Abstract
A simple, accurate, precise, and economical Q-absorbance ratio UV-spectrophotometric method was developed and validated for simultaneous estimating Rosuvastatin Calcium and Ezetimibe in combined tablet dosage form. The solvent used was a 1:1 t mixture of Isopropyl alcohol and distilled water. Two wavelengths 244nm (?max of Rosuvastatin Calcium) and 240nm (Isoabsorptive point) were selected to estimate Rosuvastatin Calcium and Ezetimibe for the Q-Absorbance ratio method. The drug concentration was determined using the ratio of absorbance at the iso-absorptive point (?1 = 240 nm) and the ?max of Rosuvastatin Calcium (?2 = 244 nm). This method is linear for both drugs in the range of 5 to 25 ?g/ml at ?1 (R2 = 0.998) and at ?2 (R2 = 0.997) for Rosuvastatin Calcium, and Ezetimibe in the range of 5 to 25 ?g/ml for found at ?1 (R2 = 0.9992) and ?2 (R2 = 0.9993). The percentage recovery was 102.11 % of Rosuvastatin Calcium and 99.72 % of Ezetimibe by standard addition method. The LOD was found to be 1.126 ?g/ml and 1.400 ?g/ml for Rosuvastatin Calcium at ?1 and ?2 respectively. The LOD was found to be 0.713 ?g/ml and 0.396 ?g/ml for Ezetimibe at ?1 and ?2 respectively. The LOQ was found to be 3.412?g/ml and 4.240?g/ml for Rosuvastatin Calcium at ?1 and ?2 respectively. The LOQ was found to be 2.162?g/ml and 1.199?g/ml for Ezetimibe at ?1 and ?2 respectively. The method was precise as % RSD was found to be less than 2 in Repeatability and Interday for Rosuvastatin Calcium and Ezetimibe. The % assay of analyte drugs in the combined tablet dosage form was found to be 101.41% of Rosuvastatin Calcium and 99.24 % of Ezetimibe which showed good applicability of the developed method.
Keywords
Rosuvastatin Calcium, Ezetimibe, Q-absorbance ratio method, iso absorptive point
Introduction
Worldwide under health sciences and for therapeutic protocol, ancient and historical literature reported huge knowledge of diseases and medication related to information provided ayurveda and folk theories [1]. According to Botanical Survey of India, near about 46,000 species of plant were identified and reported , where more than 7,000 plants species were documented for variety of medicinal features [2]. Among these plants, Ocimum sanctum (OS) commonly known as Tulsi found as sacred plant according to Hindu religion all over India . OS most respectful herb in all over the world [3].
In the phytoremedial procedure about all the part like leaves, stem, flower, root, seeds or even whole plant of OS prominently used for various treatments like bronchitis, malaria, diarrhoea, dysentery, skin disease, arthritis, eye diseases, insect bites, anti-fertility, anticancer, antifungal, antimicrobial, cardioprotective, analgesic, antispasmodic and adaptogenic actions. Biochemicaly alcoholic extract of OS reported for numerous pharmacological activities like hypoglycaemic, immunomodulatory, antistress, analgesic, antipyretic, anti-inflammatory, anti-ulcerogenic, antihypertensive, CNS depressant, radioprotective, antitumour and antibacterial activity [4, 5]. Genus Ocimum belonging to family Labiatae with variety of species with different therapeutic uses including Ocimum sanctum L. (Tulsi), Ocimum gratissium (Ram Tulsi), Ocimum canum (Dulal Tulsi), Ocimum basilicum (Ban Tulsi), Ocimum kilimandscharicum, Ocimum ammericanum, Ocimum camphora, Ocimum minimum L., Ocimum tenuiflorum L. and Ocimum micranthum etc.[6]. Since ancient period, among phytoremedial herb, genus Amaranth has notably reported for to treat conditions like diuretic, useful in cold and cough, urinary and throat troubles and gastric problems. Seed of amaranth were used in hypertension, cardiovascular disease, reducing blood pressure, lowering cholesterol, also it is used in piles, blood purify and antiscorbutic [7]. Specially Amaranthus viridis (AV) among the group has anti-inflammatory feature used in vermifuse, diuretics and also for the treatment of kidney stones as antiurolithiatic agent [8]. In traditional Indian medicines, Cystone is a polyherbal ayurvedic formula known for treatment of, burning micturition , urinary tract complications in pregnancy and other various renal disorders [9]. Each tablet form contains approximately 130 mg Didymocarpus pedicellate, 98 mg Saxifraga ligulate, 32 mg Rubia cordifolia, Cyperus scariosus , 32 mg Achyranthes aspera, 32 mg Onosma bracteatum , 32 mg Vernonia cinerea, 26 mg Purified Shilajeet and 32 mg Hajrul yahood Bhasma [10]. By taking account of available phytomedicinal literature and pathological scenario present investigation was focused to find out comparative efficiency and biochemical screening of main bioactive compounds from two different plant species, pertaining to understand and upgrade traditional knowledge for its better application against some urolithiatic condition.
