Abstract
This study focuses on the preparation and evaluation of a thermoreversible ocular in situ hydrogel of chloramphenicol, aimed at achieving sustained drug delivery in the eye. Chloramphenicol, known for its high lipid solubility and effective prophylaxis against ocular surgery infections, was formulated using poloxamer 407 as a thermoreversible polymer, with HPMC K4M and carbopol 940 as viscosity enhancers. The formulation process utilized the cold method, ensuring ease of preparation and cost-effectiveness. Characterization of the drug and excipients confirmed their purity and compatibility. The formulated in situ hydrogel exhibited satisfactory physical properties, drug release profiles, and stability over time, with HPMC K4M proving to be a superior viscosity enhancer compared to carbopol. Stability studies revealed that the gel maintained its properties at 4°C over 60 days, making it a promising option for ocular drug delivery.
Keywords
Chloramphenicol, in situ hydrogel, thermoreversible polymer, poloxamer 407, HPMC K4M, carbopol 940, ocular drug delivery, sustained release, stability, bioavailability.
Introduction
The eye is a vital and highly sensitive organ, often described as the "window to the world" because it allows us to perceive and interact with our surroundings. However, the eye is susceptible to a variety of diseases that can significantly impact vision, potentially leading to partial or complete loss of sight.1 As a result, the development of effective ophthalmic drug delivery systems is crucial for treating these conditions. Ophthalmic drug delivery systems are broadly categorized into traditional and advanced methods; with topical application being the most commonly used and widely accepted approach for administering drugs to the eye.2 Despite its popularity, topical drug delivery to the eye faces significant challenges. The protective mechanisms of the eye, including tear production, blinking, and tear drainage, limit drug absorption and result in a short duration of therapeutic action. This necessitates frequent administration of eye drops, which can lead to poor patient compliance. Conventional ophthalmic formulations, such as inserts, ointments, suspensions, and aqueous gels, often suffer from limitations such as increased precorneal drug elimination, variability in therapeutic efficacy, and issues like blurred vision.4-6 The anatomy and physiology of the eye make it particularly resistant to foreign substances, posing a challenge for pharmaceutical scientists developing ophthalmic drug delivery systems. These systems must be carefully designed to bypass the eye's protective barriers without causing permanent tissue damage.7 Over the past few decades, advancements in diagnostic techniques and the development of novel therapeutic agents have led to the creation of more effective ocular delivery systems. Traditional formulations, such as solutions, suspensions, and ointments, have been refined with the addition of viscosity-enhancing agents or mucoadhesive polymers to improve drug bioavailability. However, these improvements have not fully addressed the issue of rapid drug elimination and the need for frequent dosing.8 One promising approach to overcoming these challenges is the development of in situ-forming ophthalmic drug delivery systems. These systems utilize polymers that undergo a reversible phase transition from a liquid to a gel upon contact with the ocular surface, allowing for extended drug release and improved bioavailability. The rapid drainage of conventional ophthalmic solutions can be mitigated by using such in situ gelling systems, which provide a longer residence time in the eye and reduce the frequency of administration.
The primary challenge in ophthalmic drug delivery remains the short residence time of formulations within the eye.9,10 Tears quickly wash out instilled drugs, leading to low bioavailability—often as low as 1-10%. The normal tear volume in the eye is approximately 7 µL, while a non-blinking eye can accommodate up to 30 µL of fluid. However, most instilled solutions, with typical drop sizes of 50 µL, are rapidly drained from the eye, leading to significant drug loss. Factors contributing to poor drug bioavailability include binding to tear proteins, tear turnover, limited corneal surface area, and the impermeability of the corneal epithelium.11-13 To address these issues, there has been a growing focus on developing delivery systems that offer controlled and targeted drug release over prolonged periods. Various novel formulations, such as nanoparticles, liposomes, dendrimers, and niosomes, have been explored to enhance drug bioavailability and minimize side effects. Among these, polymeric in situ gelling systems have shown significant potential in recent years as a means to improve the therapeutic efficacy of ophthalmic drugs by providing sustained and localized drug delivery.14 The treatment of ocular infections is a significant challenge due to the unique anatomy and physiology of the eye, which limits drug absorption and retention time. Topical administration is the most common method for delivering drugs to the eye; however, it often results in low bioavailability due to rapid elimination by tear drainage and blinking. This necessitates the frequent application of eye drops, which can be inconvenient for patients and may reduce adherence to treatment regimens.15 To address these challenges, researchers have been exploring novel drug delivery systems that can enhance the retention time of drugs on the ocular surface, thereby improving therapeutic efficacy and patient compliance. One such approach is the development of in situ hydrogels, which are liquid formulations that undergo a phase transition to a gel state upon contact with the ocular surface. This phase transition is typically triggered by changes in temperature, pH, or ionic strength. Thermoreversible hydrogels, in particular, have garnered considerable attention due to their ability to transition from a liquid to a gel state at body temperature, providing prolonged drug release and increased contact time with the ocular tissues.16
Chloramphenicol, a broad-spectrum antibiotic, is commonly used to treat bacterial conjunctivitis and other ocular infections. However, the conventional delivery of chloramphenicol through eye drops is associated with limitations, including poor patient compliance due to the need for frequent dosing and suboptimal drug bioavailability. The development of a thermoreversible in situ hydrogel formulation of chloramphenicol offers a promising solution to these challenges. This formulation can provide sustained drug release, enhance drug residence time, and improve the therapeutic outcomes of chloramphenicol in treating ocular infections.
In this study, we aim to prepare and evaluate a thermoreversible ocular in situ hydrogel of chloramphenicol. The formulation will be characterized in terms of its physicochemical properties, in vitro drug release profile, and in vivo therapeutic efficacy. By optimizing the hydrogel formulation, we seek to enhance the delivery and efficacy of chloramphenicol for the treatment of ocular infections, ultimately improving patient outcomes and compliance.
MATERIALS AND METHODS
Materials
The experimental work for this study involved the use of various chemicals, each sourced from reputable suppliers. Chloramphenicol was obtained from FDC Ltd. in Mumbai. Poloxamer 407 was supplied by BASF Ltd., Mumbai. Hydroxyl propyl methyl cellulose, benzalkonium chloride, sodium chloride, sodium hydroxide, sodium bicarbonate, calcium chloride dehydrates, disodium hydrogen phosphate, and potassium dihydrogen phosphate were all procured from M/s Loba Chemie Ltd., Mumbai. These chemicals were essential for the formulation and evaluation of the thermoreversible ocular in situ hydrogel of chloramphenicol.
