Abstract
Drug-induced hepatotoxicity is the most common reason for acute liver failure in developed nations. It represents roughly 10 of cases of acute liver failure worldwide and around 40- 50 of all cases of liver injury. The end of this study is to expose the result of the incidence of drug-induced hepatotoxicity attained from a pharmacovigilance database and which are the most common medicines associated. Evidence-based information on hepatotoxicity isn't readily available in a single Source. Thus several clinical scoring systems have been enforced.[1] RUCAM( Roussel Uclaf Causality Assessment Method) or its former reverse CIOMS( Council for International Organizations of Medical Sciences) is a well-established tool in common use to quantitatively assess reason in cases of suspected medicine-induced liver injury( DILI) and condiment-induced liver injury( HILI). In this composition scoring systems are explained in detail.
Keywords
Drug-induced liver injury, Drug hepatotoxicity, RUCAM, CIOMS, VigiBase
Introduction
The recent increase in reports of serious adverse events associated with medical remedies is of important public health interest. From 1998 to 2005, reported serious and fatal adverse events increased nearly threefold, four times faster than the increase in the total number of outpatient prescriptions during the same period. Drug-induced liver injury ( DILI ) is one of the most common medicine-related adverse events and can result in death or acute liver failure( ALF) by taking an emergency liver transplant.[2] The diagnosis of DILI is challenging since the evaluation of liver histology may not be individual and sensitive/ specific biomarkers are still under development. Numerous DILI events aren't necessarily reported in the literature and, even in reported cases, rigorous adjudication or vetting may not have been employed.
Etiology -
There are patient risk factors associated with the development of DILI, which includes female sex, older age, and increased body mass indicator (BMI). Further than 1000 medications and herbal compounds are known to bring hepatotoxicity and can be set up on a searchable database maintained by the National Institute of Diabetes and Digestive, and Kidney Diseases (NIDDK) called LiverTox. Natural DILI is most generally caused by acetaminophen, while it's less frequently seen in aspirin, tetracycline, and vitamin A. Idiosyncratic DILI cases are caused by Antibiotics amoxicillin-clavulanate (most common), sulfamethoxazole-trimethoprim, ciprofloxacin, isoniazid ( INH) Nonsteroidal anti-inflammatory drugs( NSAIDs). Herbal and dietary supplements ( HDS) green tea extract, anabolic steroids, and multi-ingredient nutritional supplements. Cardiovascular medicines statins, amiodarone d. Central nervous system( CNS) agents valproate, phenytoin e. Antineoplastic medicines tyrosine kinase inhibitors, tumor necrosis factor impediments, nascence impediments, and methotrexate.
Data Sources and Data Collection-
The data sources used to identify drugs associated with hepatotoxicity include( i ) databases created by ongoing prospective studies to collect cases of DILI or ALF; ( ii) databases created using regulatory resources;( iii) published literature; and other public disciplines, including websites of the regulatory agencies. Publications reporting DILI related to drugs linked in this study were linked using Cantina-Med and other intimately available search engines( e.g. Google, Google, Scholar), as well as a publication that provides periodic updates of the literature on hepatotoxic drugs.
Research-
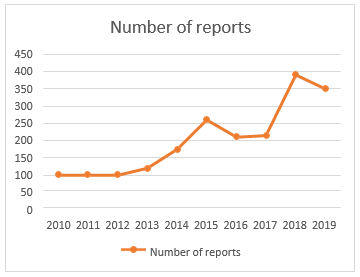
In this issue of the Allergy, Asthma & Immunology Research, Kang [3] and associates have reported a multicentre experimental study that investigated DILI developed during hospitalization. They enrolled 256,598 subjects who were rehabilitated for 1 time in 3 tertiary university hospitals in Korea. The algorithm is composed of 2 ways using both laboratory criteria and individual codes. First, the algorithm barred subjects with no laboratory data or high position of serum ALT> 3 times upper normal limit( UNL) of or total bilirubin> 2 times UNL and included subjects with high serum ALT > 3 × UNL plus total bilirubin> 2 × UNL or ALT> 5 × UNL afterward during hospitalization. Next, they barred subjects with individual codes recorded at the time of discharge suggesting liver conditions other than DILI. As the causative medicines for DILI, they reported antibiotics, similar to piperacillin- tazobactam, and chemotherapeutic agents. The utility of algorithms detecting DILI based on laboratory findings and/ or individual codes has been tested in several studies [4] the novelty of these studies is that the algorithms were applied to a larger number of inpatients with advanced positive prophetic value than the previous According to the last census data in Portugal, which was concluded in 2011, the total population was inhabitants. The rate between males to females was 0.9149. In the period between 1 January 2010 to 31 December 2019, the PPS entered 2038 reports related to liver ADRs. As shown in Figure 1, there was an adding trend over time in the number of reports entered by the PPS. The 3 times with the loftiest number of reports ( 2015, 2018 – 2019) correspond to nearly half of the total number of reports.
