Abstract
Prostate cancer–specific positron emission tomography (pcPET) has been shown to detectsites of disease recurrence at serum prostate-specific antigen (PSA) levels that are lower than those levels detected by conventional imaging. Commonly used pcPET radiotracers in the setting of biochemical recurrence are reviewed including carbon 11/fludeoxy glucose 18 (F-18) choline, gallium 68/F-18 prostate-specific membrane antigen (PSMA), and F-18 fluciclovine. Review of the literature generally favors PSMA-based agents for the detection of recurrence as a function of low PSA levels. Positive gallium 68/F-18 PSMA positron emission tomography/computed tomography scans detected potential sites of recurrence. Sensitive pc PET imaging has provided new insight into the early patterns of disease spread, which has prompted judicious reconsideration of additional local therapy after either prostatectomy, definitive radiation therapy, or postprostatectomy radiation therapy. This review discusses the literature, clinical utility, availability, and fundamental understanding of pc PET imaging needed to improve clinical practice. Prostate cancer is the most frequent tumor found in men worldwide and in Mexico in particular. Age and family his-tory are the main risk factors. The diagnosis is made by prostate biopsy in patients with abnormalities detected in their prostate-speci?c antigen (PSA) levels or digital rectal exam (DRE). This article reviews screening and diagnostic methods as well as treatment options for patients diagnosed with prostate cancer.
Keywords
Prostate Cancer; Risk Factors; Diagnosis; Treatment; Prevention; Positron Emission Tomography(PET);Prostate Imging; MRI
Introduction
Prostate cancer remains one of the most common malignancies affecting men worldwide [1,2]. Prostate cancer recurrence following primary treatment is usually signaled by a rising serum prostate-specific antigen (PSA) level,which can be quite anxiety-provoking for patients and clinicians.[3.4.5]Fortunately, advances in prostate cancer–specific positron emission tomography (pcPET) have demonstrated new insights into patterns of disease recurrence.[6.7.8]Emerging pcPET radiotracers including carbon 11 (C-11) choline, gallium 68 (Ga-68) prostate specific membrane antigen (PSMA), C-11 acetate, and18F-fluorocyclobutane-1-carboxylic acid fluciclovine(FACBC) provide opportunities to localize prostate cancer recurrence at an earlier state in the disease course when the PSA level is low, to inform medical decision-making, and to study PET-directed local therapy.[9-13]In anticipation of in cris of interest eased use and availability of pc PET radiotracers, a critical review of the following : (1) fundamentals of PET; (2) current systematic reviews and meta-analyses of commonly used pcPET radiotracers; (3) comparative studies evaluating pc PET radiotracers; (4) future directions of pc PET technology in the management of prostate cancer.We limit the scope of our discussion to pc PET radiotracers that image both soft tissue and bone and do not address other novel methods such as F-18 sodium fluoride PET other use of whole body magnetic resonance imaging (MRI). Despite rapid and signi?cant advances in our understanding of all aspects of prostate cancer, it remains the most diagnosed malignancy among US men, accounting for ap-proximately 290,000 new diagnoses and 35,000 deaths [14]. Prostate cancer is far from a uniform diagnosis. It can be suf?ciently indolent to warrant surveillance alone, or it can be so aggressive and disseminated at diagnosis that aggressive multi-disciplinary cancer care is critical to maximizing outcomes. Prostate cancer(Pca) is the second most common cancer among men, repersenting a common cause of cancer death worldwide[15] Advanced imaging methods including positron-emission tomography/computed tomography (PET/CT) and positron-emission tomography/magnetic resonance imaging(PET/MRI) using different radiopharmaceuticals have showed relevant results for early detection of local and systemic spread of PCa, according to available evidence-based data [16].In particular, hybrid imaging methods combining functional and morphological information have the potential to overcome the limitations of conventional imaging methods in some clinical situations of PCa staging [17,18].
Fundamentals of PET
A comprehensive literature search was performed using electronic databases, including: MEDLINE, EMBASE, PubMed, ScienceDirect, Web of Science, Cochrane Library, and Google Scholar. Search keywords included, but were not limited to: prostate, prostate cancer, prostate malignancy, prostate recurrence, recurrent prostate cancer, biochemical recurrence, positron emission tomography, PET, prostate specific membrane antigen, PSMA, choline, C-11 or F-18 choline PET, C-11 acetate PET, fluciclovine, FACBC, and Axumin. Additional articles were identified by searching bibliographies of relevant literature. A review approach was utilized using collective information from a broad review of the existing scientific literature sourced from PubMed search with relevant keywords and input from a multidisciplinary team of experts in medical physics, radiation treatment planning, nuclear medicine, and radiation therapy. PET is a type of functional imaging technique used to localize metabolic processes. A radionuclide produced from either a cyclotron or a generator is attached to a biologically active molecule forming a PET radiotracer. The PET radiotracer is then introduced into the patient by injection, ingestion, or inhalation. In modern practice, the functional information from PET is almost always acquired simultaneously with anatomic information provided via computed tomography (CT) scanning or MRI. Once the PET radiotracer is administered, the patient is positioned so that detectors can register incident gamma rays, 2 511 keV photons traveling in opposite directions, produced as the radionuclide decays resulting in an annihilation event from a positron combining with an electron after traversing a short distance. The detector’s electronics are synced in such a way that the 2 photons emitted are detected on opposite sides and are called coincident and therefore must have originated from the same annihilation event. These coincident projections are assigned to a line of response and are then reconstructed using standard tomographic techniques to identify the location of the annihilation event. By using modern “time of flight” information in PET image reconstruction with very fast scintillators, the origin of the annihilation event along the line of response is detected with improved accuracy.[19] More recent advancements in PET imaging and spatial resolution have been further improved by the use of iterative reconstruction algorithms such as the Ordered Subsets Expectation Maximization and Bayesian penalized-likelihood reconstruction algorithms.[20] Newer reconstruction algorithms have mean standardized uptake value levels 2 to 3 times higher than conventional Ordered Subsets Expectation Maximization technology, which should be considered when comparing studies of intergenerational scanners.[21] Properties of important pcPET radiotracers are shown in Table 1.[22]
Table 1 Properties of important prostate cancer-specific positron emission tomography radiotracers
Prostate cancer–specific PET scans are performed uniquely. Unlike standard F-18 PET scans, which are usually imaged starting at the head and scan toward the feet, pcPET scans typically image the pelvis first. Imaging is initiated 3 to 5 minutes after radiotracer administration and scanning begins at the mid-thigh and proceeds to the base of skull. This is done primarily to minimize urinary tract contamination, but also because of the short half-life of isotopes such as C-11. Urinary tract contamination is the primary reason pcPET protocols are performed in this manner, including those involving radiotracers with longer half-lives such as F-18 fluciclovine.
Background for choline PSMA and fluciclovine.
This review will focus on 3 PET radiotracers of interest: C-11/F-18 choline, Ga-68/F-18 PSMA, and F-18 fluciclovine. Choline metabolism has been shown to be altered in prostate cancer cells. Increased levels of choline compounds concentrate preferentially in human prostate cancer cells derived from metastases. Alteration of choline metabolites within the cancer cell relates to choline transport, incorporation, and utilization within the cell.[23], [24], [25] Preclinical data conflict on the theory of augmented choline use by the cell because of increased cell membrane synthesis and proliferation.[26], [27], [28] Multiple metabolomic studies on prostate cancer have shown permutations in choline metabolism not related to cell membrane ogenesis; however, it is well accepted that choline is used via a 3-step process known as the Kennedy pathway for the de novo synthesis of phosphatidylcholine, which is an essential component of the cell membrane.[29]Preclinical data have shown that there is an increase in the expression of choline transporters and an increase in the choline transport rate in malignant prostate cells when compared with normal prostate tissues[.30] Interestingly, preclinical data have also shown that treatment of prostate cancer cells leads to changes in energetic metabolism and choline metabolism.[31] This notion is consistent with what experienced centers have observed after administration of systemic therapy to patients with C-11/F-18 choline PET-positive lymph node(s), wherein the nodes are no longer choline-avid (Fig 1) Figure 1. Carbon 11 (C-11) choline positron emission tomography/computed tomography scan of a 75-year-old man status post radical prostatectomy for prebiopsy prostate-specific antigen (PSA) 5.3 ng/mL, Gleason 8, pT2c,N0,M0, R0 resection who experienced a rising PSA postoperatively to 0.55 ng/mL and was treated with salvage prostatic fossa only radiation therapy in 7 months later.