METHODS
Characterization of drug chloramphenicol:
The characterization of the drug chloramphenicol was conducted through a series of analytical techniques. First, UV spectroscopy was performed to determine the maximum absorbance wavelength (?max). Chloramphenicol was accurately weighed and dissolved in distilled water to prepare a 10 µg/ml solution, and the UV spectrum was recorded across the 200-400 nm range using a UV spectrophotometer. Next, IR spectrum interpretation was carried out to analyze the chemical structure. A dry sample of chloramphenicol, along with a blend of the drug and polymers, was placed on the transparent surface of an FTIR spectrophotometer, and the IR spectrum was recorded using diffuse reflectance. Lastly, the melting point determination was performed using the capillary method to confirm the purity and identity of the drug. These methods collectively ensured the accurate characterization of chloramphenicol for its intended use in formulation studies.
Construction of calibration curve for chloramphenicol:
Preparation of phosphate buffer pH7.4:
Potassium phosphate monobasic solution:
Dissolve 27.22g of monobasic potassium phosphate (KH2PO4) in water & dilution with water to 1000ml.
Place 50ml of Potassium phosphate monobasic solution in a 200ml volumetric flask add the 39.ml 0.2M of sodium hydroxide solution then adjust volume with water.
Calibration curve of chloramphenicol:
Accurately weighed 10 mg pure drug was dissolved in 100ml of phosphate buffer to get the stock solution of 100µg/ml. From this stock solution aliquots of 0.2, 0.4, 0.6, 0.8, 1.2 & 1.4 ml were withdrawn and further diluted to 10 ml with buffer to obtain a concentrations range of 2 to 14 µg/ml. The absorbance of the dilution was checked using UV/Visible spectrophotometer at absorbance maximum (lmax) of 278 nm.
Preparation of simulated tear fluid (STF):
Dissolve 0.670g of sodium chloride, 0.200g of sodium bicarbonate, 0.008g of calcium chloride dehydrate in 100g purified water.
Calibration curve of chloramphenicol:
Accurately weighed 10 mg pure drug was dissolved in 100ml of simulated tear fluid (STF) to get the stock solution of 100µg/ml. From this stock solution aliquots of 0.2, 0.4, 0.6, 0.8, 1, 1.2 & 1.4 ml were withdrawn and further diluted to 10 ml with STF to obtain a concentrations range of 2 to 14 µg/ml. The absorbance of the dilution was checked using UV/Visible spectrophotometer at absorbance maximum (lmax) of 278 nm.
Formulation development of Thermoreversible in situ ocular hydrogel of chloramphenicol:
Method of preparation:
Ten formulas were prepared as shown in table 1 using different ratios of poloxamer 407(pluronic F127) (12%, 18%) as gelling agent in combination with carbapol 940 (0.02%, 0.04%) or HPMC (0.5%, 1.0%) using as viscosfying agents. Thermoreversible gels were prepared using cold technique. First of all the aqueous dispersions of selected concentrations of carbapol 940 (0.02%, 0.04%) for formulas (F2, F3, F7, and F8) and HPMC (0.5%, 1.0%) for formulas (F4, F5, F9, and F10) and pluronic F127 for formulas (F1 and F6) in phosphate buffer pH 7.4 were prepared. The pluronic/carbapol combination and the pluronic/HPMC combination were prepared by dispersing the pluronic in the desired concentration of respective polymer solutions. Then the partially dissolved solutions were refrigerated until thoroughly mixed (approximately 24 hrs). An appropriate amount of drug dissolved in phosphate buffer pH 7.4, then benzalkonium chloride 0.01?ded with continuous stirring until uniform drug solution was obtained. The drug solution was finally added to polymer solution with continuous stirring. The developed formulations were filled in amber glass containers. The formulations and their final pack were subjected to thermal sterilization by autoclaving at temp.1210C and 15 psi pressure for 20 minutes. Then stored in refrigerator prior to further characterization.
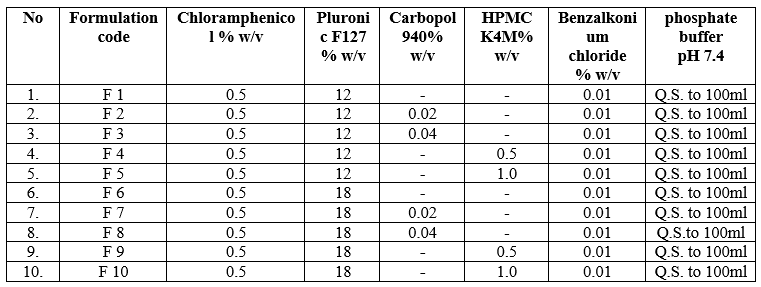
Table 1: Formulation Table
Evaluation of formulations:
Determination of Visual Appearance and Clarity:
Clarity is a crucial characteristic of ophthalmic formulations, ensuring that the product is free from particulate matter, which could cause discomfort or harm to the eye. To evaluate clarity, the formulations were visually examined against both white and black backgrounds. This simple observation method helps detect any visible particles or cloudiness, ensuring the formulation is suitable for ocular use.17
pH Determination:
The pH of an ophthalmic formulation is vital for both the stability of the formulation and patient comfort. The pH affects the solubility and stability of the active ingredients, and it is essential that the formulation is non-irritating to the eye. Ideally, the pH of ophthalmic products should fall within the range of 5 to 7.4. The pH of the developed formulations was measured using a digital pH meter to ensure that they are within the acceptable range and are stable and safe for ocular application.18
Measurement of Gelation Temperature of the Gel:
The gelation temperature is the temperature at which the liquid formulation transitions into a gel, a critical property for in situ gels. To determine this, 10 mL of the sample solution was placed in a transparent vial with a magnetic stir bar, and the vial was immersed in a low-temperature water bath. As the solution was heated at a rate of 2 °C/min with continuous stirring at 500 rpm, the temperature at which the magnetic bar stopped moving, indicating gelation, was recorded. This measurement was performed in triplicate to ensure accuracy.19,20
Effect of Dilution on Phase Transition Temperature:
To simulate the in vivo phase transition of the thermoreversible gel when diluted with tear fluid, the formulation was diluted with simulated tear fluid (STF) in a 40:7 ratio. The diluted formulation was then placed in a transparent beaker with a magnetic stirrer, and the temperature was gradually increased in 1 °C increments while stirring at 100 rpm. The phase transition temperature, at which gelation occurred and the magnetic bar stopped moving, was recorded. This test helps understand how the formulation will behave when administered to the eye.21,22
Drug Content:
The drug content in the chloramphenicol in situ gel was determined to ensure the correct dosage. An accurately weighed amount of the gel, equivalent to 4 mg of chloramphenicol, was dissolved in simulated tear fluid (STF, pH 7.4) and stirred on a magnetic stirrer until fully dissolved. The solution was then filtered, and a 10 mL aliquot was further diluted to 100 mL with STF. The absorbance of this sample was measured at 278 nm against a blank STF solution to quantify the drug content. This step ensures that the formulation contains the intended amount of the active drug.23
In vitro permeation study:24
Franz diffusion cell assembly:
Chloramphenicol permeation from the in-situ hydrogel was tested using a Franz diffusion cell. A cellophane membrane was mounted on the receptor compartment, filled with 20 ml of simulated tear fluid (STF), and maintained at 37±1°C. The gel containing 0.5% chloramphenicol was applied to the membrane. Samples were taken every 30 minutes up to 3 hours, then hourly up to 8 hours, and analyzed via UV spectroscopy.