The incidence of DILI in cases with COVID-19 was more advanced than in cases hospitalized for other causes. The presence of DILI after COVID-19 was associated with longer hospital stays. Most cases were mild, hepatocellular in medium, and with posterior recovery. We observed that a high frequency of former COVID-19 hepatitis was observed in DILI. The most constantly associated medicines were hydroxychloroquine, azithromycin, tocilizumab, ceftriaxone, lopinavir/ ritonavir, paracetamol, remdesivir and enoxaparin, with RUCAM reason analysis defined as probable in 51.2 of cases. The highest incidence rate of DILI per 10,000 DDDs was for remdesivir ( 992.7/ 10,000 DDDs). A vulnerable mechanism has been demonstrated in DILI in a small subset of DILI cases Remdesivir had the highest incidence rate of DILI per administration in our study. The hepatotoxicity of remdesivir has been subject to debate. Although randomized trials in COVID-19 demonstrate original liver enzyme elevations between treatment and control groups, screening of the WHO safety reports database showed that, of the total 387 reports of ADRs for this medicine, 130 were hepatic, whereas the maturity was hepatobiliary. Azithromycin had the alternate loftiest prevalence rate of DILI in this study. [ 5] Large clinical trials using azithromycin reported acute, flash, and asymptomatic increases in serum ALT situations in 1 – 2 of cases. Azithromycin has been associated with DILI in cases with COVID-19 infection, with <6>
Methods –
1. A Comprehensive Analysis Using the Roussel Uclaf Causality Assessment Method.
Evidence-grounded information on hepatotoxicity, still, isn't readily available from a single source. Several clinical scoring systems have been enforced since 1992 to give a standardized reason assessment on suspected DILI cases. [12] Presently, the Roussel Uclaf Causality Assessment Method( RUCAM)( also designated the CIOMS scale) is extensively used to assess the probability of DILI, although this system has limitations. [13] The RUCAM/ CIOMS scoring system assigns weighted scores to clinical and laboratory data in seven disciplines time to onset ( from launch and conclusion of the intertwined drug), time to enzyme normalization after the conclusion of the drug(> 50 enhancement), threat factors, attendant medicine use, indispensable non-drug-affiliated causes of liver injury, former information on hepatotoxicity of the medicine, The probability of DILI( i.e. definite/ largely probable, probable, possible, doubtful or barred) is also determined grounded on the total scores from all the disciplines. We used lists of ( i) drugs arbitrated as causes of DILI by study groups in Spain, Sweden, and the US;( ii) drugs intertwined as causes of ALF by the study groups in these three countries; and( iii) in Europe or the US, drugs that have been subordinated to serious nonsupervisory conduct due to hepatotoxicity and response tore- administration, which can be either purposeful or accidental. [14] Classification of liver injury is needed for reason assessment of suspected DILI and HILI cases by the streamlined RUCAM. Note the above peak from hepatic origin only. Abbreviations peak, alkaline phosphatase; ALT, Alanine aminotransferase; DILI, Drug-induced liver injury; HILI, Herb-induced liver injury; N, Upper limit of normal; R, rate; RUCAM, Roussel Uclaf Causality Assessment Method
Limitations-
RUCAM has not been designed for chronic DILI and HILI or when a suspected injury occurs on pre-existing liver complaint, both complex conditions where an expert panel of hepatologists would give a more accurate approach, especially for the timing of the events and the rejection of indispensable causes. Drugs linked as Causes of Liver Injury at the Three DILI Registries Overall, 369 drugs were linked in the three DILI registries as causes of liver injury. Among the 369 drugs, 50 drugs classified as herbal drugs, salutary supplements, or indispensable drugs were set away for unborn investigation and barred from this analysis, leaving 319 pharmaceuticals. Drugs were linked as a cause of liver injury in five or further cases in the three registries( 76 drugs). Drugs linked at the three spots showed Indigenous divergence. Thirty- one medicines were linked in all three registries. Among the 107 pharmaceuticals, 62 drugs were linked in arbitrated medicine-convinced ALF cases either in Spain or Sweden, using the RUCAM/ CIOMS scoring system. Regional divergence of intertwined medicines in the ALF cases was apparent. Only 9 medicines were intertwined in all three countries( 13 in both the US and Spain, 9 in both Spain and Sweden, and 25 in both the US and Sweden). Of the 107 medicines intertwined as a cause of ALF, 6 were associated with serious nonsupervisory conduct( suspense or pullout) either in the US or Europe, and 82 were linked in the three. DILI registries and 102) have been reported in the published literature as being a cause of liver injury. 104 medicines were reported in the WHO database as being associated with at least one liver injury case, and 101 medicines were reported as being associated with at least one ALF case. Among these, 52 were associated with a disproportionally advanced reporting frequency of liver injury events( 44 medicines for ALF) compared with the anticipated frequency [15]
2. Vigi Base –
Vigi Base is the WHO global database of adverse event reports for drugs and vaccines. It's the largest database of its kind in the world, with over 35 million reports of suspected adverse effects of drugs submitted by member countries of the WHO PIDM since 1968. VigiBase is a World Health Organization's ( WHO) global Individual Case Safety Report( ICSR) database that contains ICSRs submitted by the sharing member countries enrolled under WHO's transnational medicine monitoring program. It's the single largest medicine safety data depository in the world. Since 1978, the Uppsala Monitoring Centre ( UMC; established in Uppsala, Sweden) on behalf of WHO, has been maintaining VigiBase. Vigibase is used to gain information about the safety profile of a medicinal product.
