction between product and container or label should be considered.
Seven Steps of QbD:
The best way to assess how to implement QbD in your organization in a simple seven-step process:
- Hire an independent Quality design expert.
- Audit your organization and processes with the expert conducting a gap analysis.
- Hold a basic QbD workshop with all your personnel – the expert should lead this and design it to speak to multiple levels, from the factory floor to the board room.
- Review the expert's report and recommendations.
- Draft an implementation plan, timelines, and estimated costs.
- Assign the resources.
- Retain the independence expert as your project assurance advisor.
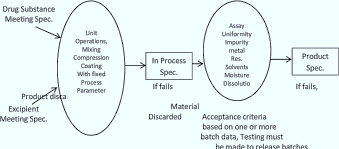
Fig: quality diagram by QbD
- Product design goal:
After outlining the quality target product profile (QTPP), it is necessary to determine the different critical quality characteristics (CQA) that the product must have. The QTPP contains defining factors for the product, such as dosage form, dose strength, delivery mechanism, route of administration, and intended use, among many other factors. CQA refers to a product's physical, chemical, biological, or microbiological characteristics that must fall within a given range, limit, or distribution in order for the product to meet the intended quality standards. Both QTPP and CQA offer a framework for product design and understanding, which is accomplished by conducting trials on various formulations, such as gels, ointments, and sprays, to characterize a substance's solubility, stability, compatibility, etc.
- Process design space:
Within the pharmaceutical sector, batch differences both within and between batches are commonplace. The robustness of the design space can help to reduce these variations. A "established multidimensional combination and interaction of material attributes and/or process parameters demonstrated to provide assurance of quality," according to the ICH Q8, is what is meant by design space. Identification of key process parameters (CPPs) is aided by assessing the potential impact of a process variation on the final product's quality. Defined design space makes it feasible to anticipate problems and achieve improved process control. With the aid of real experimental data, product experience, or literature guidance, the bounds of the sets of the parameter that has to be refined can be stated.
- Control space:
The process design space can serve as the foundation for defining a control space. It enables us to comprehend the processes in a way that makes it possible to guarantee product quality despite the known production process' variability, giving us greater control over complex production processes. Consider a dataset of a reference product with closely clustered data points that show the result of a procedure under strict control to help visualise this idea. Whether or not the process is in control will be shown when the output of this process is plotted and contrasted with a reference. When a product is studied early in its development, a number of deviations can be prevented in addition to the benefits that come with it. This will eliminate a number of wasted efforts and enable early identification of the underlying causes of unanticipated negative consequences.
- Operating space
Operating space is the optimal set of statistically determined parameters that can readily account for any variability that naturally arises in both CPPs and CQAs. For generics, the operating space should be contained within the control space's bounds, enabling the testing of a reference product with identical parameters; for new products, the operating space should be contained within the design's bounds in accordance with legal requirements.
By thoroughly exploring the design space and testing multiple formulation batches in an effort to improve their product, the innovators can obtain a competitive edge.
Elements of QbD
-
-
-
- Control strategies -A planned set of controls, derived from current product and process understanding that ensures process. performance and product quality
- Critical process parameters (CPP)- A process parameter whose variability has an impact on a critical quality attribute and therefore should be monitored or controlled to ensure the process produces the desired quality.
- Critical quality attributes (CQA)- A physical, chemical, biological or microbiological property or characteristic that should be within an appropriate limit, range or distribution to ensure the desired product quality.
- Design space- The multidimensional combination and interaction of input variables (e.g., material attributes) and process parameters that have been demonstrated to provide assurance of quality.
- Proven acceptable range (PAR)- A characterized range of a process parameter for which operation within this range, while keeping other parameters constant, will result in producing a material meeting relevant quality criteria.
- Quality risk management- A systematic process for the assessment, control, communication and review of risks to the quality of the drug (medicinal) product across the product lifecycle.
- Target Product Profile (TPP) - Explains the main purpose of a drug development programme and provides information regarding the development process.
- Quality target product profile (QTPP) -A prospective summary of the quality characteristics of a drug product that ideally will be achieved to ensure the desired quality, taking into account safety and efficacy of the drug product.