Ex Vivo Corneal Permeation Study Using Goat’s Cornea:
Permeation studies were conducted using goat corneas obtained from a slaughterhouse, with the corneas kept in cold STF. The corneas were mounted on a Franz diffusion cell, with the corneal side in contact with the formulation. The receptor compartment, filled with STF and maintained at 37±1°C, was stirred continuously. Samples were taken at similar intervals as in the in vitro study and analyzed by UV spectroscopy.25
Rheological Study:
Rheological properties of the gelling solutions were evaluated using a Brookfield R/S-CPS rheometer with spindle number S25. Measurements were taken at 50 rpm and shear rates from 0.03/s to 14.6/s over 10 minutes, with the temperature set to 37°C to simulate physiological conditions. This assessed the viscosity of the poloxamer and HPMC/carbopol-chloramphenicol formulations.26
Antimicrobial Efficiency of Controlled Release Gel:
The antimicrobial efficiency of the chloramphenicol gel was tested against Staphylococcus aureus using the agar diffusion test. After incubating the gel-filled wells in nutrient agar for 24 hours at 37°C, the zones of inhibition were measured and compared to those produced by a marketed chloramphenicol eye drop, evaluating the gel's effectiveness.27,28
Stability Studies:
Stability of the in-situ gelling formulations was tested by storing them in sealed glass vials at refrigeration (4°C), ambient (25°C), and elevated (40°C) temperatures for two months. Samples were withdrawn at 15-day intervals to assess appearance, pH, gelation temperature, drug content, and in vitro release, ensuring the formulation's stability over time.29
RESULTS AND DISCUSSION:
Preformulation Studies: Characterization of Chloramphenicol
The preformulation studies of chloramphenicol included an assessment of its physical appearance and melting point. Chloramphenicol was observed to be a white powder, consistent with its known physical properties. The melting point of chloramphenicol was determined and found to be within the range of 150-151°C, which aligns with the expected range for this compound. These findings confirm the identity and purity of the chloramphenicol used in the formulation.
Spectroscopic studies:
UV spectroscopy: (Determination of l max.)
l max of chloramphenicol was found to be 278 nm in phosphate buffer (F7.4)
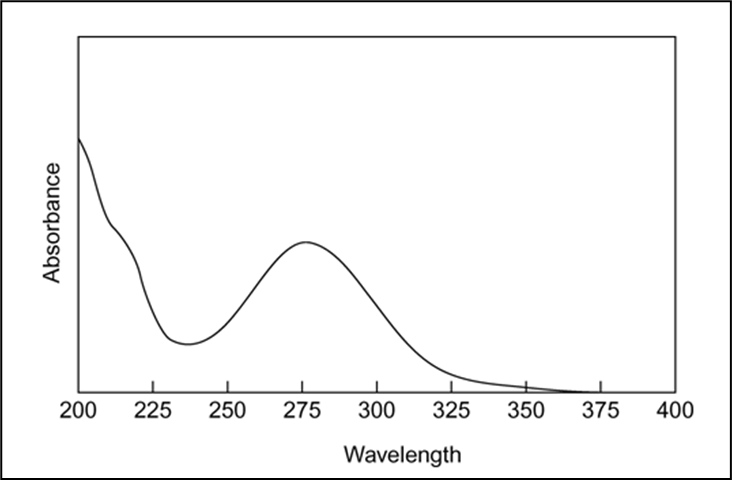
Figure 1: U.V.spectra of chloramphenicol in PB F7.4
l max of chloramphenicol was found to be 278 nm in simulated tear fluid -STF

Figure 2: U.V.spectra of chloramphenicol in STF
Preparation of calibration curve for Chloramphenicol in phosphate buffer pH7.4
Calibration curve observation at 278 nm

Table 2: Calibration curve observation in PB pH7.4

Figure 3: Calibration curve in PB pH7.4
Preparation of calibration curve for Chloramphenicol in simulated tear fluid.
Calibration curve observation at 278 nm
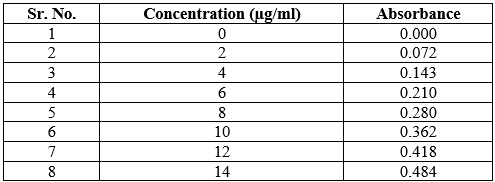
Table 3: Calibration curve observation in STF
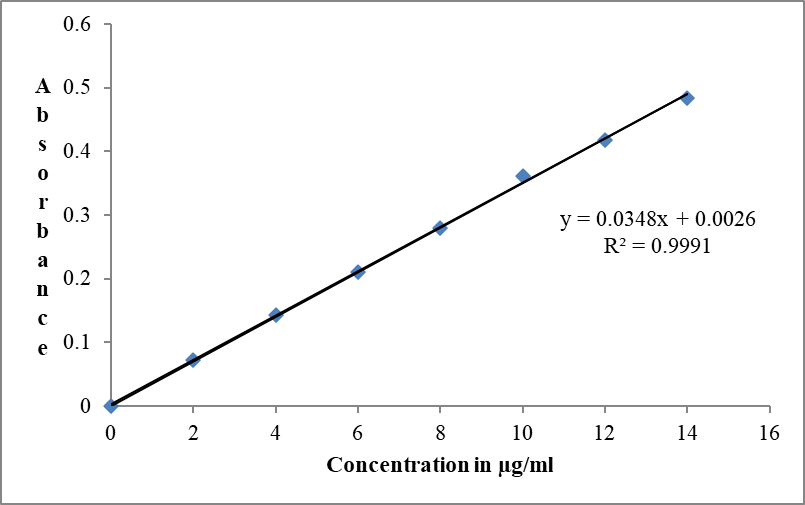
Figure 4: Calibration curve in STF
IR SPECTRUM INTERPRETATION:
The infrared spectrum of pure Chloramphenicol was recorded and spectral analysis was done.
