Abstract
The most common toxic effect of aniline is splenotoxicity which is produced via the generation of oxidative stress. The present study examines the protective effect of Maqui Berry Extract on spleen weight, body weight, endogenous antioxidants and membrane bound ATPases in aniline hydrochloride (AH) induced spleen toxicity in rats. Adult male albino rats were divided into five groups, each group consists of six rats. Toxicity was induced by administration of AH (200 ppm, p. o) in drinking water for 30 days. Body weight, markers of oxidative stress and haematological parameters were assessed at the end of treatment period. Treatment rats received Maqui Berry Extract (25, 50 and 100 mg/kg/day/p.o) for 30 days. AH toxic rats showed a significant elevation of spleen weight, WBC count, iron content, LPO level, NO level and Ca++ ATPase whereas a significant decrease in body weight, haemoglobin, RBCs level, Protein content, GSH and Na+/ K + and Mg++ ATPase were observed. Administration of Maqui Berry Extract (25, 50 and 100 mg/kg/day/p.o) for 30 consecutive days shows a significant decrease in spleen weight, WBC level, iron content, LPO level, NO level whereas significant increase in body weight, hemoglobin level, RBCs level Protein content, GSH level, Na+/ K+ ATPase, Ca++ and Mg++ ATPase when compared with aniline treated group. These findings indicate greater protective effect of Maqui Berry Extract (100 mg/kg/day/p.o) in aniline induced spleen toxicity in rats compared to Maqui Berry Extract (25 and 50 mg/kg/day/p.o).
Keywords
Spleen toxicity, Antioxidant, Maqui Berry Extract, Aniline HCl
Introduction
The poisonous aromatic amine aniline is made up of an amino group joined to a phenyl group. Among its many uses in the industrial chemical industry are resins, dyes, perfumes, pigments, fungicides, varnishes, hydroquinone, and rubber compounds. Exposure to aniline can cause cyanosis, weakness, vertigo, headache, stupor, loss of coordination, and coma, among other clinical signs [1]. Rats exposed to aniline have been shown to develop spleen toxicity. Aniline causes toxicity through a number of processes, including oxidative stress, protein oxidation, iron excess, and the spleen's production of hemoglobin. An early sign of aniline poisoning is the formation of methemoglobin (MetHb), which disrupts the blood's ability to carry oxygen [2]. Spleen poisoning is largely caused by oxidative stress, and early indications of spleen toxicity may be avoided with treatment with high-quality antioxidants [3, 4]. Maqui berries are small, dark purple fruits that are endemic to South America. The scientific name for them is Aristotelia chilensis. They are renowned for providing several health advantages and having a high nutritional content. The maqui berry is well-known for having strong antioxidant qualities. Many polyphenols are present in it, with anthocyanins, flavonoids, and phenolic acids being the most prevalent. These substances exhibit potent antioxidant properties. In maqui berries, delphinidin-3-sambubioside-5-glucoside and delphinidin-3,5-diglucoside are the most prevalent anthocyanins. Antioxidants included in maqui berries can affect the functioning of different oxidative stress-related enzymes. ROS production can rise as a result of persistent inflammation. The anti-inflammatory qualities of maqui berries aid in reducing this oxidative stressor. Maqui berry also has anti-aging, anti-cancer, and cardiovascular potential, according to several research [5].
The goal of the current study was to evaluate the effects of Maqui berry on AH-induced spleen toxicity in rats, taking into account the role of antioxidants and oxidative stress in spleen toxicity.
MATERIALS AND METHODS
Drugs and chemicals
Aniline HCL was procured from Sigma Aldrich Chemical, Mumbai. Maqui Berry Extract was procured from Shree Sai Biotech, Indore (M.P.). All the other chemicals used in the study were of analytical grade and procured from standard supplier.
Experimental animals
Male wistar rats (200-250g) were used in the study. The rats were maintained under standard laboratory conditions at temperature 23±1?C, relative humidity 45–55% and 12 h light and 12 h dark cycles throughout the experiments as per CPCSEA guidelines. The experimental protocol was approved by Institutional Animal Ethics Committee (IAEC) of SSDJ College of Pharmacy, Neminagar, Chandwad.
Experimental protocol
The rats were divided into five groups of six rats each. Group 1: Served as control and received distilled water. Group II: Rats received AH (200ppm in drinking water) for 30 days. Group III: Rats received AH (200ppm in drinking water) and Maqui berry extract (25mg/kg/p.o.) for 30 days. Group IV: Rats received AH (200ppm in drinking water) and Maqui berry extract (50mg/kg/p.o.) for 30 days. Group V: Rats received AH (200ppm in drinking water) and Maqui berry extract (100mg/kg/p.o.) for 30 days.