These data are used by medicinal diligence, academic institutions, and nonsupervisory authorities for statistical signal discovery, streamlining periodic reports, ICSR comparisons with company databases, and studying the reporting patterns. The data(pre-dominantly post- marketing serious and non-serious cases) is collected from each of its 110 member countries which presently comprises over 10 million ICSRs. About a hundred thousand ICSRs are added each time. [16] (ICSR) database system, VigiBase, was used to gain reporting frequency of the liver events related to the medicines linked in this study. The WHO International Drug Monitoring Programme started in 1968 and is presently developed and maintained by the Uppsala Monitoring Centre (UMC), Uppsala, Sweden, on behalf of the WHO. The database holds further than 5 million ICSRs since 1968 from further than 80 countries worldwide and has been used as a data source for DILI exploration. The maturity of ICSRs in the database were entered from Europe and North America, and include both nonsupervisory and voluntary sources of reporting, depending on each country’s pharmacovigilance system. Some of the medicines are linked by further than one name( i.e. general and brand name) in the WHO database. In similar cases( e.g. amoxicillin/ clavulanic acid), data were combined and anatomized as pooled data 2001- launch of Vigibase Online design, - 100th country joins the WHO Programme for International Drug Monitoring. 2014- Over 10 million adverse responses were reported in VigiBase. Also started to include a larger volume of further regular cessions from China. The reporting frequency data in VigiBase were calculated as Empirical Bayes Geometric Mean( EBGM) with 90CI using the Multi- Item Gamma Poisson Shrinker ( MGPS) system. MGPS is an empirical Bayes data- mining system that uses all of the data on medicines and events in a particular database to describe safety signals.
Limitations and strengths This study has major strengths. First, to the stylish of our knowledge, no other former study has explored Swiss DILI cases in the WHO global pharmacovigilance database VigiBase ™. Due to indigenous differences in defining and healthcare systems, or ethical differences, medicine- related hepatic diseases vary from country to country. A study using DILI registries from different countries set up significant differences in the linked medicines causing DILI in the colorful regions. Data from other countries may therefore not inescapably apply to Switzerland. Second, analysis of a global pharmacovigilance database gives perceptivity into real-world data and therefore into events being in diurnal routine use. still, studies grounded on a robotic reporting system have some limitations. It needs to be emphasized that the VigiBase ™ data aren't applicable for judging the reason between a drug and a response. Only limited data can be uprooted and delved with this type of database. therefore, assessing an unproductive relationship isn't possible since data regarding the temporal relationship, as well as information concerning de-/ rechallenges, are missing. also, information on possible comorbidity or other factors affecting hepatic function( e.g., alcohol consumption) is constantly not available. Underreporting or picky reporting is a further limitation of the robotic reporting system increased reporting doesn't inescapably indicate that a drug is causing a certain adverse drug response more constantly. Reporting may be poisoned, for case, by media reports or by authority warnings. likewise, data are missing in numerous ICSRs. therefore, the results need conservative interpretation owing to the limited information available. In our study, information regarding the suggestion/ administration of medicines and “ conduct taken ” was especially meager. Another constraint of pharmacovigilance databases is the threat of. Duplicates. still, the algorithm vigiMatch, as well as the homemade hunt for duplicates, allowed us to exclude as numerous duplicates as possible. Another limitation is the miscellaneous attestation quality of the submitted reports. Grounded on some principles of the original RUCAM as the first liver-specific system, three other liver-specific styles were developed including the scale of Maria and Victorino( MV), the TKK scale named after the first three authors Takikawa, Takamori, Kumagi et al., and the DILIN system of the DILIN group. For colorful reasons, each of these three styles is still limited to the use of their authors due to major failings. MV Scale In an attempt to ameliorate the original RUCAM, the MV scale was developed by deleting laboratory particulars and adding clinical rudiments, along with simplifying and changing the relative weight of rudiments in their algorithm, As a docked and modified interpretation of the original RUCAM, the MV scale has smaller specific criteria; evaluates dechallenge as the time necessary for ALT or peak to fall below 2N; and considers a shorter quiescence period. It also asks for less accurate rejection criteria of indispensable causes; ignores attendant drug or condiment use; emphasizes drugs with more than five times of marketing without published hepatotoxicity and overestimates extrahepatic instantiations. Despite these major variations, the performance pointers( particularity and perceptivity) including prophetic values, and confirmation using a gold standard aren't available for the MV scale. Compared to the streamlined RUCAM, the MV scale shows major differences. Considering also critical commentary on failings the MV scale isn't generally recommended for assessing reason in suspected DILI and HILI cases and is not a cover for the RUCAM [17] . TTK Scale The TTK scale was established for DILI cases specifically in Japan [18] and is another attempt to modify the original RUCAM with different evaluations of the report, rejection of comedication, addition of the medicine lymphocyte stimulation test( DLST), and eosinophilia in their assessment. The TTK scale is extensively used in Japan, as recently reviewed.