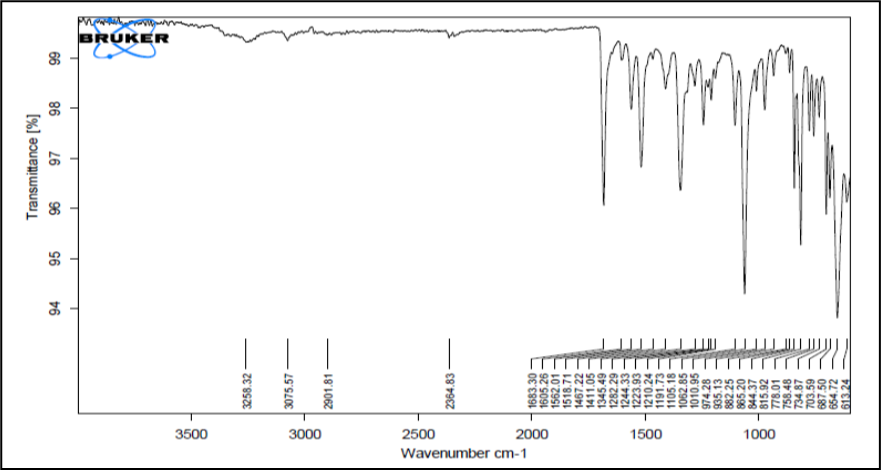
Figure 5: IR Spectrum of Chloramphenicol
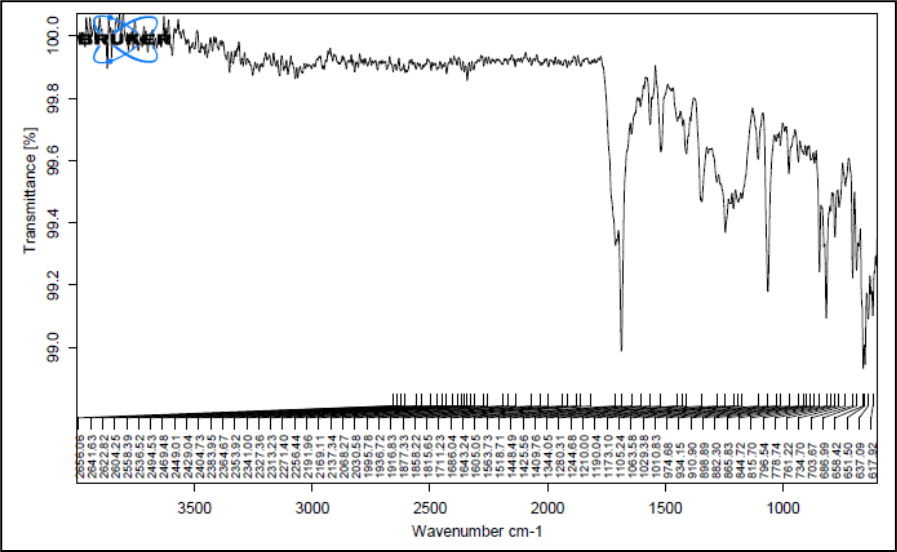
Figure 6: IR spectrum of formulation blend chloramphenicol, poloxamer 407 and carbopol 940
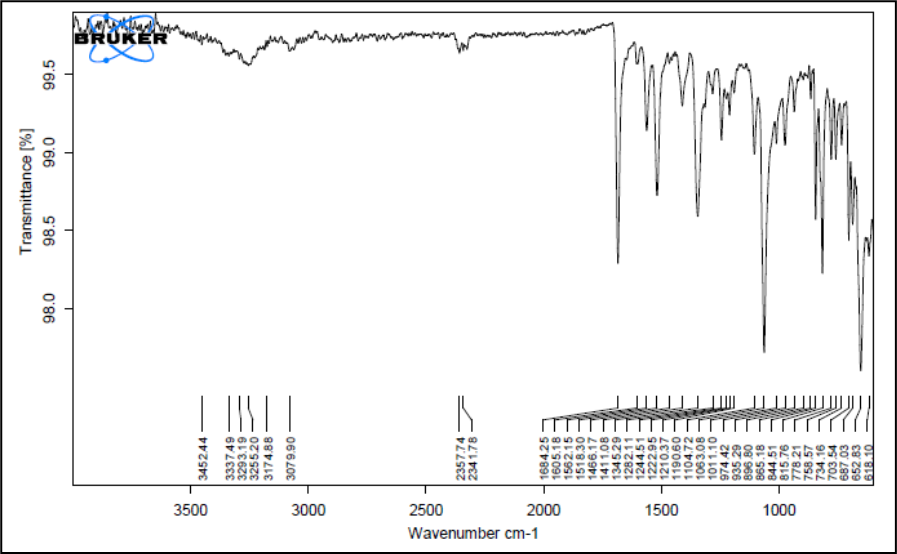
Figure 7: IR spectrum of formulation blend chloramphenicol,Poloxamer 407 and HPMC
Figure 5 shows the FTIR spectra of pure Chloramphenicol and Figure 6 & 7 shows its physical mixtures with poloxamer 407 and HPMC K4M & ploxamer 407 and carbopol and with other excipients & poloxamer 407 and carbopol respectively with other excipients. The IR spectra did not show any difference in wavelength from those obtained for their physical mixture with polymers as compared to drug. These obtained results indicate that there was no interaction between Chloramphenicol, polymers and the other excipients. Thus, Chloramphenicol can be used in combination with poloxamer 407 and HPMC K4M/carbopol for the preparation of controlled release thermoreversile in situ hydrogel.