- Design of Experiments (DoE) -Parameters that should be included or excluded in multiple variable analysis and/or model development should be assessed and chosen by considering the significant differences between the process requirements for large and small molecules in addition to the differences between active ingredients and finished product processes.
Future Applications of Analytical QbD Approaches
It is possible to foresee extensions of analytical QbD approaches, like the MDS approach, for other widely used analytical techniques. To find metals in API and medicinal products at low concentrations, for instance, inductively coupled plasma optical emission spectroscopy (ICPOES) and related techniques are employed. Even though the basic objective is to avoid using metals testing in a manufacturing setting, there are circumstances in which ICP-OES or a comparable technique is necessary.
In addition to complicated instrumentation needs and sample preparation techniques, matrix effects can also be a major problem with these procedures. All of these things could be methodically developed and then contested later on during the risk assessment step. In certain situations, testing of APIs and medicinal products requires the use of solid form analytical techniques, such as X-ray powder diffraction (XRPD). However, the inherent complexity of XRPD procedures can be rather high. Can profit from methods based on QbD. Another area where analytical QbD could result in better techniques is particle size analysis (PSA), as these techniques are essential to particle engineering strategies and the particle size distribution is frequently specified in the API for oral and inhaled products.
The user would be assisted in choosing between dynamic light scattering and laser diffraction methods, for example, and in evaluating the risk of the selected method using the structured DoE and MSA tools if an organised approach was applied to PSA method creation. Although there are existing structured ways, such as decision trees, to help guide the method developer in the development of PSA methods, no example that demonstrates the application of risk assessment tools to the evaluation of a PSA method has surfaced.
The advancement of analytical QbD depends on industry participants' sustained investment and their avoidance of generic approaches unless their hazards are already well acknowledged.
CONCLUSION
With the implementation of a Question Based Review (QBR) structure, the Office of Generic Drugs (OGD) has taken serious steps to incorporate QbD concepts into its ANDA drug filing process. The focus behind QbD needs to change into a business proposition that appeals to the generics industry as a basis for corporate competitiveness if the rate of QbD adoption in the market is to increase. For Quality-Based Purchasing to be effective, it must support a generic product development team whose main goal is to submit applications first. The quantity and timing of ANDA filings, rather than the quality of the ANDA, are the primary metrics used by many R&D organisations in the generics business. The risk of inadequate process and product expertise is palpable when we consider the Agency's efforts to guarantee that bioavailability promises made during development are upheld in commercial medicines that have already received approval and are on the market. The technique known as Quality by Design, or QbD, is becoming more and more prominent in the creation of pharmaceutical products. improves production while giving patients access to high-quality medications. The requirements of the contemporary production process are indicated by Quality by Design (QbD) and its instruments. Designing for quality is a time- and money-efficient method of production and design. QbD has a broad application in biotechnological products, including enzymes, vaccines, monoclonal antibodies, and other products. Future regulatory flexibility could be greatly increased with the implementation of this new Quality by Design (QbD) process. Furthermore, Quality by Design is becoming a widely used production paradigm. The product quality is not checked at the conclusion of the production process when using the QbD technique. Rather, quality is included into the end product within the product design process. When it comes to quality management, quality assurance is the better approach. In QbD, quality is guaranteed instead of controlled. QbD has arisen as a technique where the manufacturing process performance for process validation is continuously monitored, evaluated, and adjusted as continuous process improvements have become available. This new method allows judgements to be made based on information that is both scientific and risk based.
REFERENCE
- Woodcock J, The concept of pharmaceutical quality. American Pharmaceutical Review, 7(6), 2004, 10–15.
- Q9: Quality Risk Management. ICH Harmonized Tripartite Guidelines. International Conference on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use, 2006.
- Q10: Pharmaceutical Quality System, ICH Tripartite Guidelines. International Conference on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use, 2007.
- Lionberger RA, Lee LS, Lee L, Raw A, Yu LX, Quality by design: Concepts for ANDAs, The AAPS Journal, 10, 2008, 268–276.
- FDA Guidance for Industry and Review Staff: Target Product Profile – A Strategic Development Process Tool (Draft Guidance).
- Q8 (R1): Pharmaceutical Development, Revision 1, ICH Harmonized Tripartite Guidelines, International Conference on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use, 2007.