MATERIALS AND METHODS :
Plant Sample:
The best quality and non infected seeds of Ocimum sanctum and Amaranthus viridis was collected from the local market from Kolhapur, Maharashtra, India. The tablet Cystone (Himalaya, Batch No.106221154 ) was procured from local pharmacy.
Crude Extraction :
Among the collected seeds 300 gm of the seeds of Ocimum sanctum (OS) and Amaranthus viridis (AV) were cleaned and grinded in mortar and pestle followed by Cystone. Absolute ethanol was used as solvent for Soxhlet extraction method for 6 hr. The prepared seed extract was evaporateed and dry under reduced pressure at 40° C [11].

GCMS Analysis protocol:
The ethanolic extracts obtained from seeds of Ocimum sanctum, Amaranthus viridis and Cystone were subjected to GC-MS analysis . GC-MS analysis of this extract was performed using a Shimadzu GP 2010 system and Gas Chromatograph interfaced to a Mass Spectrometer (GC-MS) equipped with a RTX TQ 2010 column (60 m X 0.25 mm ID X 1 iMdf, composed of 100% D imethyl polysiloxane) for GC-MS detection, an electron ionization system with ionization energy of 70 eV was used. Specially pure Helium gas (99.99 %) was used as the carrier gas at a constant flow rate of 1ml/min and an injection volume of 2 Dl was employed. Injector temperature 250ºC; Ion-source temperature 280º C. The oven temperature was programmed from 80ºC (isothermal for 2 min.) with an increase of 10ºC/min to 200º C, then 5ºC/min to 280ºC, ending with a 9 min. isothermal at 2800º C. Mass spectra were taken at 70 eV; a scan interval of 0.5 seconds and fragments from 45 to 650 m/z. Total GC running time was 34 minutes the relative percentage amount of each component was calculated by comparing its average peak area to the total areas. Software adapted to handle mass spectra and chromatograms was GC-MS Real Time Analysis [12].
Identification of Phytochemical compounds :
Phytochemical screening for active compounds was performed using standard biochemical protocols as -
- Alkaloids by Dragendorff’s test:
Detection of alkaloids was done by using Dragendorff’s reagent. The reagent was prepared by adding 0.85 gram of basic Bismuth nitrate in10 ml acetic acid followed by adding 40 ml distilled water and named as solution A. 8 gm of Potassium iodide was added in 20 ml distilled water and named as B. 5ml from solution A and B was added in 20 ml of Acetic acid followed by 100 ml of water and named as C or Dragendorff’s reagent [13]. 4 to 5 drops of Dragendorff’s reagent was added in the 1ml of extract in a test tube. A reddish-brown precipitate was observed which indicated the presence of alkaloids [14].
- Flavonoids by Shinoda test:
1 ml of extract was added with Magnesium followed by few drops of concentrated Hydrochloric acid. Appearance of reddish to pink colour indicates the presence of flavonoids [15].
- Glycosides by Liebermann’s test.:
2 ml of acetic acid and 2 ml of Chloroform was added in 2 ml of extract followed by concentrated Sulphuric acid. Appearance of green colour showed presence of glycosides [16]
- Proteins by Biuret test:
Few drops of extract was added in the 1 ml 3% Copper sulphate followed by few drops of 10 % Sodium hydroxide. Appearance of violet or red colour formation indicating that proteins are present [17].