Formulation Study:
Determination of visual appearance and clarity:
The appearance of all gelling solution and gel was observed, that the formulation from F1 to F5 were transparent and clear & the formulation from F6 to F10 were cloudy. The following observation concludes that, the formulations containing more concentration of poloxamer are cloudy in nature. Poloxamer concentration more and low concentration of HPMC/Carbapol that shift the pH towards acidic this may affect transparency and clarity.
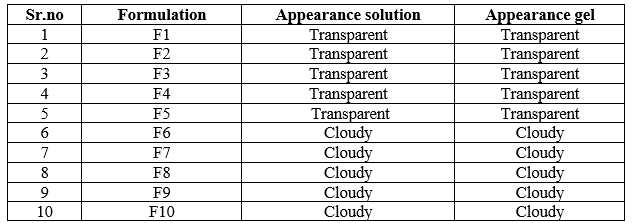
Table 4: Determination of visual appearance
Determination pH of gel:
The ophthalmic preparation pH mostly near to the ocular pH i.e.7.4 which was not gave irritation to the eye. The pH of above formulations range from 6.8 to 7.4. Formulation F1, F2, F4 gave the pH near to the ocular ph. From above observations it is concluded that, the formulations F1, F2 & F4 have pH within acceptable range and hence would not cause any irritation upon administration of formulations.
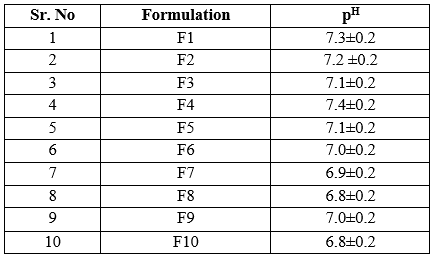
Table 5: Determination of pH
Determination of phase change (gelation) temperature of the gel:
As these are thermoreversible in situ gel formulations, they must be liquid at room temperature and gave gelation near to body temperature. From the above observation, F1, F2, F3, F4 & F5 shows the gelling temperature near to the body temperature. These observations concluded that, the low concentration of poloxamer has the gelling temperature near to body temperature and high concentration of poloxamer has the low gelling temperature than the body temperature. As conc.of HPMC/carbopol increases the gelling temperature decreases.
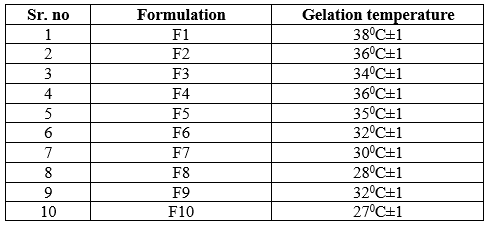
Table 6: Determination of gelling temperature
Effect of Dilution on Phase TransitionTemperature:
From the following observation, the gelling temperature of formulation after dilution was increased by 2 to 50C than gelling temperature before dilution. From that it is concluded that, when formulation instilled in eye mixed with tear that shift the gelling temperature up to 50C. Formulation F2, F3, F4, F5 was gave appropriated gelling temperature after dilution, which was near to the body temperature.
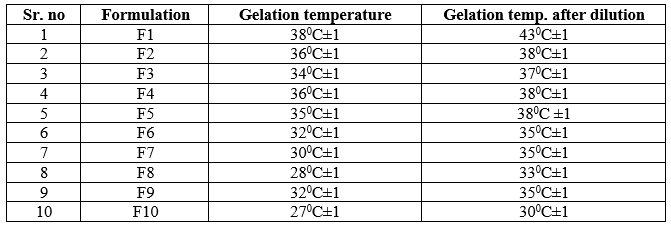
Table 7: Determination of effect of dilution on gelling temperature
Drug content:
The drug content was found to be in acceptable range for all the formulations. Percent drug content of formulations was found to between range of 96.38 to 99.65 indicating uniform distribution of drug.
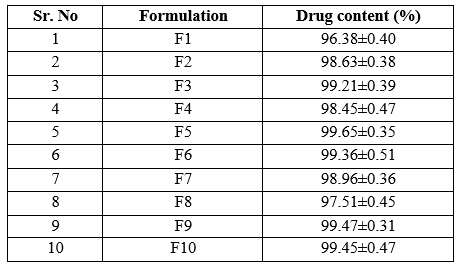
Table 8: Determination of drug content
In vitro permeation study:
In vitro drug release study of F 1 to F 5 in situ gel batches were carried out. From following observation it was found that Chloramphenicol released from the gel by diffusion mechanism. It was also observed that gel shows controlled release activity. Poloxamer 407 and HPMC/carbopol gel was used as a vehicle for Chloramphenicol, which is provide proper residence time for the drug which was adhere to the eye. The percentage drug release after 8 hrs of gels was ( F1-91.125,F2-87.75,F3-85.5,F4- 4.5,F5-92.25,F6-85.5,F7-82.125.55,F8-78.55,F9-75.37,F10-73.125). When the concentration of HPMC and Carbopol increase i.e. 0.5 & 1.0 %w/w and 0.02 & 0.04%w/w respectively with appropriate concentration of poloxmer then there is decrease in release from the formulations. From these observations it is concluded that, for thermoreversible in situ occular hydrogel , HPMC/Carbopol should be in lower range with appropriate conc. of poloxamer. Increase in conc. of HPMC in F5 there is decrease in release as compared to F4 where increase in conc. of carbopol in F3 decrease in release as compared to F2.