Assessment of spleen toxicity
Body weight, spleen weight, water consumption, and feed intake were recorded at the conclusion of the treatment period. Using a glass capillary, blood was extracted from the retroorbital plexus, and a high-speed centrifuge was used to separate the serum. Blood was used to measure the amount of hemoglobin (using Sahli's hemometer method), the number of red blood cells (RBCs), and the number of white blood cells (WBCs) using a hemocytometer. Using a Span diagnostic kit, serum was utilized to estimate the total iron contents [6] and protein contents on a biochemistry analyzer. Euthanasia was used to sacrifice the animals. The spleen was quickly placed in ice-cold water, homogenized, centrifuged, and the supernatant was used to measure endogenous antioxidants including tissue lipid peroxidation (LPO) [7], reduced glutathione (GSH) [8], nitrite level [9], and superoxide dismutase (SOD) [10], catalase (CAT) [11].The estimation of membrane-bound ATPases, including Na+/K+ [12], Ca++ [13], and Mg++ [14], was done using the spleen sediment.
Statistical analysis
Values are expressed as Mean ± SEM (n=6). Analysis was performed by applying ANOVA followed by Dunnett‘t’ test. *p<0>**p<0>***p<0>#p<0>##p<0> ###p<0>
RESULTS
Effect of different concentration of Maqui berry extract on body weight, feed intake, water intake, and spleen weight
Following the treatment period, each group's body weight, food consumption, and water intake were tracked. Comparing the AH-treated group to the control group, it was discovered to have changed somewhat. When compared to the AH-treated group, the 30-day administration of varying concentrations of Maqui berry extract (25, 50, and 100 mg/kg/day) resulted in a substantial (p<0>
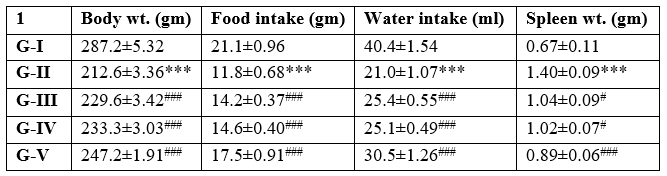
Table 1: Effect of different concentration of Maqui berry extract on body weight, food intake, water intake and spleen weight
Values are expressed as Mean ± SEM (n=6). Analysis was performed by applying ANOVA followed by Dunnett‘t’ test. *p<0>**p<0>***p<0>#p<0>##p<0> ###p<0>
3.2 Effect of different concentration of Maqui berry extract on hemoglobin, RBCs and WBCs counts
WBC, RBC, and hemoglobin levels were tracked for each group. Rats treated with AH had considerably lower levels of hemoglobin and red blood cells (RBCs) than control rats (p<0>
The WBC count of the rats receiving AH treatment was found to be significantly higher in the current investigation. When compared to rats treated with AH, rats treated with Maqui berry extract (25, 50 mg/kg/day) had a substantial (p<0>
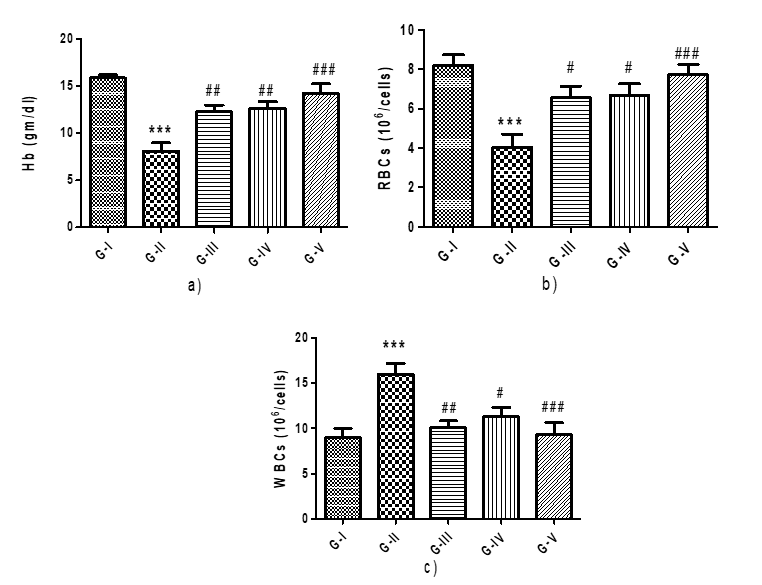