- Saponin by Foam test :
few drops extract was added in the few drops of water . Foam produced on shaking and persists for 10 to 15 min, indicates presence of Saponins [18].
- Phytosterols by Sulphuric acid test :
2 ml of extract was added in 2 ml Chloroform followed by concentrated Sulphuric acid. The solution was diluted in acetic acid. Finaly 3 ml Acetic anhydride was added. Appearance of bluish green color showed the presence of phytosterols [19]
- Tannin by Ferric chloride test :
2 ml of extract was diluted in distilled water. Few drops of Ferric chloride was added . Blue-green or black coloration indicates presence of tannins [20]
- Carbohydrates by Benedict’s Test :
Detection of carbohydrate was done by using Benedict’s reagent. The reagent was prepared by adding 173 gm of Sodium citrate in 100 gm of Sodium carbonate and diluted with 800 ml distilled water and boiled and named as solution A. 17.3 gm of Copper sulphate dissolved in 100 ml distilled solution B. 10 ml from solution A and B was named as C or Benedict’s reagent [21]. 2 ml of extract was added in 2 ml of Benedict’s reagent and boiled. Appearance of reddish brown precipitate indicating the presence of carbohydrate (Reducing sugar) [22].
- Phenol by Ferric chloride test :
1ml of the extract was added with three drops of Ferric chloride followed by few drops of Potassium ferricyanide. Apiarence of greenish- blue confirmed the presence of phenols [ 23].
- Terpenoids by Salkowski test :
2 ml of extract was mixed with 2 ml Chloroform followed by 3 ml concentrated H2SO4. appearance of reddish brown colour of indicating presence of terpenoids [24].
RESULTS:
Phytochemical screening of seed extract of Ocimum sanctum showed presence of flavonoids, proteins, saponin, phytosterols, carbohydrates, phenol and terpenoids. The alkaloid, glycosides and tannins were found absent. The Seed extract of Amaranthus viridis showed presence of alkaloid, flavonoids, proteins, saponins, tannins, carbohydrates and phenols where as glycosides, phytosterols and terpenoids were absent. Cystone extract showed presence of alkaloids, proteins, saponins, carbohydrates and phenols where flavonoids, glycosides, phytosterols, tannins and tannin were found absent. The GC – MS chromatogram of ethanolic extract of Ocimum sanctum, Amaranthus viridis and Cystone showed many major peaks ( graph 2 a. 3 a and 4 a). The chromatogram of Ocimum sanctum extract found were total 23 chemical constituents as 2-Hepten-4-ol, 2-Decenal, (E) -, 2,4-Decadienal, (E,E)-, 1- Tetradecanol, Tetradecane, Caryophyllene, Benzene, 1-(1,5-dimethyle-4hexenyl)-4-methyl, (1S,5S)-2-Methyl-5-(1,5R-6-methylhept-5en-2), 1H-Benzocycloheptene,2, 4a, 5, 6,7, 8, 9, 9a -octa, Cyclohexene, 3-(1, 5-dimethyl-4-hexenyl)-6m, 1-Hexadecanol, Hexadecane, 1,1’:4’1”- Tercyclohexane, Khusimyl methyl ether, Tetradecanoic acid, 1-Nonadecene, Heneicosane, Eicosanal, i-Propyl 12- methyltetradecanote, n-Hexadecanoic acid, Linoleic acid ethyl ether, 9,12,15- Octadecatrienoic acid and ethyl ester. Out of 23 , Hexadecenoic acid (23 %), i-Propyl 12- methyltetradecanote ( 14 %) found maximum. The chromatogram of Amaranthus viridis extract found total 15 chemical constituents as 1- Tetradecanol, Tetradecane, 1-Nonadecene, Hexadecane, Cyclohexane decyle, Eicosane, Dodecylcyclohexane, n-Hexadecanoic acid,, Hexadecanoic acid, ethyl ester, oleic acid, linoleic acid ethyle ester, ( E )-9-Octadecenoic acid ,15-methyl-, ethyl ester and Ethyl 14- methyl-hexadecanoate in which Hexadecenoic acid ( 21%) and linoleic acid ethyle ester ( 20 %) found maximum The chromatogram of Cystone extract found total 5 chemical constituents as (3ar,4R,7R)-1, 4, 9, 9 -1 tetramethyl-3, 4, 5, 6, 7, 8, n-Hexadecanpic acid,6- Ocadecenoic acid, Octadecanoic acid and Octadecanoic acid,17 methyl-,methyl ester where hexadecenoic acid ( 54 %) showed maximum area

+ : Present , - :Absent.