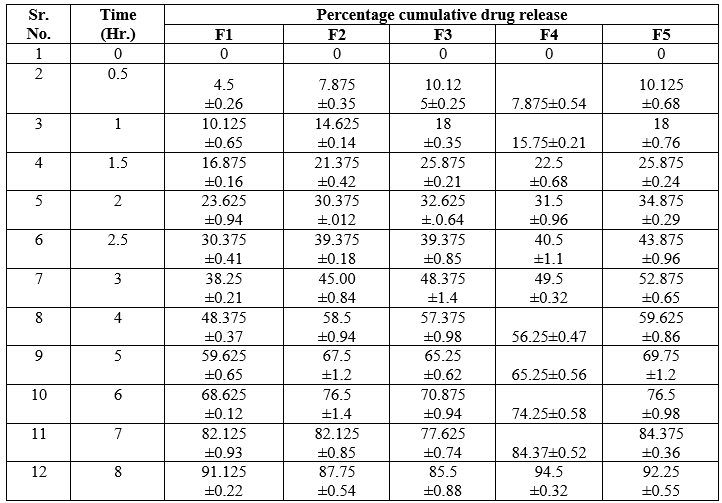
Table 9: In vitro permeation study of formulation
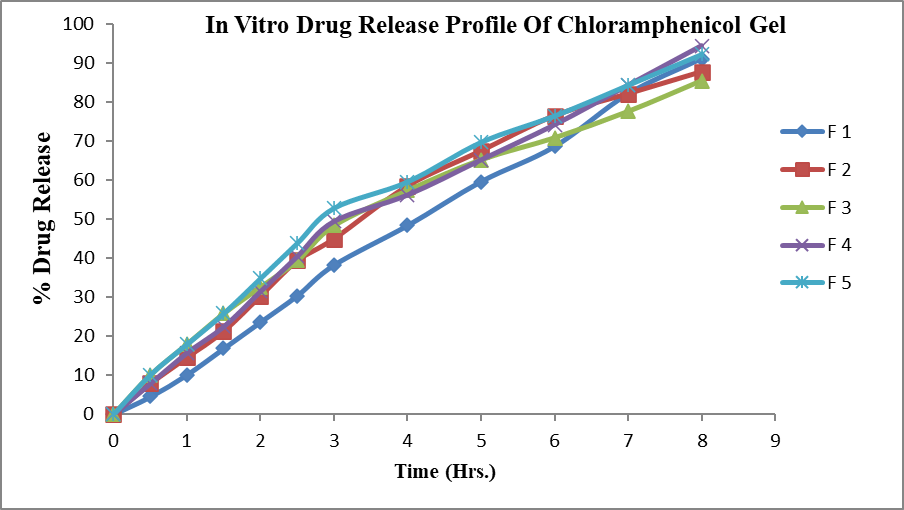
Figure 8: In vitro drug release profile of chloramphenicol gel
Ex-vivo corneal permeation studies using goat’s cornea:
The formulations F1, F2, F3, F4 and F5 were subjected to ex vivo release studies. These ex vivo release studies were carried out using simulated tear fluid (STF) of pH 7.4 as the dissolution medium. The ex-vivo drug release data obtained for formulations F1, F2, F3, F4 and F5 was as shown in the table 10. From the plot of percent drug released as a function of time for formulation , It was found that formulation F1, F2, F3, F4 and F5 having percent drug release was 82.125%,74.25%,70.875%,78.75%,and 76.00 % respectively after 8 hours. The ex vivo release data indicated that the formulation F1 showed better control effect than other four formulations. Formulations contain HPMC in batch F4 and F5 shows greater ex vivo release than formulations containing carbopol. The ex-vivo drug release conditions may be very different from those likely to be encountered in the eye. However, the results clearly show that the gels have the ability to retain drug for prolonged period of time (8 hour) and that premature drug release will not occur. In the cul-de-sac, the gels will probably undergo faster dissolution due to the shearing action of the eyelid and eyeball movement. It is also observed that the dissolution in the cul-de-sac will proceed more slowly than that seen in the in-vitro experiments, as the normal resident volume of the lachrymal fluid in the human eye is 7.5-10µl.

Table 10: Ex-vivo permeation study of formulation through goat cornea
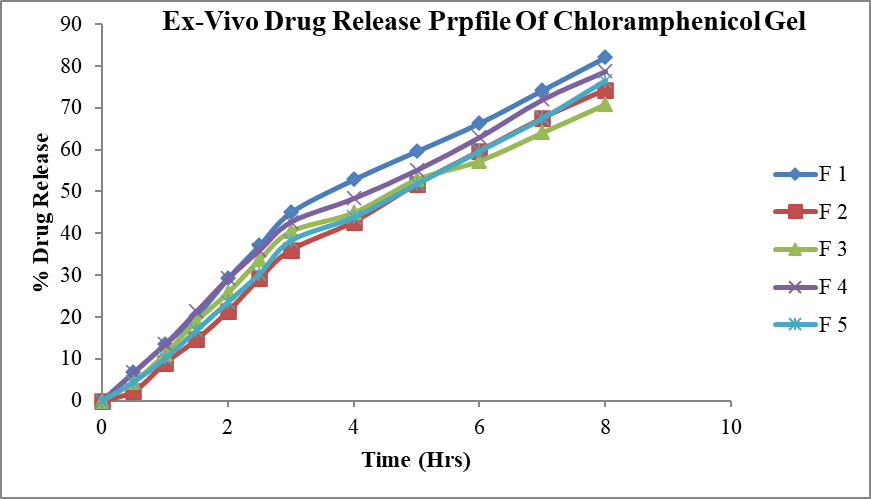
Figure 9: Ex-vivo drug release profile of Chloramphenicol gel
Rheological studies:
For the thermosetting gel, the viscosity at various conditions is an important rheological parameter involved in its utilization and its in-vivo performance. For example, if viscosity is too high it will lead to difficult instillation; on the contrary, if viscosity is too low it will give rise to increased drainage. The dynamic viscosity of poloxamer formulation was measured as the change of shear rate under a physiological condition. The gels formed at physiological condition, and the viscosity of the formulation decreased as the shear rate increased, which showed the character of pseudoplastic fluid. Because the shear rate is very large ranging from 0.03/second to 28500/second during blinking, viscoelastic fluid with a viscosity that is high under high shear rate condition and low under high shear rate condition is preferred. The pseuodoplastic property of poloxamer formulation under physiological condition is in favor of controlled drainage of drug from the conjunctival sac of the eye, simultaneously without the unwanted blinking tendency for undergoing shear thinning. From above observations it is concluded that the viscosity of F1 to F5 formulations is within normal range and Formulations containing carbopol i.e.F2 and F3 have more viscosity than formulations containg HPMC i.e. F4 and F5 & Formulation without HPMC/Carbopol has lower viscosity than those compared to formulations containing HPMC/Carbopol.