Figure 1: Effect of different concentration of Maqui berry extract on hemoglobin, RBCs and WBCs counts
Values are expressed as Mean ± SEM (n=6). Analysis was performed by applying ANOVA followed by Dunnett‘t’ test. *p<0>**p<0>***p<0>#p<0>##p<0> ###p<0>
Effect different concentration of Maqui berry extract on total protein and total iron content
Additionally, the level of total protein content was tracked. Rats treated with AH were shown to exhibit a significant (p < 0> Rats treated with AH had a considerably higher total iron level (p < 0>
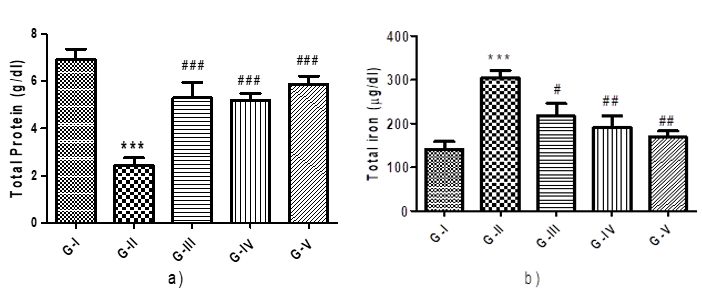
Figure 2: Effect different concentration of Maqui berry extract on total protein and total iron content
Values are expressed as Mean ± SEM (n=6). Analysis was performed by applying ANOVA followed by Dunnett‘t’ test. *p<0>**p<0>***p<0>#p<0>##p<0> ###p<0>
Effect of different concentration of Maqui berry extract on Lipid Peroxidation (LPO), Nitric Oxide (NO) and Reduced Glutathione (GSH), Superoxide dismutase (SOD) and Catalase (CAT) in spleen tissue:
All groups had their levels of lipid peroxidation, nitric oxide, reduced glutathione, superoxide dismutase and catalase checked. When AH-treated rats' spleen tissue homogenate was compared to control rats, it was shown that the concentration of LPO and NO was significantly (p<0>
AH treated rats showed significant (p<0>) reduction in level of SOD and CAT due to alteration in oxidative stress markers when compared to the control groups. Treatment with Maqui berry extract in different concentration of (25, 50, 100 mg/kg/day) displayed significant (p<0>) increased the concentration of SOD and CAT when compared with AH treated groups (Figure 3 d & e).
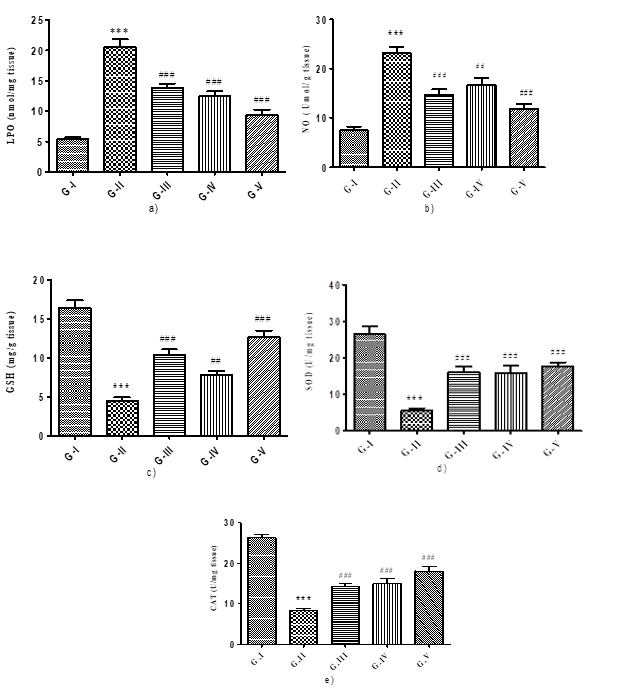
Figure 3: Effect of different concentration of Maqui berry extract on Lipid Peroxidation (LPO), Nitric Oxide (NO) and Reduced Glutathione (GSH) Superoxide dismutase (SOD) and Catalase (CAT) in spleen tissue
Values are expressed as Mean ± SEM (n=6). Analysis was performed by applying ANOVA followed by Dunnett‘t’ test. *p<0>**p<0>***p<0>#p<0>##p<0> ###p<0>
Effect of different concentration of Maqui berry extract on Na+/K+, Ca2+ and Mg2+ ATPases:
The levels of the ATPases Na+/K+, Ca++, and Mg++ in the spleen homogenate were observed in each group. According to (Fig. 4 a, b, and c). Rats treated with AH showed a significant (p<0>+/K+, Ca++, and Mg++ when compared to the control group. When compared to the AH treatment group, AH rats given varying doses of Maqui berry extract (25, 50, and 100 mg/kg/day) for 30 days demonstrated a significant (p<0>+/K+ and Ca2++.