The patient then received a course of concurrent androgen suppression and consolidative radiation therapy to the pelvic lymph nodes, including simultaneous integrated boost to the pre chemo hormonal prostate cancer–specific positron emission tomography avid lymph node, with radiation portals abutting his previously irradiated prostatic fossa. The patient’s PSA remains undetectable (<0>

Figure 1. Prostate-specific membrane antigen (PSMA) structure with common PSMA small-molecule inhibitors that target the substrate recognition site that are combined with a radioisotope to form a clinically useful pc PET radiotracer.
There appears to be growing interest in developing an 18-F–labeled PSMA agent. Some experts argue that it would offer advantages with respect to availability, production amount, and image resolution. This approach was first explored at Johns Hopkins University where F-18 DCFBC, the first-generation F-18 PSMA radiotracer, was developed and is currently licensed to Cyclotek for clinical use in Australia and New Zealand.[40], [41] Since the development of F-18 DCFBC, second-generation tracers such as F-18 DCFPyL have been developed.[42] Currently, there are a number of groups working to develop the most clinically useful next-generation F-18–labeled PSMA radiotracer.[43], [44], [45], [46], [47], [48] PSMA’s unique expression differential between cancer and normal cells coupled with its large extracellular domain provides an excellent target for imaging, but also for therapeutics such as the ranostic applications with lutetium 177 PSMA. Less than 10% of prostate cancers have no uptake on PSMA PET.[49] Additionally, the short half-life of Ga-68 (68 minutes) results in low radiation exposure to patients. Furthermore, the agent is rapidly cleared from nontarget tissue. On average, patients receive 3.0 mSv from the PET component of 150 MBq of Ga-68-PSMA-11, which is lower than most other pcPET agents such as C-11 and 18-F choline scans.[50], [51]
Evidence synthesis
1. The PSMA gene (FOLH1) and protein
PSMA is encoded by the FOLH1 gene located on chromosome 11p11.12 [52]. Consisting of 19 exons and 18 intron within a 60-kb region, the gene is under the control of an upstream promoter and an enhancer region present within the third intron. It has been shown that SOX-7 (repres-71sor), the TMPRSS2-ERG gene fusion (repressor), and NFATC-1 (activator) regulate FOLH1 gene expression [53-55]. However, none of these transcription factors are entirely responsible for PSMA expression, suggesting that additional factors contribute to the regulation of PSMA in PCa.PSMA is a glycosylated, transmembrane carboxypeptidase subdivided into three major regions: a short cytoplasmic tail, a transmembrane segment, and a large extracellular portion [56]. The role of PSMA depends on the site of expression. In glial cells, PSMA catalyses the synthesis of81glutamate from the neuropeptide N-acetyl-aspartyl-glutamate (NAAG), thereby promoting excitatory neural transmission ]. In the duodenum, PSMA cleaves glutamate moieties from dietary polyglutamated folates to produce monoglutamated folates that are more readily absorbed[57]
2. Regulation of PSMA expression in PCa
Regulation by the androgen receptor
The dichotomous reltionship between PSMA and androgen receptor(A) signalling has been described in the preclinical and clinical setting . studies using hormone-sensitive prostate cancer(HSPC)cell lines and xenografts showed that traeatment with testosterone dihydrotestosterone, or the synthetic analogue R1881 reduces PSMA experssion, while androgen deprivation therapy(ADT) inscreased PSMA experssion[58,59]
By contrast, a clinical imaging study using68Ga-PSMA Positron emission tomography (PET) showed that ADT acutely down regulated PSMA [removed]maximum [SUV-max] and mean [SUVmaean] standarized uptake values in the majority of patients with HSPC who also experienced a marked decrease in prostate- specific antigen(PSA)[60].

Figure 2. PRISMA flow diagram PCa=prostate cancer; PSMA=prostate-specific membrane antigen
Fluciclovine is a synthetic amino acid, and an analog of l-leucine, which is preferentially taken up by prostate cancer cells and gliomas via specialized amino acid transporters, namely alanine-serine-cysteine transporter 2 (ASCT2) and LAT-1.[61], [62], [63], [64], [65] Its chemical name is anti-1-amino-3-FACBC, and is commonly known by its trade name Axumin. Amino acid transporters such as ASCT2 play a critical role in amino acid metabolism in prostate cancer cells. ASCT2 is an important transporter of glutamine, which is known to be an essential tumor nutrient and has been implicated in cancer signaling pathways.[66], [67] Fluciclovine is predominantly transported by ASCT2 and transports in a manner similar to glutamine[68] Unlike glutamine, however, 18-F fluciclovine does not undergo additional metabolism in the cell, which lends to its intracellular accumulation particularly in prostate cancer cells and at major sites of amino acid metabolism such as the liver and pancreas.[69] Additional pcPET radiotracers used in prostate cancer imaging have been developed as previously noted. These include C-11 acetate and F-18 sodium fluoride. In addition, F-18 PET may be useful in imaging prostate cancer patients who have developed dedifferentiated neuroendocrine tumors of the prostate,which conversely may not image well using these pcPET agents.[70]
Detection Rates as a Function of PSA
PSA are regularly monitored. Nevertheless, clinicians sometimes disagree on the best time to perform PCT imaging when a patient's PSA is rising. Fig. 3 provides an overview of data on detection rates as a function of PSA. Reviews of 10 trials examining detection rates as a function of PSA for choline, 6 PSMA, and 4 fluciclovine are presented. Over each histogram cluster, the bolded figure indicates the median percentage of patients with positive pcPET scans. In terms of recurrence detection at PSA levels of 2.0 ng/mL, the overall trends indicate that Ga-68 PSMA performs better than both C-11/F-18 choline and F-18 fluciclovine. This comparative review is not without significant limitations, though. First, there aren't many data points in the fluciclovine data. Second, there are significant variations in radiotracer dose between investigations, which has an impact on detection rates, sensitivity, and specificity. For instance, the C-11 choline dose in the Mitchell et al. trial, which had relatively high detection rates, varied from 555 to 740 MBq.[71] This dose was substantially higher than the choline dose—typically 3.4 to 3.5 MBq/kg [72,73] given in prospective comparison studies with lower choline PET detection rates.This equals 280 MBq for a patient weighing 80 kg, basically.