Table 11: Rheological study
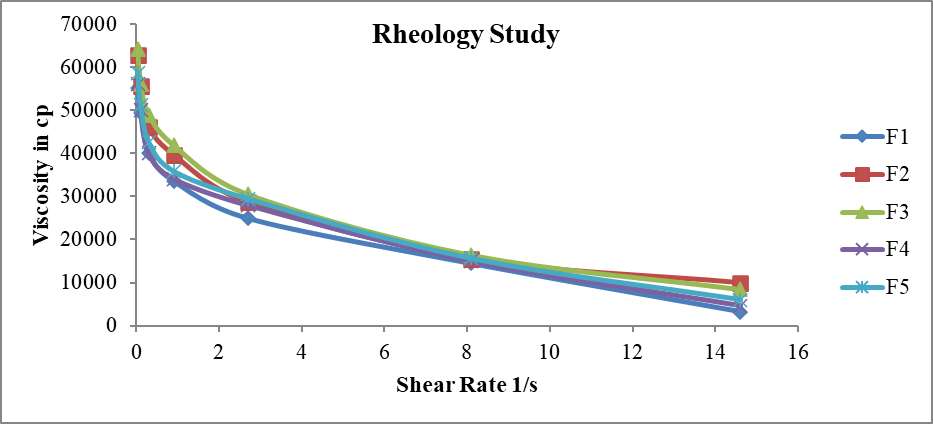
Figure 10: Rheological profile of Chloramphenicol gel
Antimicrobial efficiency of controlled release gel:
From result of antimicrobial efficiency test as shown in above figure 11 & 12 it can be concluded that zone of inhibition of formulation F2 was 3.8 and F4 was 4.1 which was better than other batches. All formulations shows zone of inhibition value in between 3.5 to 4.1. High zone of inhibition of formulation F4 could be justified by the slow and prolonged diffusion of drug from the polymeric solution due high concentration of HPMC.

Table 12: Antimicrobial efficiency of controlled release gel

Figure 11: Antimicrobial efficiency of F2
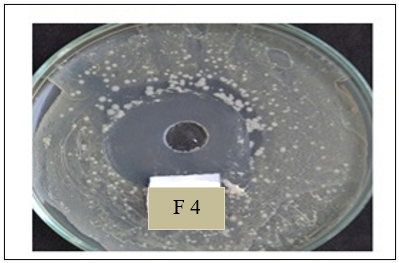
Figure 12: Antimicrobial efficiency of F4
Stability study:
The formulations F2 and F4 shows in refrigerated temperature all day appearance was transparent, pH near ophthalmic Fi.e 7.4 ,gelation temperature same as that of evaluated gelling temperature and also same drug content and percent drug release.
The formulations F2 and F4 shows in room temperature 15, 30, 45 day after appearance was transparent, pH near to ophthalmic pH, gelling temperature same as that of evaluated gelling temperature and also same drug content and percent drug release but last 15 day disturb stability not showed proper appearance and pH drug get precipitate out from formulation.
The formulations F2 and F4 shows in elevated temperature 15, 30 day after appearance was transparent, pH near to ophthalmic pH gelling temperature same as that of evaluated gelling temperature and also same drug content and percent drug release but last 30 day disturb stability not showed proper appearance and pH drug get precipitate out from formulation.
From following discussion it concluded that in situ gel formulation no.F2 and F4 stored at room temperature or elevated temperature showed less stability than those that were stored at the refrigerated temperature.
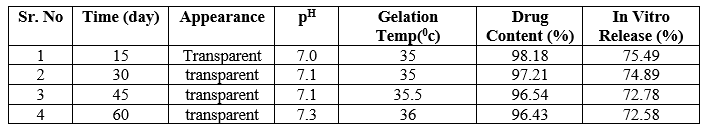
Table 13: -F2 Stability Study at 40C
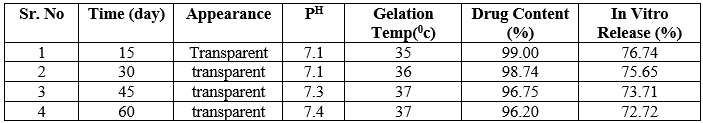
Table 14: -F4 Stability Study at 40C

Table 15: F2- Stability Study at 250C

Table 16: -F4 Stability Study at 250C
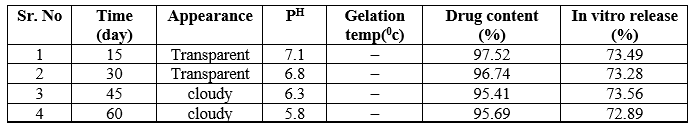
Table 17: -F2 Stability Study at 400C

Table 18: -F4 Stability Study at 400C
CONCLUSION:
The study successfully formulated a thermoreversible ocular in situ gel system for chloramphenicol, using poloxamer 407 as a gelling agent and HPMC K4M or carbopol 940 as viscosity enhancers. Chloramphenicol's high lipid solubility makes it effective for ocular drug delivery. The formulation showed good stability, controlled drug release, and efficient antimicrobial activity. Evaluation confirmed that the formulation remained liquid at cold temperatures and gelled at body temperature, with HPMC K4M proving to be a better viscosity enhancer than carbopol. The optimized gel demonstrated excellent clarity, appropriate pH, and consistent drug release in vitro and ex vivo. Stability studies indicated that the formulation was most stable at 4°C, maintaining its properties over 60 days. Thus, HPMC K4M was concluded to be more suitable for the preparation of the ocular in situ gel of chloramphenicol.
Funding:
Nil
Authors Contributions:
All authors have contributed equally.
Conflicts Of Interests:
All authors have declared no conflict of interest.
REFERENCE
- S Jitendra, Sharma P.K. Banik A. And Dixit S.; “A New Trend: Ocular Drug Delivery System”, Pharma Science Monitor, International Journal of Pharmaceutical Sciences, 2011, 2(3), 1-25.
- Hosoyaa K, Vincent HL, Kim KJ; “Roles of the conjunctiva in ocular drug delivery: a review of conjunctival transport mechanisms and their regulation”, Europian Journal of Pharmaceutics & Biopharmaceutics, 2005, 2(2), 27–40.
- Vengurlekar P. Singh A. and Rathod S., “ Microspheric In situ Gel for Ocular Drug Delivery System of Bromfenac Sodium”, International Journal of Pharma. Science and Research, 2014, 5(4), 179-185.
- Gambhire S. bhalerao K. and Singh S., “Review on In situ Hydrogel: Different Approaches to Ocular Drug Delivery”, International Journal of Pharmaceutical Science, 2013, 5(2), 27-36.