The Mg++ levels of AH rats treated with 25 and 50 mg/kg/day of Maqui berry extract for 30 days showed a significant (p<0>++ levels of rats treated with 100 mg/kg/day of Maqui berry extract showed a more notable increase than the Mg++ levels of the AH treated group.
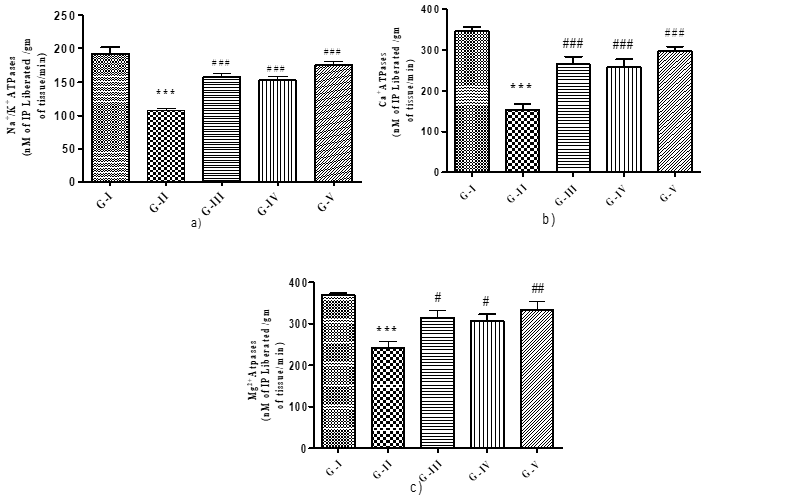
Figure 4: Effect of different concentration of Maqui berry extract on Na+/K+, Ca++ and Mg++ ATPases
alues are expressed as Mean ± SEM (n=6). Analysis was performed by applying ANOVA followed by Dunnett‘t’ test. *p<0>**p<0>***p<0>#p<0>##p<0> ###p<0>
DISCUSSION
Maqui berry extract was utilized as a potent antioxidant in this work, and its ability to prevent spleen damage caused by aniline was evaluated. Rats are known to develop selective splenic toxicity when exposed to aniline and substituted aniline [15]. Rats that are exposed to aniline suffer splenic poisoning. Aniline toxicity is mostly indicated by the production of methaemoglobin (MetHb). Aniline produces its secondary metabolites during hepatic clearance, which aid in the creation of MetHb. The production of MetHb significantly impairs the blood's ability to carry oxygen. The two metabolites of aniline that contribute to its toxicity, particularly its splenic toxicity, are metHb and phenyl hydroxyl amine (PHA) [16]. The observed harm is caused by the phagocytes primarily the macrophages becoming activated during the erythrocyte scavenging procedure. This enhanced creation of highly reactive species, like hydroxyl radical and ferrocyanide, is the outcome [17].
Changes in body weight, feed and water intake, as well as spleen hypertrophy, suggested AH toxicity in the current investigation. In the current investigation, rats exposed to AH had significantly lower levels of hemoglobin and red blood cells (RBCs), while their white blood cell counts were much higher than those of normal control rats. These alterations in blood parameters were tightly linked, in a time-dependent manner, to a concurrent enlargement of the spleen; splenomegaly seems to be caused by an excess of chemically damaged erythrocyte deposits [18]. Hemoglobin levels, RBC counts, and WBC counts were significantly altered after treatment with Maqui berry (100 mg/kg/p.o.). This change may have been caused by the fruit's potent antioxidant and free radical scavenging properties.