POSITRON EMISSION TOMOGRAPHY/MAGNETIC RESONANCE IMAGING PET/MRI
contrasts the benefits of PET's metabolic imaging with MRI's soft-tissue contrast resolution. The two methods perform comparably in lesion detection in tests with radiotracers, 11C and 18F-choline. Nonetheless, PET/MRI provided a more accurate anatomic localization of lesions, particularly in the case of pelvic and bone abnormalities.[74] For the two modalities, the maximum and mean SUV values varied, most likely as a result of various attenuation correction methods. It has been noted that 68Ga-PSMA PET/MRI performs better in lesion detection and localization than either mp-MRI or PET alone. Gleason score/PSA values and quantitative PET/MRI parameters, however, did not correlate.[75]It was found that PET/MRI was superior to PET/CT for lesion characterisation because of its higher contrast resolution.[76] According to Lake et al.'s evaluation of the best MRI sequences for 68Ga-PSMA PET/MRI, pelvic lymph nodes were best assessed using small-field-of-view T2-weighted images, while prostatic bed lesions were best detected using dynamic contrast imaging.[77] When sclerosis is not visible on CT, MRI, particularly when combined with DWI, may boost the confidence in classifying PET-positive bone lesions as metastases. PET/MRI, however, is highly unsophisticated and has several disadvantages, including higher costs, longer examination times, and the requirement for optimal MRI attenuation correction.[78]
CLINICAL APPLICATION OF PROSTATE CANCER-SPECIFIC PET IMGING-
"Where is the origin of my rising PSA?" is a therapeutically relevant question frequently posed by patients experiencing a rising PSA following decisive therapy.Prior to the extensive clinical use of multiparametric MRI and pcPET radiotracers, clinicians used less-than-ideal methods, mainly bone scans and CT scans, to investigate this subject.Even at PSA levels as low as 1.0 ng/mL, pcPET radiotracers can identify recurrence sites, as illustrated in Fig 3. In contrast, osseous metastases are detected by bone scans at a median PSA level of 40 ng/mL.[79]In a study of 23 trials, Abuzallouf et al. found that the frequencies of osseous detection were 2.3% for PSA b10 ng/mL, 5.3% for PSA 10.1 to 19.9 ng/mL, and 16.4% for PSA 20.0 to 49.9 ng/mL.[80]0% of patients with PSA b20 ng/mL and 1.1% of patients with PSA N20 ng/mL had lymph node metastases, according to 25 studies analyzing CT scans included in the review. Further data from a prospective population-based investigation of patients with recently discovered prostate cancer revealed that 15% of patients had CT scan detection rates when their PSA levels were between 4 and 20 ng/mL.[81]Due to these low positive yields, pretreatment evaluation has seen a decrease in the general usage of bone scans and CT imaging, which has also resulted in a reduction in their use in the recurrent setting[82] The origin of PSA relapse is being identified at lower PSA levels than ever before thanks to advancements in pcPET radiotracers. With Ga-68/F-18 PSMA, a median of 51.5% of patients have possible recurrence sites identified when their PSA level is b1.0 ng/mL. This is illustrated in Figure 2. The detection rate climbs to 74% for a PSA of more than 1.0 ng/mL and reaches 90% at a PSA of N2.0 ng/mL. With radiotracers based on choline and fluciclovine, comparable, if smaller, patterns are seen. Sensitive functional imaging has led to research on recurrence patterns that shed light on how prostate cancer spreads in a range of clinical settings, such as postprostatectomy, post-definitive radiation therapy, and postprostatectomy radiation therapy, early in the metastasis process.6-8Studies showing recurrence patterns like these and others have sparked more debate about aggressive metastasis-directed therapy or extra local therapy targeted at the nodal basins that are at risk. Functional imaging has entered its own period, and doctors all around the world are utilizing it to create unique radiation treatment regimens. 66% of patients receiving radiation therapy were included in a recent meta-analysis by Ost et al. that examined metastasis-directed therapy to localized and distant recurrences.
Diagnosis:
A PC diagnosis is made five to ten years prior to the onset of symptoms in the modern PSA era. Patients typically have no symptoms or symptoms of urine voiding and/or storage associated with PC. These include vesical tenesmus, pushing, frequency, urgency, and reduced urine stream. Bone pain, renal failure, hematuria, pathological bone fractures, physical weariness, and weight loss are examples of advanced PC symptoms. PSA values (>4 ng/ml) and a suspicious digital rectal examination (DRE) (e.g., increased consistency or nodules) are the most significant instruments for PC diagnosis. But in the absence of PC, other factors such as ejaculation, trauma (such as the insertion of a transurethral catheter or a rectal one), inflammation, infection (such as acute prostatitis), and prostatic hyperplasia can also raise PSA level.
It is necessary to take at least two measures, separated by at least three weeks, because there may be significant inter-individual variance. The high sensitivity and limited specificity of PSA screening might result in false positives, which is one of its drawbacks. Due to the reference value being set at 2.5 ng/ml in some countries, 80% of occurrences of unwarranted prostate biopsies (BxP) and overtreatment have occurred. It's also important to note that DRE might be the only useful diagnostic tool because up to 5% of PCs do not have elevated PSA levels. In PC cases, DRE diagnoses up to 18% of cases. There have also been descriptions of various PSA-derived metrics, including PSA density, transition zone PSA density, and other molecular techniques. Nevertheless, these PSA-derived measures offer little usefulness in practice.
If prostate cancer screening detects an abnormality, your doctor may recommend further tests to determine whether you have prostate cancer, such as:
Ultrasound. During a transrectal ultrasound, a small probe, about the size and shape of a cigar, is inserted into your rectum. The probe uses sound waves to create a picture of your prostate gland.
Magnetic resonance imaging (MRI). In some situations, your doctor may recommend an MRI scan of the prostate to create a more detailed picture. MRI images may help your doctor plan a procedure to remove prostate tissue samples.
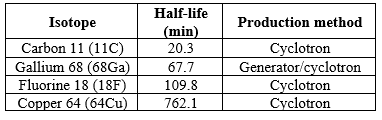
Collecting a sample of prostate tissue. To determine whether there are cancer cells in the prostate, your doctor may recommend a procedure to collect a sample of cells from your prostate (prostate biopsy). Prostate biopsy is often done using a thin needle that's inserted into the prostate to collect tissue. The tissue sample is analyzed in a lab to determine whether cancer cells are present.
Fig.3 Transrectal biopsy of the prostate
During a transrectal biopsy, a biopsy gun quickly projects a thin needle into suspect areas of the prostate gland, and small sections of tissue are removed for analysis.
Screening:
The results of two multicenter studies that evaluated PSA screening as a diagnostic tool were published independently in 2009 by the American group PLCO (Prostate, Lung, Colorectal and Ovarian Screening Trial) and the European group ERSPC (European Randomized Study on Screening for Prostate Cancer).[83]The outcomes were incoherent. After 11 years of follow-up, the PLCO group came to the conclusion that there was no difference in the reduction of PC-related mor-tality between the control and screened groups.[84,85] In contrast, after nine years of follow-up, the ERSPC group demonstrated a twenty percent reduction in PC-related mortality among one thousand patients.[86,87]Mexico lacks the necessary hospital and medical infrastructure to support all PC-diagnosed cases, which makes screening impossible.It is necessary to go over the indications for performing the PSA test with the patient, including the benefits, drawbacks, and cost-benefit ratio. The current issue is that we lack a marker or imaging test that would enable us to distinguish between PC cases that are aggressive and those that are indolent and require monitoring. We recommend PSA testing for individuals who are 40–45 years of age or older, with more frequent follow-up monitoring for results > 1 ng/ml (>40 years), as there is a larger risk of PC development in these patients.4 It should be mentioned that because PC mortality is often low (3%), people with PC typically live longer than ten years.and that some comorbidities, such cardiovascular disease, may increase the patient's mortality.