Limited access and lack of standardization have averted general clinical use of the DLST and accordingly TTK scale operations outside Japan; this may be due to methodological difficulties with false positive and false negative DLST [19] For clinicians, the TTK scale can not replace the original RUCAM [20]
3. DILIN Method
Members of the DILIN group handed their assessment system [21] which is liver- specific since it used numerous core particulars of the original RUCAM still, it ignores missing important particulars [22] and lacks a system that scores crucial rudiments, as does the original RUCAM or the streamlined RUCAM Since individual importing and scoring of crucial particulars are lacking and undiscussed, results published by the DILIN group using their system aren't transparent and not available for reassessment. This lack of scoring is one of the most disturbing failings of this system as bandied in detail before. With limited use by other authors in the DILI literature, the DILIN system requires an expert panel on the expert’s opinion, in discrepancy with the original RUCAM and the streamlined RUCAM. Accordingly, the DILIN system isn't available to croakers in need of early results for remedial opinions and is in no way an applicable cover for RUCAM . The DILIN system wasn't validated by any established gold standard as the original RUCAM. Liver Unspecific Styles As opposed to the liver-specific core rudiments of the original RUCAM and the streamlined RUCAM, similar rudiments aren't part of other styles that were established to assess reason in cases with all kinds of adverse events but not specifically those being in the liver.
Among these liver unspecific styles are the Naranjo scale [23] the WHO global soul- searching system, the WHO system in short [24] the ad hoc approach [25] , and the KL system named after Karch and Lasagna [26] Unexpectedly, all four styles were applied in suspected DILI or HILI cases, with a preference for the first three , which thus were compared with the liver- specific styles. Regulatory agencies appear to prefer these three unspecific styles in liver injury cases since this ensures high case figures attributed to medicines or sauces despite low data quality, as bandied in detail for the Naranjo scale, the WHO system [27] And the ad hoc approach, and as to be bandied for the recent ad hoc approach. All liver unspecific styles are obsolete for reason assessment of DILI and HILI cases. Likewise, largely questionable are assessments grounded on MedWatch cases [28] with their known poor data quality as reported in the maturity by professionals. Thus, inadequately proved cases and the use of unhappy reason assessment styles constitute a dangerous combination that doesn't meet the conditions of solid nonsupervisory work. Naranjo Scale The use of the liver unspecific Naranjo scale in suspected DILI and HILI cases is problematic as criteria of hepatotoxicity and re exposure conditions, specific time to onset, criteria for recovery time, and critical judgments to count aren't indeed unplanned. It also relates poisonous medicine responses to general pharmacological medicine conduct rather than to idiosyncratic responses like rare DILI or HILI. The particulars include medicine attention and monitoring, cure relationship including dwindling cure, placebo response, and cross-reactivity, using unidentified objective substantiation. Since these particulars are inapplicable for DILI and HILI, they've lower perceptivity for rare and idiosyncratic responses current in liver injury. In addition, this scale results in a possible reason indeed in the absence of essential data, by the case of simply taking the questionable agent. Problems related to the Naranjo scale were also not resolved when the United States Pharmacopeia ( USP) used its own modified, docked, and not validated Naranjo interpretation with only five rather than the original ten particulars. Lacking test validity and reproducibility, the use of this system has raised concern about judgment validity by the USP, In substance, the use of the Naranjo scale for suspected DILI and HILI shouldn't be recommended presently. Naranjo Algorithm for assessing medicine reason in a medical adverse event – The scoring is 9, certain ADR; 5- 8, probable ADR; - 4, possible ADR; 0, doubtful. [29]
|
Questionnaire
|
Yes
|
No
|
Don’t Know
|
|
Have there been previous
conclusive reports on this reaction?