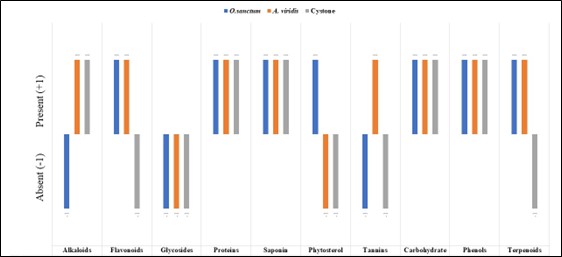
Figure 2 . Graphical representation of phytochemical analysis of ethanolic extract of seeds of Oscimum sanctum , Amaranthus viridis and Cystone
3.2 - GC-MS analysis of ethanolic extract of seeds of Oscimum sanctum , Amaranthus viridis and Cystone .
3.2.1 - GC-MS analysis of Ocimum sanctum :

Figure 3: GC-MS spectrum of Ocimum sanctum

Figure 4: Graphical representation of percentage of area detected by GC -MS in seeds of Oscimum sanctum.

Table No. 2 : Component identified in the seed extract of Oscimum sanctum.
3.2.2 - GC-MS analysis of Amaranthus viridis

Figure 5: GC-MS spectrum of Amaranthus viridis.

Figure 6 : Graphical representation of percentage of area detected by GC -MS in seeds of Amaranthus viridis.
Table No. 3 : Component identified in the seed extract of Amaranthus viridis.

3.2.2 - GC-MS analysis of Cystone:

Figure 7: GC-MS spectrum of Amaranthus viridis

Figure 8 : Graphical representation of percentage of area detected by GC -MS in Cystone.
DISCUSSION :
By the therapeutic point of view generally phytochemicals were reported as biologically active compounds, which protect animals from natural or induced toxic chemicals, pathogen where disease causing microbes are in association of fruits, leaves , seeds, grains herbs and some time total plant spices [25] . Along with food nutrients, foods source, plants have rich source of bioactive phytochemicals or bio nutrients which includes preventing chronic diseases like cancer, diabetes, coronary heart disease and hyper-cholesterolaemia. Some group of phytochemicals have disease-preventing antioxidants, detoxification , immunological and neuropharmacological activators [26]. In modern pharmacological and nutraceutical industries, phytochemicals played an important role because of wide range of its application like cofactors, modulators, inhibitors, antioxidant. The contents like carotenoids, catechins, curcumin, diosgenin , polyphenol and flavonoids have wide range against treatment of various human disease [27]. [28] documented various beneficiary effects of flavonoid like antioxidant, anti-inflammatory, antiallergic, anti-microbial, effective in hepatotoxicity, cardiovascular diseases, gastric ulcer, rheumatic disease, thrombosis, memory cognition and in cardiovascular disease. In the present study ethanolic extract of seed of Ocimum sanctum, Amaranthus viridis and Cystone, the GC MS analysis conforms variety of pharmacogenic constituents like, phytol, octadecanoic acid, Palmitic acid, oleic acid common in all three seed extract. Also Cetane and isocaryophyllene were identified. Along with precursor for vitamin E and vitamin K, phytol showed anxiolytic, metabolism-modulating, cytotoxic, antioxidant, autophagy and apoptosis inducing, antinociceptive, anti-inflammatory, immune-modulating, and antimicrobial effects [29, 30]. [31] reported 9, 12-Octadecadienoic acid has the property of antioxidant, anti-inflammatory and antiarthritic in his work. Cetane number is a indicators of the quality of diesel fuel. Variety of feedstock vegetables oils with K45 – K67are useful for the production of biodiesel [32]. [ 33] reported ?-caryophyllene has antioxidant, anti microbial and anti bacterial activities. [ 34.] reported phenols and alkaloids have antioxidants properties. [35] reported flavonoid, alkaloid, phenol, tannin and saponin have significant level of antioxidant.