Prostate Biopsy:
Prostate biopsy (BxP) is the current standard for the diagnosis of PC. There are two main criteria for a patient to be considered a candidate for a BxP: a suspicious DRE and a PSA result higher than 4 ng/ml, obtained under ideal conditions and conrmed with two separate mea-surements at least 3 weeks apart. It is important to take into consideration the size of the prostate as measured by DRE or ultrasound. For example, if the prostate is small and the PSA is high, the possibility of PC is higher. If the prostate is larger (> 40 grams) and PSA values are between 4 and 10 ng/ml, the PH likelihood is 80%. The patient must be informed of all steps of the procedure, the diagnostic and therapeutic implications and the risks that this entail. It is imperative to treat the patient with a broad-spectrum antibiotic prior to the procedure. The most commonly used antibiotics are quinolones. However, in our own center in the National Institute of Medical Sciences and Nutrition, this group of antibiotics shows a higher resistance rate. We therefore use Piperacillin/Tazobactam with good results.20 The BxP is performed by transrectal ultrasound and under sedation. At the beginning, a conventional ultrasound should be performed to visualize the prostate gland, the seminal vesicles and the bladder surface. The volume of the prostate gland is then calculated, and sextant biopsies are performed. Samples are obtained by puncturing the entire gland surface, attempting to obtain samples from the peripheral zone (75% of adeno-carcinomas depend on this area). A total of 12 fragments are conventionally obtained, six from each lobe.21 These symptoms present in approximately 2 to 20% of all procedures worldwide. In our institution, these complications do not exceed 4.6%.(22) The most common prostate tumor is adenocarcinoma. The Gleason scale of histolo-gical differentiation is used for classication. This scale is fundamental for dening the stage and prognosis of PC patients. The scale is applied additively, with the rst number representing the predominant histologic grade and the second number the secondary histologic grade. According to this reasoning, a Gleason value of 7 can reect a 3+4 tumor (where 3 is the rst number, i.e., less aggressive) or a 4+3 tumor (where 4 is the rst number, i.e., more aggressive)

Fig.4 Digital rectal exam :During a digital rectal exam, your doctor inserts a gloved, lubricated finger into your rectum and feels the back wall of the prostate gland for enlargement, tenderness, lumps or hard spots.
Staging:
For prognostic as well as therapeutic purposes, the illness stage is crucial. Generally speaking, we categorize clinical and pathological stages using the TNM scale. Other validated scales, the most popular of which is the D'Amico scale, can also be used to classify patients based on their mortality and recurrence risk.23 In order to categorize individuals into low risk (Gleason £ 6, PSA < 10> 20 ng/ml and a stage greater than T2c), this scale assesses PSA levels, the Gleason biopsy scale, and the DRE.The different 10-year survival rates for each group—83% for the low-risk group, 46% for the intermediate-risk group, and 29% for the high-risk group—elucidate the significance of this classification. To assess extraprostatic extension and extension to lymph nodes (locally advanced illness) and other structures, like bone (metastatic disease), a variety of imaging modalities have also been employed. MRIs, bone scans, and computed tomography of the chest, belly, and pelvis are a few of these methods.It is advised that patients in the intermediate- and high-risk categories use these procedures. It is also advised to evaluate alkaline phosphatase levels, as they are linked to bone metastases.
Treatment:
Your options for treating prostate cancer will vary depending on a number of circumstances, including how quickly the cancer is progressing, if it has spread, how well you are overall, and any possible advantages or disadvantages of the treatment.
Immediate treatment may not be necessary-
Treatment for low-grade prostate cancer might not be necessary immediately away. Some people may never require medical attention. Alternatively, physicians may suggest active surveillance. Regular follow-up blood tests, rectal exams, and prostate biopsies may be carried out as part of active surveillance to track the advancement of your cancer. You may choose to get radiation therapy or surgery for prostate cancer if testing reveal that the disease is spreading. If the cancer is limited to a small area of the prostate, is not predicted to grow rapidly, and is not causing any symptoms, active surveillance may be a viable treatment choice. Active monitoring may also be taken into consideration for those who are older and more difficult to treat for cancer, or who have another significant medical problem
Surgery to remove the prostate:
Surgery for prostate cancer involves removing the prostate gland (radical prostatectomy), some surrounding tissue and a few lymph nodes.Surgery is an option for treating cancer that's confined to the prostate. It's sometimes used to treat advanced prostate cancer in combination with other treatments.
To access the prostate, surgeons may use a technique that involves:
cutting multiple tiny cuts on your abdomen: In a robot-assisted laparoscopic prostatectomy, a few tiny abdominal incisions are used to insert surgical equipment that are coupled to a robot. Using hand controls, the surgeon directs the robot to maneuver the instruments while seated at a console. Using this method, the majority of prostate cancer surgeries are completed. cutting your abdomen in a single, lengthy incision: In order to access and remove the prostate gland, the surgeon performs a single, lengthy incision in your lower abdomen during retropubic surgery. Although somewhat less prevalent, this strategy might be required in some circumstances.

Fig 5.Prostatectomy incisions
During an open prostatectomy, one large incision is made in your abdomen (left). During a
robotic prostatectomy, several smaller incisions are made in the abdomen (right).
Radiation therapy:
Radiation therapy uses high-powered energy to kill cancer cells. Prostate cancer radiation therapy treatments may involve:
Radiation that comes from outside of your body (external beam radiation).
When receiving external beam radiation therapy, you lie on a table with a machine circling your body to target the prostate cancer with powerful energy beams like protons or X-rays. Usually, you have treatments with external beam radiation five days a week for a few weeks. Higher radiation doses spaced out over fewer days are used in a shorter radiation therapy course offered by certain medical facilities. One treatment option for prostate-specific cancer is external beam radiation. In cases when there is a chance that the cancer could spread or return, it can also be utilized following surgery to eradicate any cancer cells that could still be present.
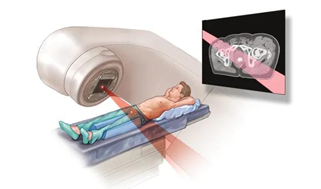
Fig 6. External beam radiation for prostate cancer
During external beam radiation treatment for prostate cancer, you lie on a table while a linear accelerator moves around you to deliver radiation from many angles. The linear accelerator delivers the precise dose of radiation planned by your treatment team.
Radiation placed inside your body (brachytherapy)-
Inserting radioactive sources into your prostate tissue is known as brachytherapy. Usually, radioactive seeds the size of rice are used to inject radiation into the prostate tissue. Over an extended period, the radiation exposure from the seeds is modest. One treatment option for cancer that hasn't progressed past the prostate is brachytherapy.

Fig 7. Permanent prostate brachytherapy
Permanent prostate brachytherapy involves placing many radioactive seeds within the prostate to treat prostate cancer. During the procedure, an ultrasound probe is placed in the rectum to help guide the placement of seeds. The seeds emit radiation that dissipates over a few months.
Freezing or heating prostate tissue:
Ablative therapies destroy prostate tissue with cold or heat. Options may include:
Freezing prostate tissue:Cryoablation or cryotherapy for prostate cancer involves using a very cold gas to freeze the prostate tissue. The tissue is allowed to thaw and the procedure repeats. The cycles of freezing and thawing kill the cancer cells and some surrounding healthy tissue.
Heating prostate tissue:High-intensity focused ultrasound (HIFU) treatment uses concentrated ultrasound energy to heat the prostate tissue and cause it to die.
Hormone therapy:
Hormone therapy is treatment to stop your body from producing the male hormone testosterone. Prostate cancer cells rely on testosterone to help them grow. Cutting off the supply of testosterone may cause cancer cells to die or to grow more slowly.
Hormone therapy options include:
Medications that stop your body from producing testosterone: Certain medications — known as luteinizing hormone-releasing hormone (LHRH) or gonadotropin-releasing hormone (GnRH) agonists and antagonists — prevent your body's cells from receiving messages to make testosterone. As a result, your testicles stop producing testosterone.
Medications that block testosterone from reaching cancer cells:
These medications, known as anti-androgens, usually are given in conjunction with LHRH agonists. That's because LHRH agonists can cause a temporary increase in testosterone before testosterone levels decrease.
Chemotherapy:
- Chemotherapy uses drugs to kill rapidly growing cells, including cancer cells. Chemotherapy can be administered through a vein in your arm, in pill form or both.