|
+1
|
+1
|
0
|
|
Did the adverse event appear
after the suspected drug was given?
|
+2
|
-1
|
0
|
|
Did the adverse reaction improve when the drug was discontinued
or a specific antagonist was given?
|
+1
|
0
|
0
|
|
Did the adverse reaction appear
when the drug was re- administered?
|
+2
|
+1
|
0
|
|
Are there alternative causes that
could have caused the reaction?
|
+1
|
+2
|
0
|
|
Did the reaction reappear when
the placebo was given?
|
+1
|
+1
|
0
|
|
Was the drug detected in any
body fluid in a toxic concentration?
|
+1
|
0
|
0
|
|
Was the reaction more severe when the dose was increased, or less severe when the dose was
decreased?
|
+1
|
0
|
0
|
|
Did the patient react similarly to the same or similar drugs in any
previous exposure?
|
+1
|
0
|
0
|
|
Did any objective evidence
confirm the adverse event?
|
+1
|
0
|
0
|
4. WHO Method
The WHO method was developed for general adverse events and isn't liver- specific, wasn't developed or validated for DILI or HILI cases, and doesn't consider hepatotoxicity-related characteristics. These failings have raised major enterprises and led to the conclusion that this scale is neither applicable for reason assessment in suspected hepatotoxicity cases nor has its advantages over other reason algorithms. The WHO system is heavily disputed and wasn't specifically mentioned, addressed, or bandied as a reason assessment system for hepatotoxicity cases in applicable reports including a recent statement of the NIH LiverTox. This system is obsolete for a hepatotoxicity case assessment. . announcement Hoc Approach No specific reason assessment system was used in two-thirds of published DILI cases( 30], inferring that some kind of a clinical ad hoc approach tried to classify the reason of a case with its given failings including lack of specific and validated hepatotoxicity criteria and missing a scoring system. When using this ad hoc approach, the croaker may note the coexistence of herbal or chemical medicine use and will estimate the liability of a hepatotoxic response.
Results of these prima outlook evaluations are fragile, disputed, not transparent, and not assessable, as shown by the assessments by the German nonsupervisory agency Bundesinstitut für Arzneimittel und Medizinprodukte, Federal Institute for Medicines and Medicinal Products and the FDA in the USA. Although disputed in connection with the German BfArM, FDA controllers also applied this dubious ad hoc approach retrospectively in MedWatch cases, known for their poor data quality as reported substantially by non-professionals generally not familiar with case details and apprehensive of the specific issues. The most dependable assessment would have been to give transparent data and to include RUCAM with a focus on many well-proved cases, reducing the threat of overreporting and furnishing scientific quality rather than likely unjustified case volume. Reports grounded on the ad hoc approach are rather disappointing and the system shouldn't be applied nor recommended for suspected DILI and HILI reason assessment. Drug- convinced hepatotoxicity is in the general population a rare adverse drug reaction but is the most common cause of acute liver failure and has been the leading cause of pullout of medicines from the request.
RESULTS-
Drugs linked as Causes of Liver Injury at the Three DILI Registries Overall, 369 medicines were linked in the three DILI registries as causes of liver injury. Among the 369 drugs, 50 drugs classified as herbal drugs, salutary supplements, or indispensable drugs were set away for unborn disquisition and barred from this analysis, leaving 319 medicinals. Medicines linked at the three spots showed Indigenous divergence. Thirty- one medicines were linked in all three registries. Concordance of The linked medicines was advanced between European registries The number of linked cases for each suspect medicine at the three registry spots also varied. amoxicillin/ clavulanic acid , diclofenac , cotrimoxazole , isoniazid ) and disulfiram , Flucloxacillin and erythromycin were also constantly linked, but not constantly among the three spots. Flucloxacillin, erythromycin, and disulfiram were especially current in the Swedish registry, while in the Spanish registry and DILIN, amoxicillin/ clavulanic acid was the most current . In the US, nitrofurantoin was also current, but not in the other registries. Similarly, flutamide, ibuprofen, fluvastatin and the combination remedy for tuberculosis( i.e. rifampicin( rifampin)- isoniazid- pyrazinamide) were especially current in the Spanish registry. Medicines Subject to Serious Regulatory Conduct in the US or Europe Forty-seven medicines were suspended or withdrawn primarily due to hepatotoxicity in the US or Europe . medicines suspended or withdrawn( 21- 24) due to other( primary) reasons( but also flaunting hepatotoxicity) were n't included in this analysis. These included amineptine dependence), amodiaquine( agranulocytosis), glafenine( severe antipathetic responses), chlormezanone ( severe cutaneous responses), sulfacarbamide ( renal, dermatological and hematological responses) and zimeldine( acuity, neurological response). The 47 medicines were suspended or withdrawn due to hepatotoxicity in the US or Europe. Among the 47 medicines, 45 were retailed in at least one European country, while only 16 were retailed in the US. Fourteen medicines were available both in the US and Europe. Among these, 11 were suspended or withdrawn both in the US and Europe. Another three medicines( tolcapone, alatrofloxacin, and trovafloxacin) were suspended or withdrawn in Europe, while in the US, the product information for these three medicines included either confined suggestions or a ‘ black’ warning. Grounded on our hunt, several medicines other than the three mentioned above are still available away( e.g. benzarone, iproniazide, fipexide, moxisylyte, nimesulide, nialamide, and tolcapone). By remedial class, anesthetics were the most current( 11 medicines), followed by antidepressants ( 6 medicines). Only six medicines of the 47 medicines were included in the list of medicines linked in all three DILI registries( see former section). Forty were reported as causes of hepatotoxicity in the published literature, and 30 were reported in the WHO database.