Rats given AH demonstrated a marked rise in iron load and fall in protein levels. As a modulator of aniline-induced splenotoxicity, iron is important. Iron builds up as a result of AH toxicity, which can lead to the overproduction of reactive oxygen species and harm proteins, lipids, and nucleic acids [17]. Aniline exposure may induce lipid peroxidation in the spleen, which may be followed by morphological changes such as increased cellularity in the red pulp due to an increase in sinusoidal cells and fibroblasts, thickening of the capsular layer, and formation of fibrous tissue in the parenchyma and capsule [19]. Greater MDA-protein adduct formation in the spleen was observed after AH treatment, indicating that MDA produced as a result of lipid peroxidation produces native protein structural modification, which can change the protein's functional properties and therefore contribute to aniline-induced splenic toxicity. In rats given AH, increased NO levels could be the result of inos stimulation. An important component of many biological processes, including cellular growth and development, protein and nucleic acid synthesis, signal transduction, and maintaining redox balance in cells, is reduced glutathione, an endogenous antioxidant [20]. GSH can produce a second strong hydrophilic antioxidant [21]. Superoxide dismutase play an essential function in oxidative damage by triggering quick dismutation of O2•? species, thus lowering the risk of •OH production in reaction that are metal-catalyzed [22]. CAT catalyzes the depletion of H2O2 and guards against extremely reactive hydroxyl radicals in cell.
The LPO and NO levels in the AH-induced group were significantly higher, whereas the spleen's GSH level was significantly lower. The oxidative stress biomarker level was dramatically restored to normal by maqui berry (25, 50, 100 mg/kg/p.o.). Aniline's free radical production can also change the level of ATPases [23]. Maqui fruit has been shown to have pharmacological properties, including antihypertensive action, and is a potent antioxidant that may scavenge free radicals, shielding cells from oxidative stress [24]. It is claimed to have a protective effect on cells due to its antioxidant qualities [25]. It has been demonstrated that maqui berries provide other antioxidants, including vitamin E, electrons [26]. As a result, the current study demonstrated the protective impact of antioxidants, such as Maqui berry extract, against rat spleen damage caused by aniline.
CONCLUSION
The current investigation demonstrated the Maqui berry's splenoprotective properties in rats with spleen toxicity treated with aniline. The potent antioxidant properties of antioxidants may be the cause of this impact. The maqui berry demonstrated a more potent antioxidant synthesis pathway. Through attenuation of produced oxidative stress and restoration of antioxidative enzyme, the administration of Maqui berry, which targets multiple pathogenic mechanisms, led to the prevention and progression of Aniline-induced spleen damage. The current investigation discovered that a dose of 100 mg/kg of Maqui berry had a greater splenoprotective impact. The spleen toxicity will be avoided with the use of antioxidant therapy. Confirming the antioxidant's response mechanism will require more in-depth molecular research.
REFERENCE
- Pouran, M., Hooshyar, H., Ghulam, M., & LM. (2019). Molecular mechanism of aniline induced spleen toxicity and neuron toxicity in experimental rat exposure: A review. Current Neuropharmacology, 7(3), 201–213.
- Okazaki, Y., Yamashita, K., Sudo, M., Tsuchitani, M., Narama, I., Yamaguchi, R., et al. (2001). Neurotoxicity induced by a single oral dose of aniline in rats. Journal of Veterinary Medical Science, 63(5), 539–546.
- Wang, J., Wang, G., Ansari, G. A., & Khan, M. F. (2008). Activation of oxidative stress-responsive signaling pathways in early splenotoxic response of aniline. Toxicology and Applied Pharmacology, 230(2), 227–234. https://doi.org/10.1016/j.taap.2008.02.022
- Upasana, K., Upaganlawar, A., & Upasani, C. (2016). Ameloriative effect of chronic supplementation of protocatechuic acid alone and in combination with ascorbic acid in aniline hydrochloride induced spleen toxicity in rats. Scientifica (Toxicology), 4306984. https://doi.org/10.1155/2016/4306984
- Wu, X., Kannan, S., Ramanujam, V. M., & Khan, M. F. (2005). Iron release and oxidative DNA damage in splenic toxicity of aniline. Journal of Toxicology and Environmental Health, Part A, 68(8), 657-666.