- Chemotherapy may be a treatment option for treating prostate cancer that has spread to other areas of the body. Chemotherapy may also be an option for cancers that don't respond to hormone therapy.
- Immunotherapy:
- Immunotherapy uses your immune system to fight cancer. Your body's disease-fighting immune system may not attack your cancer because the cancer cells produce proteins that help them hide from the immune system cells. Immunotherapy works by interfering with that process.
Prostate cancer immunotherapy can involve:
Engineering your cells to fight cancer: The Sipuleucel-T (Provenge) treatment involves injecting a small portion of your own immune cells back into your body via a vein after genetically modifying them in a lab to combat prostate cancer. When hormone therapy is no longer effective for treating advanced prostate cancer, this is an option.
Helping your immune system cells identify cancer cells:For advanced prostate tumors that no longer respond to hormone therapy, immunotherapy medications that assist the immune system's cells in locating and eliminating cancer cells represent a treatment alternative.
Prevention:
Reducing the incidence of PC by medication-assisted prevention is the goal. Numerous agents have been researched that lessen PC growth. The first trial, known as the PCPT (Prostate Cancer Prevention Trial), was double blind and examined the administration of finasteride or a placebo. In this experiment, finasteride (5-?-reductase type 2 inhibitor) reduced the incidence of PC by 6.4%. This medication relieves lower urinary tract symptoms (LUTS) and prevents testosterone from being converted to dihydrotestosterone, which lowers the need for surgery. But in this investigation, the diagnosis of PC was more aggressive histologically, with a higher proportion of high-grade tumors (1.3%). In the second research, called REDUCE (Reduction by Dutasteride of Cancer Events), dutasteride (5-?-reductase type 1 and 2 inhibitor) was compared with a placebo. In this study, there was a 23?crease in PC incidence without any correlation with more aggressive PC. Furthermore, there have been reports of lower PC rates associated with soy, lycopene, green tea, and statin use; however, these findings are dubious. There was no decrease in PC risk, according to the results of the SELECT (Selenium and Vitamin E Cancer Trial) research. Without a doubt, PC is a condition with a number of hazy prevention targets (like metformin).[88] As of now, the only drugs that have strong evidence to lower PC incidence are those that reduce PC incidence; there isn't any solid evidence to support the idea that dietary supplements can lower PC incidence.Lastly, healthy lifestyle modifications that can prevent PC and other comorbidities include exercise, diets low in saturated fat with an increase in antioxidants and omega 3 and 6 fatty acids, and other healthy practices[89].
CONCLUSION:
The treating oncologist frequently faces a therapeutic hurdle when a patient with prostate cancer experiences a biochemical recurrence. Data indicate against prolonged monitoring of detectable postprostatectomy PSA levels and support early management with salvage radiation therapy following prostatectomy.Even at very low PSA levels, patients in this clinical scenario may still benefit from pcPET imaging to detect the location of recurrence. Additionally, imaging using a multiparametric MRI scan in addition to a pcPET scan can offer complementary information regarding the location of recurrence. However, not every patient who sees a treating oncologist fits within this quite typical clinical picture of a rising PSA soon after a prostatectomy. It is true that some people show up with increasing PSA following definitive radiation therapy, while others show up following radiation therapy following a prostatectomy, and yet others show up years after their first operation following a late PSA rise.
PCT imaging has significant clinical usefulness in these difficult patients. In the context of biochemical recurrence, a review of the literature suggests PSMA-based imaging in most cases. However, more comparison studies are required to elucidate which pcPET radiotracer is most appropriate in each of a range of clinical presentations. It may be possible to determine which patients will benefit most from additional local therapy and which ones will benefit most from PCPET imaging by doing functional imaging studies that integrate genetic profiling. In patients with biochemical failure, prospective studies are being conducted to evaluate the effectiveness of pcPET-directed local treatment.36 MRI and prostate cancer-speci?c PET represent two widely applicable, rapidly devel-oping technologies that are becoming increasingly important to prostate cancer diagnosis and management. While the adoption of these techniques will help us make the most informed decisions with our patients, it is important to recognize that the clinical bene?t sand cost-effectiveness of their use are still being evaluated and debated. With continued prospective evaluation, advanced imaging for prostate cancer will continue to improve the personalization and ef?ciency of care.
REFERENCES:
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA: a cancer journal for clinicians. 2018 Jan;68(1):7-30.
- Cooperberg MR, Carroll PR. Trends in management for patients with localized prostate cancer, 1990-2013. Jama. 2015 Jul 7;314(1):80-2.
- Dale W, Bilir P, Han M, Meltzer D. The role of anxiety in prostate carcinoma: a structured review of the literature. Cancer: Interdisciplinary International Journal of the American Cancer Society. 2005 Aug 1;104(3):467-78.
- Lofters A, Juffs HG, Pond GR, Tannock IF. “PSA-itis:"Knowledge of serum prostate specific antigen and other causes of anxiety in men with metastatic prostate cancer. J Urol. 2002;168:2516-2520.
- Clark JA, Talcott JA. Confidence and uncertainty long after initial treatment for early prostate cancer: survivors' views of cancer control and the treatment decisions they made. Journal of clinical oncology. 2006 Sep 20;24(27):4457-63.
- Sobol I, Zaid HB, Haloi R, Mynderse LA, Froemming AT, Lowe VJ, Davis BJ, Kwon ED, Karnes RJ. Contemporary mapping of post-prostatectomy prostate cancer relapse with 11C-choline positron emission tomography and multiparametric magnetic resonance imaging. The Journal of urology. 2017 Jan;197(1):129-34.
- Parker WP, Davis BJ, Park SS, Olivier KR, Choo R, Nathan MA, Lowe VJ, Welch TJ, Evans JD, Harmsen WS, Zaid HB. Identification of site-specific recurrence following primary radiation therapy for prostate cancer using C-11 choline positron emission tomography/computed tomography: a nomogram for predicting extrapelvic disease. European urology. 2017 Mar 1;71(3):340-8.
- Parker WP, Evans JD, Stish BJ, Park SS, Olivier K, Choo R, Nathan MA, Welch BT, Karnes RJ, Mynderse LA, Pisansky TM. Patterns of recurrence after postprostatectomy fossa radiation therapy identified by C-11 choline positron emission tomography/computed tomography. International Journal of Radiation Oncology* Biology* Physics. 2017 Mar 1;97(3):526-35.
- Evangelista L, Briganti A, Fanti S, Joniau S, Reske S, Schiavina R, Stief C, Thalmann GN, Picchio M. New clinical indications for 18F/11C-choline, new tracers for positron emission tomography and a promising hybrid device for prostate cancer staging: a systematic review of the literature. European urology. 2016 Jul 1;70(1):161-75.
- Perera M, Papa N, Christidis D, Wetherell D, Hofman MS, Murphy DG, Bolton D, Lawrentschuk N. Sensitivity, specificity, and predictors of positive 68Ga–prostate-specific membrane antigen positron emission tomography in advanced prostate cancer: a systematic review and meta-analysis. European urology. 2016 Dec 1;70(6):926-37.
- Ost P, Bossi A, Decaestecker K, De Meerleer G, Giannarini G, Karnes RJ, Roach III M, Briganti A. Metastasis-directed therapy of regional and distant recurrences after curative treatment of prostate cancer: a systematic review of the literature. European urology. 2015 May 1;67(5):852-63.
- Supiot S, Rio E, Pacteau V, Mauboussin MH, Campion L, Pein F.OLIGOPELVIS - GETUG P07: a multicentre phase II trial ofcombined salvage radiotherapy and hormone therapy in oligometastaticpelvic node relapses of prostate cancer. BMC Cancer. 2015;15:646.
- Mohsen B, Giorgio T, Rasoul ZS, Werner L, Ali GR, Reza DK, Ramin S. Application of 11 C-acetate positron-emission tomography (PET) imaging in prostate cancer: systematic review and meta-analysis of the literature. BJU international. 2013 Dec 1;112(8).