CONCLUSION :
On the basis of result obtained under present investigation study revealed that, comparatively extract of seeds of Oscimum sanctum , Amaranthus viridis and Cystone has variety of bioactive compound which were screened by assay including flavonoids, alkaloids, phenols , tannin, saponin, phytol, etc. All these compound known for biological role in the animal body as antioxidant, antimicrobial, anticancer, anti-ulcer, anti-inflammatory and hepatoprotective. Comparatively OS, AV and CS has hexadecenoic acid chemical compound found prominently which play important role in in dissociation of calcium carbonate content. Both the phytoextract were seems to be useful for the protection of cells from accumulation of crystals and relevant pathological condition in comparison to cystone. The further advance study such as qualitative determination, purification and characterization of phytochemical constituents all extract may help to formulate herbal preparation of medical use.
ABBREVIATIONS
GC-MS : Gas Chromatography Mass Spectrometry.
OS : Oscimum sanctum
AV : Amaranthus viridis
CS : Cystone
CNS : Central Nervous System
mg : Milli Gram
ml : Milli litter
gm : Gram
CFC : Central Facility Centre
ºC : Degree Celsius
eV : Electron ionization
m/z : Mass to Charge ratio
DL : Detection limit.
H2SO4 : Sulphuric acid
+ : Present
- : Absent
gm/mol : Gram per Molecule
ACKNOWLEDGEMENT
Authors are thankful to Head, Department of Zoology, Shivaji University, Kolhapur for support during the present research work.
CONFLICT OF INTEREST
There are no conflicts of interest.
REFERENCES
- Vaidya AD, Devasagayam TP (2007) Current status of herbal drugs in India: an overview. J Clin Biochem Nutr. Jul;41(1):1-11. doi: 10.3164/jcbn.2007001. PMID: 18392106; PMCID: PMC2274994.
- Bisoi SS & Panda D (2015. Ethno-Medicinal Plants Present In Sacred Groves Of Koraput District Of Odisha, India. Acta Biomedica Scintia. 2. 39-42.
- Kulkarni KV, Belvotagi VA (2018) A review on: Indian traditional shrub Tulsi (Ocimum sanctum): The unique medicinal plant, Journal of Medicinal Plants Studies 2018; 6(2): 106-110.
- Pattanayak P, Behera P, Das D, Panda SK (2010 ) Ocimum sanctum Linn. A reservoir plant for therapeutic applications: An overview. Pharmacogn Rev. 2010 Jan;4(7):95-105. doi: 10.4103/0973-7847.65323. PMID: 22228948; PMCID: PMC3249909.
- Das SK & Vasudevan D (2006) Tulsi: The Indian Holy power Plant. Natural Product Radiance. 5. 279-283.
- Das AK , Hossain M & Deb N (2010). Chemical Compositions of Different Extracts of Ocimum basilicum Leaves. Journal of Scientific Research. 3. 197-206. 10.3329/jsr.v3i1.5409.
- Rastogi A, Shukla S. (2013) Amaranth: a new millennium crop of nutraceutical values. Crit Rev Food Sci Nutr. 2013;53(2):109-25. doi: 10.1080/10408398.2010.517876. PMID: 23072528.
- Asha S, & Thirunavukkarasu P (2013). Antiurolithiatic Activity of Amaranthus viridis on Ethylene Glycol Induced Male Rats. 2013. 13-17.
- Rao M, Rao MN ( 1998) Protective effects of cystone, a polyherbal ayurvedic preparation, on cisplatin-induced renal toxicity in rats, Journal of Ethnopharmacology, Volume 62, Issue 1, 1998,Pages 1-6, ISSN 0378-8741, https://doi.org/10.1016/S0378-8741(98)00003-8.
- Mohanty N.K. & Nayak, R.L. & Patki, Pralhad. (2010). Safety and Efficacy of an Ayurvedic Formulation Cystone in Management of Ureteric Calculi: A Prospective Randomized Placebo Controlled Study. American Journal of Pharmacology and Toxicology. 5. 58-64. 10.3844/ajptsp.2010.58.64.