Treatment/ Management-
The top treatment for medicine-convinced hepatotoxicity is the junking of the offending agent. N- N-acetylcysteine ( NAC) is the treatment for natural DILI secondary to acetaminophen toxin, as this promotes the rejuvenescence of glutathione, leading to the detoxification of the poisonous metabolite. The other specific remedy that's available is L- carnitine for valproic acid overdose. Glucocorticoid remedy is generally used when the histological appearance of DILI resembles that of autoimmune hepatitis. For this reason, it has a limited part and generally doesn't change the course of recovery. Characteristic curves are similar to corrosiveness acid sequestrants for cholestatic DILI or antihistamines for pruritis and can be used with some efficacity. Hospital admission is needed for cases with signs or symptoms of DILI progression or ALF. However, early liver transplant consideration is essential because there's high mortality with ALF If ALF is suspected. An important fresh aspect of the operation is reporting cases of DILI to nonsupervisory bodies to estimate if the suspected medicine needs to be withdrawn from the request. The Drug- Induced Liver Injury Network( DILIN) was developed to advance the exploration of DILI through a prospective clinical trial of cases. It created the DILIN Causality Scoring System to understand better the etiology, pathogenesis, threat factors, and issues of medicine-convinced hepatotoxicity.
DISCUSSION-
DILI is a rare circumstance, although it can be serious and occasionally indeed fatal. As similar, nonstop monitoring of liver adverse medicine responses is necessary. The performance of the discovery algorithm for DILI could be bettered in the future. In addition to laboratory findings and individual canons, the other signals have been tested for their utility in detecting DILI. CDW is a useful database on active surveillance of ADR and DILI, it's limited to a single institution. Thus, to assess the threat of DILI, a rare event of ADR, in a large population, the discovery algorithm needs to be applied in multiple institutions. A current and comprehensive list of medicines associated with hepatotoxicity was created by uniting clinical DILI information from different sources. This multifaceted analysis of hepatotoxicity revealed several important findings, which give a base for implicit unborn disquisition. The VigiBase data resource was used to associate the medicines linked as causes of hepatotoxicity with reporting frequency of liver safety events( ‘ overall liver injury’ and ‘ ALF’). There are several well-known essential limitations to the operation of the reporting frequency data in pharmacovigilance systems. The limitations include reporting bias, quality of data, and confounding goods by medications and/ or other reported adverse events. A high relative reporting rate doesn't inescapably indicate a high prevalence of the event or suggest an unproductive relationship between the medicine and the event since the values can be told by reporting bias, reporting frequency of other events( e.g. liver vs order and skin events), and constantly used-medications. For cases, pharmacovigilance data are subject to influence by mindfulness of adverse events( i.e. reporting bias) through publication of cases and reports of nonsupervisory conduct. In agreement, our analysis showed that the presence of case reports or serious nonsupervisory conduct among the hepatotoxic medicines linked in this design was associated.