- Siegel RL, Miller KD, Wagle NS, Jemal A. Cancer statistics, 2023. Ca Cancer J Clin. 2023 Jan 12;73(1):17-48.
- Hassanipour-Azgomi S, Mohammadian-Hafshejani A, Ghoncheh M, Towhidi F, Jamehshorani S, Salehiniya H. Incidence and mortality of prostate cancer and their relationship with the Human Development Index worldwide. Prostate international. 2016 Sep 1;4(3):118-24.
- Annunziata S, Pizzuto DA, Treglia G. Diagnostic performance of PET imaging using different radiopharmaceuticals in prostate cancer according to published meta-analyses. Cancers. 2020 Aug 4;12(8):2153.
- Schiavina R, Chessa F, Borghesi M, Gaudiano C, Bianchi L, Corcioni B, Castellucci P, Ceci F, Ceravolo I, Barchetti G, Del Monte M. State?of?the?art imaging techniques in the management of preoperative staging and re?staging of prostate cancer. International Journal of Urology. 2019 Jan;26(1):18-30.
- Fanti S, Minozzi S, Antoch G, Banks I, Briganti A, Carrio I, Chiti A, Clarke N, Eiber M, De Bono J, Fizazi K. Consensus on molecular imaging and theranostics in prostate cancer. The lancet oncology. 2018 Dec 1;19(12):e696-708.
- Surti S. Update on time-of-flight PET imaging. Journal of Nuclear Medicine. 2015 Jan 1;56(1):98-105.
- O’Doherty J, McGowan DR, Abreu C, Barrington S. Effect of Bayesian-penalized likelihood reconstruction on [13N]-NH3 rest perfusion quantification. Journal of Nuclear Cardiology. 2017 Feb 1;24(1):282-90.
- Evans JD, Jethwa KR, Ost P, Williams S, Kwon ED, Lowe VJ, Davis BJ. Prostate cancer–specific PET radiotracers: A review on the clinical utility in recurrent disease. Practical radiation oncology. 2018 Jan 1;8(1):28-39.
- Cherry SR. Fundamentals of positron emission tomography and applications in preclinical drug development. The Journal of Clinical Pharmacology. 2001 May;41(5):482-91.
- Hernández-Alcoceba R, Saniger L, Campos J, Núñez MC, Khaless F, Gallo MA, Espinosa A, Lacal JC. Choline kinase inhibitors as a novel approach for antiproliferative drug design. Oncogene. 1997 Nov;15(19):2289-301.
- Katz-Brull RA, Degani H. Kinetics of choline transport and phosphorylation in human breast cancer cells; NMR application of the zero trans method. Anticancer research. 1996 May 1;16(3B):1375-80.
- Janardhan S, Srivani P, Sastry GN. Choline kinase: an important target for cancer. Current medicinal chemistry. 2006 Apr 1;13(10):1169-86.
- Ackerstaff E, Pflug BR, Nelson JB, Bhujwalla ZM. Detection of increased choline compounds with proton nuclear magnetic resonance spectroscopy subsequent to malignant transformation of human prostatic epithelial cells. Cancer research. 2001 May 1;61(9):3599-603.
- Roberts MJ, Schirra HJ, Lavin MF, Gardiner RA. Metabolomics: a novel approach to early and noninvasive prostate cancer detection. Korean journal of urology. 2011 Feb 1;52(2):79-89.
- Lima AR, de Lourdes Bastos M, Carvalho M, de Pinho PG. Biomarker discovery in human prostate cancer: an update in metabolomics studies. Translational oncology. 2016 Aug 1;9(4):357-70.
- Awwad HM, Geisel J, Obeid R. The role of choline in prostate cancer. Clinical biochemistry. 2012 Dec 1;45(18):1548-53.
- SA M. Characterization of choline uptake in prostate cancer cells following bicalutamide, docetaxel treatment. Eur J Nucl Med Mol Imaging. 2009;36(9):1434-42.
- Lodi A, Ronen SM. Magnetic resonance spectroscopy detectable metabolomic fingerprint of response to antineoplastic treatment. PloS one. 2011 Oct 12;6(10):e26155.
- Leek J, Lench N, Maraj B, Bailey A, Carr IM, Andersen S, Cross J, Whelan P, MacLennan KA, Meredith DM, Markham AF. Prostate-specific membrane antigen: evidence for the existence of a second related human gene. British journal of cancer. 1995 Sep;72(3):583-8.
- Maurer T, Eiber M, Schwaiger M, Gschwend JE. Current use ofPSMA-PET in prostate cancer management. Nat Rev Urol. 2016;13:226-235.
- Birtle AJ, Freeman A, Masters JR, Payne HA, Harland SJ, contributors to the BAUS Section of Oncology Cancer Registry. Tumour markers for managing men who present with metastatic prostate cancer and serum prostate?specific antigen levels of< 10>
- Evans MJ, Smith-Jones PM, Wongvipat J, Navarro V, Kim S, Bander NH, Larson SM, Sawyers CL. Noninvasive measurement of androgen receptor signaling with a positron-emitting radiopharmaceutical that targets prostate-specific membrane antigen. Proceedings of the National Academy of Sciences. 2011 Jun 7;108(23):9578-82.
- Silver DA, Pellicer I, Fair WR, Heston WD, Cordon-Cardo C. Prostate-specific membrane antigen expression in normal and malignant human tissues. Clinical cancer research: an official journal of the American Association for Cancer Research. 1997 Jan 1;3(1):81-5.
- Bostwick DG, Pacelli A, Blute M, Roche P, Murphy GP. Prostate specific membrane antigen expression in prostatic intraepithelial neoplasia and adenocarcinoma: a study of 184 cases. Cancer: Interdisciplinary International Journal of the American Cancer Society. 1998 Jun 1;82(11):2256-61.
- Maurer T, Eiber M, Schwaiger M, Gschwend JE. Current use ofPSMA-PET in prostate cancer management. Nat Rev Urol. 2016;13:226-235.
- Eder M, Scha?fer M, Bauder-Wu?st U, Hull WE, Wa?ngler C, Mier W, Haberkorn U, Eisenhut M. 68Ga-complex lipophilicity and the targeting property of a urea-based PSMA inhibitor for PET imaging. Bioconjugate chemistry. 2012 Apr 18;23(4):688-97.
- Foss CA, Mease RC, Fan H, Wang Y, Ravert HT, Dannals RF, Olszewski RT, Heston WD, Kozikowski AP, Pomper MG. Radiolabeled small-molecule ligands for prostate-specific membrane antigen: in vivo imaging in experimental models of prostate cancer. Clinical cancer research. 2005 Jun 1;11(11):4022-8.
- Cho SY, Gage KL, Mease RC, Senthamizhchelvan S, Holt DP, Jeffrey-Kwanisai A, Endres CJ, Dannals RF, Sgouros G, Lodge M, Eisenberger MA. Biodistribution, tumor detection, and radiation dosimetry of 18F-DCFBC, a low-molecular-weight inhibitor of prostate-specific membrane antigen, in patients with metastatic prostate cancer. Journal of Nuclear Medicine. 2012 Dec 1;53(12):1883-91.
- Szabo Z, Mena E, Rowe SP, Plyku D, Nidal R, Eisenberger MA, Antonarakis ES, Fan H, Dannals RF, Chen Y, Mease RC. Initial evaluation of [18 F] DCFPyL for prostate-specific membrane antigen (PSMA)-targeted PET imaging of prostate cancer. Molecular imaging and biology. 2015 Aug;17:565-74.