- Lim SN, Cheung PC, Ooi VE, Ang PO (2002) Evaluation of antioxidative activity of extracts from a brown seaweed, Sargassum siliquastrum. J Agric Food Chem. 2002 Jun 19;50(13):3862-6. doi: 10.1021/jf020096b. PMID: 12059172.
- Phukan H, Bora C & Mitra P. (2017) Phytochemical Screening and GC-MS Analysis of Methanolic leaf Extract of an Endemic Plant Kayea assamica. IOSR Journal of Pharmacy and Biological Sciences. 12. 7-16. 10.9790/3008-1205020716.
- Sanghani Y (2017). Handbook of chemistry lab reagent.
- Samatha, T. & Srinivas, P. & Shyamsundarachary, R. & Nanna RS (2012). Phytochemical analysis of seeds, stem bark and root of an endangered medicinal forest tree Oroxylum Indicum(L)kurz. International Journal of Pharma and Bio Sciences. 3. B1063-B1075.
- Guediri, I.; Boubekri, C.; Smara, O (2020) Preliminary Phytochemical Screning From Different Extracts Of Solanum Nigrum Plant Growing In South Of Algeria. Journal Of Fundamental And Applied Sciences, [S. L.], V. 12, N. 2, P. 624–633, 2020. Doi: 10.4314/Jfas.V12i2.7.
- Gul R, Jan SU, Faridullah S, Sherani S, Jahan N (2017) Preliminary Phytochemical Screening, Quantitative Analysis of Alkaloids, and Antioxidant Activity of Crude Plant Extracts from Ephedra intermedia Indigenous to Balochistan. ScientificWorldJournal. 2017;2017:5873648. doi: 10.1155/2017/5873648. Epub 2017 Mar 13. PMID: 28386582; PMCID: PMC5366796.
- Kancherla N, Dhakshinamoothi A, Chitra K, Komaram RB (2019) Preliminary Analysis of Phytoconstituents and Evaluation of Anthelminthic Property of Cayratia auriculata (In Vitro). Maedica (Bucur). 2019 Dec;14(4):350-356. doi: 10.26574/maedica.2019.14.4.350. PMID: 32153665; PMCID: PMC7035446.
- Bhandary S, Kumari S & Bhat V, Sherly S & Bekal M (2012). Preliminary phytochemical screening of various extracts of Punica granatum peel, whole fruit and seeds. Nitte University Journal of Health Science. 2. 10.1055/s-0040-1703609.
- Ahmed Z, Aziz S, Hanif M, Mohiuddin SG, Ali Khan SH, Ahmed R, Sheikh Ghadzi SM, Naoras Bitar A (2020) Phytochemical screening and enzymatic and antioxidant activities of Erythrina suberosa (Roxb) bark. J Pharm Bioallied Sci. Apr-Jun;12(2):192-200. doi: 10.4103/jpbs.JPBS_222_19. Epub 2020 Apr 10. PMID: 32742119; PMCID: PMC7373117.
- Ukoha P, Egbuonu, Anthony C, Nnamdi O Ejikeme P (2011). Tannins and other phytochemical of the Samanaea saman pods and their antimicrobial activities. Afri. J. Pure Appl. Chem.. 5. 237-244.
- Shaikh J, & Patil M. (2020). Qualitative tests for preliminary phytochemical screening: An overview. 8. 603-608. 10.22271/chemi.2020.v8.i2i.8834.
- Deshmukh M Theng M (2018). Phytochemical Screening, Quantitative Analysis Of Primary And Secondary Metabolites Of Acacia Arabica Bark. International Journal of Current Pharmaceutical Research. 10. 35. 10.22159/ijcpr.2018v10i2.25889.
- Dubale S, Kebebe D, Zeynudin A, Abdissa N, Suleman S (2023) Phytochemical Screening and Antimicrobial Activity Evaluation of Selected Medicinal Plants in Ethiopia. J Exp Pharmacol. Feb 8;15:51-62. doi: 10.2147/JEP.S379805. PMID: 36789235; PMCID: PMC9922502.
- Sheel, D., & Nisha, K.M. (2014). Qualitative phytochemical analysis for isolation of terpens from Clerodendron infortunatum leaves. IOSR Journal of Applied Chemistry, 7, 14-18.