CONCLUSION –
In conclusion, pharmacovigilance systems are extremely important to assess the actuality, frequency, and soberness of apparent ADRs that are only known when a medicine is administered to a large population. Medicine- convinced hepatotoxicity is the most frequent cause of acute liver failure in developed countries and one of the most frequent in developing countries and has been the leading cause of pullout of medicines from the request. It’s becoming a serious health problem that affects all the actors involved in the health system In summary, this transnational cooperative work provides the most current, comprehensive list of medicines associated with liver injury in arbitrated or well-vetted cases. Known hepatotoxicity is one of the essential rudiments of the RUCAM scoring styles thus, this list should help clinicians in the clinical opinion of DILI by furnishing similar information as a single resource. Likewise, the information on reporting frequency in the WHO database provides a multifaceted ‘ weight of substantiation’ view of hepatotoxicity. To further increase the operation of this Information in clinical practice and exploration, a web- grounded searching tool for the linked hepatotoxins( www.spanishdili.uma.es) has been developed to partake the information collected in this design. In conclusion, this paper provides a multifaceted assessment of medicines intertwined with hepatotoxicity. We believe this information can grease the accurate clinical opinion of DILI and enhance exploration sweats to clarify the relationship of medicines to liver injury
[31] medicine- convinced liver injury( DILI) is the main cause of medicine pullout from the request. It is an adverse response that indeed when it wasn't observed in clinical trials, can be later detected when retailed. This study aimed to identify DILI frequency and issues assessing colorful pharmacovigilance strategies [32] Backing- The authors declare that no backing was entered for the present study Acknowledgments I would like to express my sincere gratefulness to all those who contributed to the completion of this review on drug-induced hepatotoxicity. Special thanks to my mentors for their precious perceptivity and guidance throughout the review process. I'm thankful to him for his stimulant and understanding during this bid. I also appreciate the support from “The Ashokrao Mane Institute Of Pharmacy ” Which Handed Me Access To Crucial Resources and literature.
REFERENCES
- Kim SH. Active pharmacovigilance of drug-induced liver injury using electronic health records. Allergy, Asthma & Immunology Research. 2020 May 1;12 (3):378-80.
- Moore TJ, Cohen MR, Furberg CD. Serious adverse drug events reported to the Food and Drug Administration,1998-2005. Arch Intern Med 2007Sep 10; 167 (16): 1752-9
- Kang Y, Kim SH, Park SY, ParkQ BY, Lee JH, An J, et al. Evaluation of drug-induced liver injury developed during hospitalization using electronic health record (EHR)-based algorithm. Allergy Asthma Immunol Res 2020;12:430-42
- Kim SH. Active pharmacovigilance of drug-induced liver injury using electronic health records. Allergy, Asthma & Immunology Research. 2020 May 1;12(3):378-80.
- in patients with COVID-19 Beigel, J.H.; Tomashek, K.M.; Dodd, L.E.; Mehta, A.K.; Zingman, B.S.; Kalil, A.C.; Hohmann, E.; Chu, H.Y.; Luetkemeyer, A.;
- Kline, S.; et al. Remdesivir for the Treatment of Covid-19—Final Report. N. Engl. J. Med. 2020, 383, 1813–1826.
- Mahamid, M.; Mader, R.; Safadi, R. Hepatotoxicity of tocilizumab and anakinra in rheumatoid arthritis: Management decisions.
- Muhovi´c, D.; Bojovi´c, J.; Bulatovi´c, A.; Vuk?cevi´c, B.; Ratkovi´c, M.; Lazovi´c, R.; Smolovi´c, B. The first case of drug-induced liver injury associated with the use of tocilizumab in a patient with COVID-19. Liver Int. 2020, 40, 1901–1905.
- Serviddio, G.; Villani, R.; Stallone, G.; Scioscia, G.; Foschino-Barbaro, M.P.; Lacedonia, D. Tocilizumab and liver injury. Ther. Adv. Gastroenterol. 2020, 13, 1756284820959183.
- Batteux, B.; Bodeau, S.; Gras- Champel, V.; Liabeuf, S.; Lanoix, J.P.; Schmit, J.L.; Andréjak, C.; Zerbib, Y.; Haye, G.; Masmoudi, K.;et al. Abnormal laboratory findings and plasma concentration monitoring of lopinavir and ritonavir in COVID-19. Br. J. Clin.Pharmacol. 2021, 87, 1547–1553
- Sulkowski, M.S. Drug-induced liver injury associated with antiretroviral therapy that includes HIV-1 protease inhibitors. Clin. Infect. Dis. 2004, 38 (Suppl. 2), S90–S97.
- Andrade RJ, Camargo R, Lucena MI, et al. Causality assessment in drug-induced hepatotoxicity. Expert Opin Drug Saf 2004 Jul; 3 (4): 329-44.