- Gupta P, Vermani K and Garg S.; “Hydrogels: from controlled release to pH responsive drug delivery”, Drug Discovery Today, 2002, 1(2), 69-79.
- Rathore K.S.; “In situ Gelling Ophthalmic Drug Delivery System: An Overview”, International Journal of Pharmacy and Pharmaceutical Sciences, 2010, 2(4), 30-34.
- Nanjawade B.K., Manvi F.V., Manjapa A. S.; “In situ forming hydro gels for sustained ophthalmic drug delivery”, Journal of controlled release, 2007, 1(2),119-134.
- Reddy Ravindra, Ravi Shankar M, Yadav M and Reddy Sabitha; “preparation and evaluation of Aceclofenac ophthalmic in situ gels”, Journal of chemical Biological and physical science, 2011, 1(2) 289-298.
- Zarikar N. Katedeshmukh R. Kulkarni A. and Patel R., “Ophthalmic In situ Drug Delivery System: A Review”, International Journal of Pharmaceutical Research and Development, 2013, 5(5), 48 – 55.
- Nerkar T.S. Gujarathi N.A. Bhushan R. Bakliwal S. R. and Pawar S.P., “In situ Gel: Novel Approach in Sustained and Controlled Drug Delivery System”, Pharma. Sci. monitor, 2013, 4(4), 1-18.
- Lee VHL, Robinson JR; “Topical ocular drug delivery: recent developments and future challenges”, Journal of Ocular Pharmacology, 1986, 2(2), 67–108.
- K Basavaraj, Nanjawade, Manvi F V and Manjappa AS; “In situ-forming hydrogels for sustained ophthalmic drug delivery”, Journal of Controlled Release, 2007, 1(2), 119–134.
- Khurana AK, Khurana I. Anatomy & physiology of Eye; 2nd ed. CBS publishers & Dist 2007.280-298.
- Kumar L. Singh R.P. Singh S.G. and Kumar D., “In situ Gel: A Novel System for Ocular Drug Delivery”, International Jornal of Pharmaceutical Science Review And Research, 2011, 9(2), 83-91.
- Varshosaz J., Tabbakhian M and Salmani Z.; “Designing of a Thermosensitive Chitosan/Poloxamer in Situ Gel for Ocular Delivery of Ciprofloxacin”, The Open Drug Delivery Journal, 2008, 2(1), 61-70.
- Jtirvinena K, Tomi J, Urttia SA; “Ocular absorption following topical delivery”, Advanced Drug Delivery System, 1995, 2(1), 3-19.
- Hemalata Dol, Sanket Gandhi, Dinesh Pardhi and Namrata Vyawahare; “Formulation and evaluation of in situ ophthalmic gel of moxifloxacin hydrochloride”, The Pharma Innovation Journal, 2014, 3(5): 60-66.
- B.S.Bhoyar, V.V.Agnihotri and M.M.Bodhankar; “New method for sustained ocular drug delivery: Based on polymeric combination with thermo sensitive gelling agents”, International journal of research in pharmaceutical and biomedical sciences, 2011, 2(3), 1151-1160.
- Shirish Vodihala, Sadhna Khatry, Nalini Shastri, M.Sadanandam; “Development and evaluation of thermo reversible ocular gels of ketorolac tromethamine”, International journal of Biopharmaceutics, 2010, 1(1), 39-45.
- P.G.Yeole, Dhanalakshmi Iyer; “Thermosensitive insitu gel of Timolol maleate for the treatment of open angle glaucoma”, Research journal of pharmaceutical, biological and chemical science, 2011, 2(3), 1048-1054.
- Divyesh H. Shastri Shailesh T. Prajapati Laxmanbhai D. Patel ; “Thermoreversible mucoadhesive ophthalmic in situ hydrogel: Design and optimization using a combination of polymers”, Acta Pharm.,2010, 1(1), 349–36.
- Gajanan Darwhekar, Priya Jain, Dinesh Kumar Jain and Gaurav Agrawal; “Development and Optimization of Dorzolamide Hydrochloride and Timolol Maleate in Situ Gel for Glaucoma Treatment”, Asian Journal of Pharmaceutical Anlysis, 2011, 1(4), 93-97.
- Rahul Nair, Venkatakrishnakiran. P, Dhanalakshmi.P, Prasannaraju.Y; “Formulation and evaluation of in situ gelling systems for ocular delivery of Doxycycline hyclate”, Journal Of Innovative Trends In Pharmaceutical Sciences, 2012, 3(1), 1-7.
- A.H. El-Kamel; “In vitro and in vivo evaluation of Pluronic F127-based ocular delivery system for timolol maleate”, International Journal of Pharmaceutics, 2002, 2(1), 47 – 55.
- Mahendra Singh Rathore and Dipak K. Majumdar; “Effect of Formulation Factors on In Vitro Transcorneal Permeation of Gatifloxacin From Aqueous Drops”, American Association Of Pharmacutical Scientists Pharma Sciecnce Tech, 2006, 7 (3), 57-60.
- J. Padma Preetha, K. Karthika, Rekha. NR and Khalid Elshafie; “Formulation and evaluation of in situ ophthalmic gels of Diclofenac sodium”, Journal Of Chemical And Pharmceutical Research, 2010, 2(3), 528-535.
- Manas Bhowmik, Sanchita Das, Dipankar Chattopadhyay, Lakshmi K.Ghosh; “Study of thermosensitive in-situ gel for ocular delivery”, Scientia Pharmaceutica, 2011, 1(1), 351-358.
- Hanan M. El-Laithy, Demiana I. Nesseem; “Evaluation of two in situ gelling systems for ocular delivery of Moxifloxacin:In vitro and in vivo studies”, Journal Of Chemical And Pharmceutical Research, 2011, 3(2):66-79.
- Vinod Singh, S.S. Bushetti, S Appala Raju, Rizwan Ahmad, Mamta Singh; “Glaucoma: a treatment by hydrogel”, An international journal of pharmaceutical sciences, 2011, 2(1), 174-182.


 Akash Nanaware*
Akash Nanaware*
 Pooja Shah
Pooja Shah






























 10.5281/zenodo.13368569
10.5281/zenodo.13368569