- Food and Drug Administration. Final Report on Pharmaceutical cGMPs for the 21st Century – A Risk Based Approach, http://www.fda.gov/ cder/ gmp/ gmp 2004/ GMP_ final report 2004.html.
- Nasr, N., Risk Based CMC Review Paradigm. Advisory Committee for Pharmaceutical Science Meeting 2004, July, 20-21.
- US Food and Drug Administration. Guidance for Industry. PAT—A Framework for Innovative Pharmaceutical Manufacturing and Quality Assurance. Pharmaceutical cGMPs. Rockville, MD, 2004 Sept., 1–21.
- Delasko, J.M., Cocchetto, D.M., Burke. L.B., Target Product Profile: Beginning Drug Development with the End in Mind. Update, January/February, Issue 1, 2005, http://www.fdli.org.
- US Food and Drug Administration. Guidance for industry: Q10 Quality Systems Approach to Pharmaceutical cGMP Regulations, FDA, Rockville, MD 2006.
- Lawrence X, Raw A, Lionberger R, Rajagopalan R, Lee L, Holcombe F, Patel R, Fang F, Sayeed V, Schwartz P, Adams P, and Buehler G. U.S. FDA question-based review for generic drugs: A new pharmaceutical quality assessment system. J. Generic Med 2007; 4:239–248
- Bhatt D, Rane S. International Journal of Pharmacy and Pharmaceutical Science 2011; Vol 3, Issue 1
- ICH Harmonized Tripartite Guideline on Pharmaceutical Quality Systems Q10, step 4 version, dated June 4, 2008.
- Schmitt S (2011). Quality by Design Putting Theory into Practice. LLC, USA: Davis Healthcare International Publishing Singh SK, Venkateshwaran TG, Simmons SP (2010) Oral Controlled Drug Delivery: Quality by Design (QbD) Approach to Drug Development.
- In Wen H and Park K (EDs) Oral Controlled Release Formulation Design and Drug Delivery: Theory to Practice New York: John Wiley&Sons, inc, pp. 279-303. (2008)
- International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use, Pharmaceutical Quality System (Q10) Step 4. http://www.ich.org/products/guidelines/quality/article/quality-guidelines.html. Accessed 10.05.2015. (2009)
- International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use, Pharmaceutical Development Guideline, Q8, http://www.ich.org/products/guidelines/quality/article/quality-guidelines.html. Accessed 10.05.2015. Lepore J, Spavins J (2008) PQLI Design Space J Pharm Innov., 3(2): 79-87.
- Lionberger RA, Lee SL, Lee L, Raw A, Yu LX (2008) Quality by Design: Concepts for ANDAs, AAPS J., 10(2): 268-276. Mesut B, Aksu B, Özsoy Y (2013) Design of Sustained Release Tablet Formulations of Alfuzosin HCl by Means of Neuro-Fuzzy Logic. Lat Am J Pharm. 32 (9): 1288-1297.
- Mesut B, Aksu B, Özsoy Y (2015) The Place of Drug Product Critical Quality Parameters in Quality by Design (QBD) Turk J Pharm Sci, 12(1): 75-92. Pritesh Dasare (2013) Analytical QbD Approach. http://sspcmsn.org/technical_ presentations/iipc-sspc__idma_organized_state_level_seminar_18-01-13 Accessed 12.08.2015. (2015)
- Quality by Design Specifications for Solid Oral Dosage Forms: Multivariate Product and Process Monitoring for Managing Drug Quality Attributes by the Specification Design and Lifecycle Management Working Group of the PQRI Manufacturing Technical Committee. Available online: http://www.pqri.org/pdfs/mtc/pqriqbdspecsconceptpaper. pdf Accessed 30.07.2015.
- Munson J, Gujral B, Stanfield CF, A review of process analytical technology (PAT) in the U.S. pharmaceutical industry. Current Pharmaceutical Analysis, 2, 2006, 405–414.
- Leuenberger H, Puchkov M, Krausbauer E, Betz G, manufacturing pharmaceutical granules, Is the granulation end-point a myth, Powder Technology, 189, 2009, 141–148.


 Netra Machhindra Adhav *
Netra Machhindra Adhav *

 10.5281/zenodo.12607157
10.5281/zenodo.12607157