- Lucena MI, Garcia-Cortes M, Cueto R, et al. Assessment of drug- induced liver injury in clinical practice. Fundam Clin Pharmacol 2008 Apr; 22 (2): 141-58 Kaplowitz N. Causality assessment versus guilt-by-association in drug hepatotoxicity. Hepatology 2001 Jan; 33 (1): 308-10
- Danan G, Benichou C. Causality assessment of adverse reactions to drugs: I. A novel method based on the conclusions of international consensus meetings: application to drug-induced liver injuries. J Clin Epidemiol 1993 Nov; 46
- Biour M, Ben Salem C, Chazouilleres O, et al.Drug- induced Liver injury; fourteenth updated edition of the bibliographic database of liver injuries and related drugs. Gastroenterol Biol 2004 Aug-Sep; 28 (8-9): 720-59
- DuMouchel W. Bayesian data mining in large frequency tables, with an application to the FDA spontaneous reporting system (with discussion). Am Stat 1999; 53:177-202
- Maria V.A.J., Victorino R.M.M. Development and validation of a clinical scale for the diagnosis of drug-induced hepatitis. Hepatology. 1997;26:664 –669.doi: 10.1002/hep.510260319.
- Takikawa H., Takamori Y., Kumagi T., Onji M., Watanabe M., Shibuya A., Hisamochi A., Kumashiro R., Ito T., Mitsumoto Y., et al. Assessment of 287 Japanese cases of drug-induced liver injury by the diagnostic scale of the International Consensus Meeting. Hepatol. Res. 2003;27:192–195. doi: 10.1016/S1386- 6346(03)00232-8.
- National Institutes of Health (NIH) and LiverTox: Agents Included in LiverTox by Drug Class. [(accessed on 7 November 2015)]
- Teschke R., Eickhoff A., Schulze J. Drug and herb induced liver injury in clinical and translational hepatology: Causality assessment methods, quo vadis? J. Clin. Transl. Hepatol. 2013;1:59–74. Doi 10.14218/JCTH.2013.D002X.
- Fontana R.J., Watkins P.B., Bonkovsky H.L., Chalasani N., Davern T., Serrano J., Rochon J. Drug-induced liver injury Network (DILIN) prospective study. Rationale, design, and conduct. Drug Saf. 2009;32:55–68. doi: 10.2165/00002018- 200932010-00005
- Agarwal V.K., McHutchison J.G., Hoofnagle J.H. Drug-Induced Liver Injury Network (DILIN). Important elements for the diagnosis of drug- induced liver injury. Clin. Gastroenterol. Hepatol. 2010;8:463–470. doi: 10.1016/j.cgh.2010.02.008.
- Naranjo C.A., Busto U., Sellers E.M., Sandor P., Ruiz I., Roberts E.A., Janecek E., Domecq C., Greenblatt D.J. A method for estimating the probability of adverse drug reactions. Clin. Pharmacol. Ther. 1981;30:239– 245. doi: 10.1038/clpt.1981.154
- World Health Organization (WHO) The Use of the WHO-UMC System for Standardised Case Causality Assessment. WHO Collaborating Centre for International Drug Monitoring (Uppsala Monitoring Centre, UMC), Database 2000. [(accessed on 7 November 2015)]
- Kaplowitz N. Causality assessment versus guilt-by- association in drug hepatotoxicity. Hepatology. 2001;3 3:308–310. doi: 10.1053/jhep.2001.21083.
- Karch F.E., Lasagna L. Adverse drug reaction. A critical review. JAMA. 1975;234:1236– 1241. doi: 10.1001/jama.1975.032602500 28021
- World Health Organization (WHO) Assessments of the Risk of Hepatotoxicity with Kava Products. WHO Documentn Production Services; Geneva, Switzerland: 2007
- Teschke R. Black cohosh and suspected hepatotoxicity— Inconsistencies,m confounding variables, and prospective use of a diagnostic causality algorithm: A critical review. Menopause. 2010;17:426– 440. doi: 10.1097/gme.0b013e3181c515 9c
- Naranjo CA., et al. “A method for estimating the probability of adverse drug reactions”. Clinical Pharmacology and Therapeutics 30.2 (1981): 239-245
- Tajiri K., Shimizu Y. Practical guideline for diagnosis and early management of drug-induced liver injury. World J. Gastroenterol. 2008;14:6774– 6785. doi: 10.3748/wjg.14.6774.
- Suzuki A, Andrade RJ, Bjornsson E, Lucena MI, Lee WM, Yuen NA, Hunt CM, Freston JW. Drugs associated with hepatotoxicity and their reporting frequency of liver adverse events in VigiBase™: unified list based on international collaborative work. Drug safety. 2010 Jun;33:503-22
- Becker MW, Fontoura LN, Blatt CR. Pharmacovigilance of drug- induced liver injury in search for frequency and outcomes in a Brazilian hospital: Challenges in future cases using a robust causality assessment method such as the updated RUCAM. Journal of Modern Medicinal Chemistry. 2020;8:65-73.


 Sudhir Aswale *
Sudhir Aswale *
 Pritam Salokhe
Pritam Salokhe
 Nilesh Chougale
Nilesh Chougale

 10.5281/zenodo.14247630
10.5281/zenodo.14247630