- Rowe SP, Macura KJ, Mena E, Blackford AL, Nadal R, Antonarakis ES, Eisenberger M, Carducci M, Fan H, Dannals RF, Chen Y. PSMA-based [18 F] DCFPyL PET/CT is superior to conventional imaging for lesion detection in patients with metastatic prostate cancer. Molecular imaging and biology. 2016 Jun;18:411-9.
- Kelly J, Amor-Coarasa A, Nikolopoulou A, Kim D, Williams C, Ponnala S, Babich JW. Synthesis and Harada N, Kimura H, Onoe S, Watanabe H, Matsuoka D, Arimitsu K, Ono M, Saji H. Synthesis and biologic evaluation of novel 18F-labeled probes targeting prostate-specific membrane antigen for PET of prostate cancer. Journal of Nuclear Medicine. 2016 Dec 1;57(12):1978-84.pre-clinical evaluation of a new class of high-affinity 18 F-labeled PSMA ligands for detection of prostate cancer by PET imaging. European Journal of Nuclear Medicine and Molecular Imaging. 2017 Apr;44:647-61.
- Dietlein M, Kobe C, Kuhnert G, Stockter S, Fischer T, Schomäcker K, Schmidt M, Dietlein F, Zlatopolskiy BD, Krapf P, Richarz R. Comparison of [18 F] DCFPyL and [68 Ga] Ga-PSMA-HBED-CC for PSMA-PET imaging in patients with relapsed prostate cancer. Molecular Imaging and Biology. 2015 Aug;17:575-84.
- Cardinale J, Schäfer M, Benešová M, Bauder-Wüst U, Leotta K, Eder M, Neels OC, Haberkorn U, Giesel FL, Kopka K. Preclinical evaluation of 18F-PSMA-1007, a new prostate-specific membrane antigen ligand for prostate cancer imaging. Journal of Nuclear Medicine. 2017 Mar 1;58(3):425-31.
- Harada N, Kimura H, Onoe S, Watanabe H, Matsuoka D, Arimitsu K, Ono M, Saji H. Synthesis and biologic evaluation of novel 18F-labeled probes targeting prostate-specific membrane antigen for PET of prostate cancer. Journal of Nuclear Medicine. 2016 Dec 1;57(12):1978-84.
- Bouvet V, Wuest M, Jans HS, Janzen N, Genady AR, Valliant JF, Benard F, Wuest F. Automated synthesis of [18 F] DCFPyL via direct radiofluorination and validation in preclinical prostate cancer models. EJNMMI research. 2016 Dec;6:1-5.
- Budäus L, Leyh-Bannurah SR, Salomon G, Michl U, Heinzer H, Huland H, Graefen M, Steuber T, Rosenbaum C. Initial experience of 68Ga-PSMA PET/CT imaging in high-risk prostate cancer patients prior to radical prostatectomy. European urology. 2016 Mar 1;69(3):393-6.
- Pfob CH, Ziegler S, Graner FP, Köhner M, Schachoff S, Blechert B, Wester HJ, Scheidhauer K, Schwaiger M, Maurer T, Eiber M. Biodistribution and radiation dosimetry of 68 Ga-PSMA HBED CC—a PSMA specific probe for PET imaging of prostate cancer. European journal of nuclear medicine and molecular imaging. 2016 Oct;43:1962-70.
- Afshar-Oromieh A, Hetzheim H, Kübler W, Kratochwil C, Giesel FL, Hope TA, Eder M, Eisenhut M, Kopka K, Haberkorn U. Radiation dosimetry of 68 Ga-PSMA-11 (HBED-CC) and preliminary evaluation of optimal imaging timing. European journal of nuclear medicine and molecular imaging. 2016 Aug;43:1611-20. newwwww
- O’Keefe DS, Su SL, Bacich DJ, Horiguchi Y, Luo Y, Powell CT, Zandvliet D, Russell PJ, Molloy PL, Nowak NJ, Shows TB. Mapping, genomic organization and promoter analysis of the human prostate-specific membrane antigen gene. Biochimica et Biophysica Acta (BBA)-Gene Structure and Expression. 1998 Nov 26;1443(1-2):113-27.
- Peng W, Guo L, Tang R, Liu X, Jin R, Dong JT, Xing CG, Zhou W. Sox7 negatively regulates prostate?specific membrane antigen (PSMA) expression through PSMA?enhancer. The Prostate. 2019 Mar;79(4):370-8.
- Lee SJ, Lee K, Yang X, Jung C, Gardner T, Kim HS, Jeng MH, Kao C. NFATc1 with AP-3 site binding specificity mediates gene expression of prostate-specific-membrane-antigen. Journal of molecular biology. 2003 Jul 18;330(4):749-60.
- Yin L, Rao P, Elson P, Wang J, Ittmann M, Heston WD. Role of TMPRSS2-ERG gene fusion in negative regulation of PSMA expression. PloS one. 2011 Jun 24;6(6):e21319.
- Mesters JR, Barinka C, Li W, Tsukamoto T, Majer P, Slusher BS, Konvalinka J, Hilgenfeld R. Structure of glutamate carboxypeptidase II, a drug target in neuronal damage and prostate cancer. The EMBO journal. 2006 Mar 22;25(6):1375-84.
- Halsted CH. Jejunal brush-border folate hydrolase. A novel enzyme. Western journal of medicine. 1991 Dec;155(6):605.
- Evans MJ, Smith-Jones PM, Wongvipat J, Navarro V, Kim S, Bander NH, Larson SM, Sawyers CL. Noninvasive measurement of androgen receptor signaling with a positron-emitting radiopharmaceutical that targets prostate-specific membrane antigen. Proceedings of the National Academy of Sciences. 2011 Jun 7;108(23):9578-82.
- Meller B, Bremmer F, Sahlmann CO, Hijazi S, Bouter C, Trojan L, Meller J, Thelen P. Alterations in androgen deprivation enhanced prostate-specific membrane antigen (PSMA) expression in prostate cancer cells as a target for diagnostics and therapy. EJNMMI research. 2015 Dec;5:1-1.
- Emmett L, Yin C, Crumbaker M, Hruby G, Kneebone A, Epstein R, Nguyen Q, Hickey A, Ihsheish N, O’Neill G, Horvath L. Rapid modulation of PSMA expression by androgen deprivation: serial 68Ga-PSMA-11 PET in men with hormone-sensitive and castrate-resistant prostate cancer commencing androgen blockade. Journal of Nuclear Medicine. 2019 Jul 1;60(7):950-4.
- Oka S, Hattori R, Kurosaki F, Toyama M, Williams LA, Yu W, Votaw JR, Yoshida Y, Goodman MM, Ito O. A preliminary study of anti-1-amino-3-18F-fluorocyclobutyl-1-carboxylic acid for the detection of prostate cancer. Journal of Nuclear Medicine. 2007 Jan 1;48(1):46-55.
- Sasajima T, Ono T, Shimada N, Doi Y, Oka S, Kanagawa M, Baden A, Mizoi K. Trans-1-amino-3-18F-fluorocyclobutanecarboxylic acid (anti-18F-FACBC) is a feasible alternative to 11C-methyl-L-methionine and magnetic resonance imaging for monitoring treatment response in gliomas. Nuclear medicine and biology. 2013 Aug 1;40(6):808-15.
- Oka S, Okudaira H, Yoshida Y, Schuster DM, Goodman MM, Shirakami Y. Transport mechanisms of trans-1-amino-3-fluoro [1-14C] cyclobutanecarboxylic acid in prostate cancer cells. Nuclear medicine and biology. 2012 Jan 1;39(1):109-19.
- Schuster DM, Nanni C, Fanti S. PET tracers beyond FDG in prostate cancer. InSeminars in nuclear medicine 2016 Nov 1 (Vol. 46, No. 6, pp. 507-521). WB Saunders.