- Saxena M & Jyoti S& Nema R & Dharmendra S & Abhishek, G. (2013). Phytochemistry of Medicinal Plants. J Pharm Phytochem. 1. 168-182.
- Rao BN (2023) Bioactive phytochemicals in Indian foods and their potential in health promotion and disease prevention. Asia Pac J Clin Nutr. 2003;12(1):9-22. PMID: 12737006.
- Singh MK, Singh SK, Singh AV, Verma H, Singh PP, Kumar A, (2020) Phytochemicals: Intellectual property rights, Editor(s): Bhanu Prakash, Functional and Preservative Properties of Phytochemicals, Academic Press, 2020, Pages 363-375, ISBN 9780128185933, https://doi.org/10.1016/B978-0-12-818593-3.00012-9.
- Sandhar HK, Kumar B , Prasher S. & Tiwari P & Salhan, M. & Sharma P.(2011). A review of phytochemistry and pharmacology of flavonoids. Internationale Pharmaceutica Sciencia. 1. 25-41.
- Casuga F, Castillo & Corpuz MJA (2016) GC-MS analysis of bioactive compounds present in different extracts of an endemic plant Broussonetia luzonica (Blanco) (Moraceae) leaves. Asian Pacific Journal of Tropical Biomedicine. 6. 10.1016/j.apjtb.2016.08.015.
- Islam MT , Eunüs SA , Shaikh UJ , Subrata S , Islam MA, Ahmed MI , Manik C , Utpal KK, Nagendra SY, Khan IN, Billah MM , Magdalena DP, Gokhan Z, Clemens M, Ferdinando N, Diana G, Neagoe BI , Apostol A, Maciej B, Andy Y, Demerdash A, Jianbo X, Dey P, Yele S, Jó?wik A, Strza?kowska N, Marchewka J, Rengasamy KRR, Horba?czuk J, Kamal MA, Mubarak MS , Mishra SK , Shilpi JA, Atanasov AG (2018)Phytol: A review of biomedical activities, Food and Chemical Toxicology, Volume 121, 2018, Pages 82-94, ISSN 0278-6915, https://doi.org/10.1016/j.fct.2018.08.032.
- Nishanthini A, Mohan VR, Jeeva S (2014) Phytochemical, FT-IR, and GC-MS analysis of stem and leaf of Tiliacora acuminata (lan.) hook f and Thomas(menispermaceae). Int J Pharm Sci Res 5(9):3977–3986.
- Yadav A & Mishra SP , Kendurkar P, Kumar A & Maurya R (2020). Physicochemical characterization of Jatropha oil seed and suitability as biodiesel feedstock. Tropical Plant Research. 7. 581-586. 10.22271/tpr.2020.v7.i3.071.
- Sitarek, Rijo, Patricia, Garcia, Catarina, Ska?a, Ewa, Kalemba, Danuta, Bia?as, AdamJ., Szemraj, Janusz, Pytel, Dariusz, Toma, Monika, Wysoki?ska, Halina, ?liwi?ski, Tomasz (2017)Antibacterial, Anti-Inflammatory, Antioxidant, and Antiproliferative Properties of Essential Oils from Hairy and Normal Roots of Leonurus sibiricus L. and Their Chemical Composition, Oxidative Medicine and Cellular Longevity, 2017, 7384061, 12 pages, 2017. https://doi.org/10.1155/2017/7384061
- Gan J, Feng Y, He Z, Li X , Zhang H, ( 2017) Correlations between Antioxidant Activity and Alkaloids and Phenols of Maca (Lepidium meyenii), Journal of Food Quality, 2017, 3185945, 10 pages, 2017. https://doi.org/10.1155/2017/3185945
- Nithya TG , Jayaprakash J, Ragunathan M (2016). Antioxidant activity, total phenol, flavonoid, alkaloid, tannin, and saponin contents of leaf extracts of Salvinia molesta D. S. Mitchell (1972). 9.


 Vishal Sambhaji Sutar*
Vishal Sambhaji Sutar*
 Nitin Anandrao Kamble 2
Nitin Anandrao Kamble 2










 10.5281/zenodo.13737965
10.5281/zenodo.13737965