- Ramsay, W. N. (1969). The determination of the total iron-binding capacity of serum. Clinica Chimica Acta, 2(3), 221–226. https://doi.org/10.1016/0009-8981(57)90106-7
- Slater, T. F., & Sawyer, B. C. (1971). The stimulatory effect of carbon tetrachloride and other halogenalkane or peroxidative reaction in the rat liver functions in vitro. Biochemical Journal, 123(5), 805–814. https://doi.org/10.1042/bj1230805
- Moron, M. S., & Depierre, J. W. (1979). Levels of glutathione, glutathione reductase and glutathione S transferase activities in rat lung and liver. Biochimica et Biophysica Acta, 582(1), 67–78. https://doi.org/10.1016/0304-4165(79)90289-7
- Guevara, I., Iwanejko, J., Dembinska-Kiec, A., Pankiewicz, J., Wanat, A., Anna, P., et al. (1998). Determination of nitrite/nitrate in human biological material by the simple Griess reaction. Clinica Chimica Acta, 274(2), 177–188. https://doi.org/10.1016/s0009-8981(98)00060-6
- Mishra, H.P., Fridovich, I., 1972.The role of superoxide anion in the autooxidation of epinephrine and a simple assay of superoxide dismutase. J Biol Chem . 247, 3170-3175. https://doi.org/10.1016/S0021-9258(19)45228-9
- Luck, H.,1971.Methods of enzymatic analysis (Academic Press, New York).(3),885. https://doi.org/10.1016/B978-0-12-395630-9.50158-4
- Bonting, S. L. (1970). Membrane ion transport. In T. Pembroski, T. Schmidt, & G. Blumchen (Eds.), Bio-behavioral base of coronary heart disease (Vol. 1, pp. 254–363). London: Wiley.
- Hjerken, S., & Pan, H. (1983). Purification and characterization of two forms of low-affinity calcium ion ATPase from erythrocyte membrane. Biochimica et Biophysica Acta, 728(2), 281–288. https://doi.org/10.1016/0005-2736(83)90480-7
- Ohinishi, T., Suzuki, T., Suzuki, Y., & Ozawa, K. (1982). A comparative study of plasma membrane Mg2+ ATPase activities in normal, regenerating and malignant cells. Biochimica et Biophysica Acta, 684(1), 67–74.
- Khan, M. F., Green, S. M., Ansari, G. A. S., & Boor, J. P. (1998). Phenylhydroxylamine: Role in aniline-associated splenic oxidative stress and induction of subendocardial necrosis. Toxicological Sciences, 42, 64-71.
- Gupta, S. K. (2001). Pharmacology and therapeutics in the new millennium (Vol. 18, pp. 277–278). Springer.
- Khan, M. F., Gu, Y., Alcock, W., & Boor, P. J. (1997). Oxidative stress in the splenotoxicity of aniline. Fundamental and Applied Toxicology, 35(1), 22–30. https://doi.org/10.1006/faat.1996.2259
- Khan, M. F., Kaphaha, B., & Boor, P. J. (1993). Subchronic toxicity of aniline hydrochloride in rats. Archives of Environmental Contamination and Toxicology, 24, 368-374.
- Minotti, G. (1993). Sources and role of iron in lipid peroxidation. Chemical Research in Toxicology, 6(2), 134-136.
- Bartoli, C. G., Buet, A., Grozeff, G. G., Galatro, A., & Simontacchi, M. (2017). Ascorbate-glutathione cycle and abiotic stress tolerance in plants. In Springer (pp. 177-200). https://doi.org/10.1007/978-3-319-74057-7-7
- Sharma, P., Jha, A. B., Dubey, R. S., & Pessarakli, M. (2012). Reactive oxygen species, oxidative damage, and antioxidant defence mechanism in plants under stressful conditions. Journal of Botany, 2012, 217037. https://doi.org/10.1155/2012/217037
- Bowler, C., Montagu, M. V., Inzé, D.1992. Superoxide dismutase and stress tolerance. Plant Biol. Ann. Rev. 43, 83–116. https://doi.org/10.1146/annurev.pp.43.060192.000503
- Xiuzhen, F., Jianling, W., Kizhake, V. S., Ansari, G. A. S., & Khan, M. F. (2011). Aniline induced nitrosative stress in rat spleen: Proteomic identification of nitrated proteins. Toxicology and Applied Pharmacology, 255(1), 103–112.
- Rosenfeldt, F. L., Haas, S. J., Krum, H., Hadj, A., Ng, K., et al. (2007). Coenzyme Q10 in the treatment of hypertension: A meta-analysis of the clinical trials. Journal of Human Hypertension, 21, 297-306.
- Maheshwari, R. A., Balaraman, R., Sen, A. K., & Seth, A. K. (2014). Effect of coenzyme Q10 alone and its combination with metformin on streptozotocin-nicotinamide-induced diabetic nephropathy in rats. Indian Journal of Pharmacology, 46, 627-632.
- Lass, A., & Sohal, R. S. (2000). Effect of coenzyme Q10 and ??tocopherol content of mitochondria on the production of superoxide anion radicals. The FASEB Journal, 14(1), 87-94.


 Pavan khonde*
Pavan khonde*
 Swati Jadhav
Swati Jadhav





 10.5281/zenodo.12683260
10.5281/zenodo.12683260