- Savir-Baruch B, Zanoni L, Schuster DM. Imaging of prostate cancer using fluciclovine. PET clinics. 2017 Apr 1;12(2):145-57.
- Ganapathy V, Thangaraju M, Prasad PD. Nutrient transporters in cancer: relevance to Warburg hypothesis and beyond. Pharmacology & therapeutics. 2009 Jan 1;121(1):29-40.
- Nakanishi T, Tamai I. Solute carrier transporters as targets for drug delivery and pharmacological intervention for chemotherapy. Journal of pharmaceutical sciences. 2011 Sep 1;100(9):3731-50.
- Oka S, Okudaira H, Ono M, Schuster DM, Goodman MM, Kawai K, Shirakami Y. Differences in transport mechanisms of trans-1-amino-3-[18 F] fluorocyclobutanecarboxylic acid in inflammation, prostate cancer, and glioma cells: comparison with L-[Methyl-11 C] methionine and 2-deoxy-2-[18 F] fluoro-D-glucose. Molecular imaging and biology. 2014 Jun;16:322-9.
- Asano Y, Inoue Y, Ikeda Y, Kikuchi K, Hara T, Taguchi C, Tokushige T, Maruo H, Takeda T, Nakamura T, Fujita T. Phase I clinical study of NMK36: a new PET tracer with the synthetic amino acid analogue anti-[18 F] FACBC. Annals of nuclear medicine. 2011 Jul;25:414-8.
- Spratt DE, Gavane S, Tarlinton L, Fareedy SB, Doran MG, Zelefsky MJ, Osborne JR. Utility of FDG?PET in clinical neuroendocrine prostate cancer. The Prostate. 2014 Aug;74(11):1153-9.
- Mitchell CR, Lowe VJ, Rangel LJ, Hung JC, Kwon ED, Karnes RJ. Operational characteristics of 11C-choline positron emission tomography/computerized tomography for prostate cancer with biochemical recurrence after initial treatment. The Journal of urology. 2013 Apr;189(4):1308-13.
- Nanni C, Zanoni L, Pultrone C, Schiavina R, Brunocilla E, Lodi F, Malizia C, Ferrari M, Rigatti P, Fonti C, Martorana G. 18 F-FACBC (anti1-amino-3-18 F-fluorocyclobutane-1-carboxylic acid) versus 11 C-choline PET/CT in prostate cancer relapse: results of a prospective trial. European journal of nuclear medicine and molecular imaging. 2016 Aug;43:1601-10.
- Morigi JJ, Stricker PD, van Leeuwen PJ, Tang R, Ho B, Nguyen Q, Hruby G, Fogarty G, Jagavkar R, Kneebone A, Hickey A. Prospective comparison of 18F-fluoromethylcholine versus 68Ga-PSMA PET/CT in prostate cancer patients who have rising PSA after curative treatment and are being considered for targeted therapy. Journal of nuclear medicine. 2015 Aug 1;56(8):1185-90.
- Souvatzoglou M, Eiber M, Takei T, Fürst S, Maurer T, Gaertner F, Geinitz H, Drzezga A, Ziegler S, Nekolla SG, Rummeny EJ. Comparison of integrated whole-body [11 C] choline PET/MR with PET/CT in patients with prostate cancer. European journal of nuclear medicine and molecular imaging. 2013 Oct;40:1486-99.
- .Eiber M, Weirich G, Holzapfel K, Souvatzoglou M, Haller B, Rauscher I, Beer AJ, Wester HJ, Gschwend J, Schwaiger M, Maurer T. Simultaneous 68Ga-PSMA HBED-CC PET/MRI improves the localization of primary prostate cancer. European urology. 2016 Nov 1;70(5):829-36.
- Afshar-Oromieh A, Haberkorn U, Schlemmer HP, Fenchel M, Eder M, Eisenhut M, Hadaschik BA, Kopp-Schneider A, Röthke M. Comparison of PET/CT and PET/MRI hybrid systems using a 68 Ga-labelled PSMA ligand for the diagnosis of recurrent prostate cancer: initial experience. European journal of nuclear medicine and molecular imaging. 2014 May;41:887-97.
- Lake ST, Greene KL, Westphalen AC, Behr SC, Zagoria R, Small EJ, Carroll PR, Hope TA. Optimal MRI sequences for 68 Ga-PSMA-11 PET/MRI in evaluation of biochemically recurrent prostate cancer. EJNMMI research. 2017 Dec;7:1-9.
- Lindenberg L, Ahlman M, Turkbey B, Mena E, Choyke P. Evaluation of prostate cancer with PET/MRI. Journal of Nuclear Medicine. 2016 Oct 1;57(Supplement 3):111S-6S.
- Taneja SS. Imaging in the diagnosis and management of prostate cancer. Reviews in urology. 2004;6(3):101.
- Abuzallouf S, Dayes IA, Lukka H. Baseline staging of newly diagnosed prostate cancer: a summary of the literature. The Journal of urology. 2004 Jun 1;171(6):2122-7.
- ALBERTSEN PC, HANLEY JA, HARLAN LC, GILLILAND FD, HAMILTON A, LIFF JM, STANFORD JL, STEPHENSON RA. The positive yield of imaging studies in the evaluation of men with newly diagnosed prostate cancer: a population based analysis. The Journal of urology. 2000 Apr;163(4):1138-43.
- Cooperberg MR, Lubeck DP, Grossfeld GD, Mehta SS, Carroll PR. Contemporary trends in imaging test utilization for prostate cancer staging: data from the cancer of the prostate strategic urologic research endeavor. The Journal of urology. 2002 Aug;168(2):491-5.
- Schröder FH, Hugosson J, Roobol MJ, Tammela TL, Ciatto S, Nelen V, Kwiatkowski M, Lujan M, Lilja H, Zappa M, Denis LJ. Screening and prostate-cancer mortality in a randomized European study. New England journal of medicine. 2009 Mar 26;360(13):1320-8.
- Andriole GL, Crawford ED, Grubb III RL, Buys SS, Chia D, Church TR, Fouad MN, Gelmann EP, Kvale PA, Reding DJ, Weissfeld JL. Mortality results from a randomized prostate-cancer screening trial. New England journal of medicine. 2009 Mar 26;360(13):1310-9.
- Thompson IM, Goodman PJ, Tangen CM, Lucia MS, Miller GJ, Ford LG, Lieber MM, Cespedes RD, Atkins JN, Lippman SM, Carlin SM. The influence of finasteride on the development of prostate cancer. New England journal of medicine. 2003 Jul 17;349(3):215-24.
- Andriole GL, Bostwick DG, Brawley OW, Gomella LG, Marberger M, Montorsi F, Pettaway CA, Tammela TL, Teloken C, Tindall DJ, Somerville MC. Effect of dutasteride on the risk of prostate cancer. New England Journal of Medicine. 2010 Apr 1;362(13):1192-202.
- Pinsky PF, Black A, Grubb R, Crawford ED, Andriole G, Thompson I, Parnes H. Projecting prostate cancer mortality in the PCPT and REDUCE chemoprevention trials. Cancer. 2013 Feb 1;119(3):593-601.
- Bensimon L, Yin H, Suissa S, Pollak MN, Azoulay L. The use of met-formin in patients with prostate cancer and the risk of death. Cancer Epidemiol Biomarkers Prev 2014; 23(10):211-218
- Pinsky PF, Black A, Grubb R, Crawford ED, Andriole G, Thompson I, Parnes H. Projecting prostate cancer mortality in the PCPT and REDUCE chemoprevention trials. Cancer. 2013 Feb 1;119(3):593-601.


 Mayuri N. Jagtap*
Mayuri N. Jagtap*
 Avinash B. Darekar
Avinash B. Darekar








 10.5281/zenodo.13732499
10.5281/zenodo.13732499