Validation is the process of approving documentation proof that shows the subsequent steps will reliably result in the product that will provide the desired outcome. Validation studies, according to GMP, are an essential component of GMP; they must be carried out in accordance with predetermined protocols. Process, testing, and cleaning are the bare minimum that need to be validated in order to establish control procedures that monitor output and validate manufacturing processes that might be causing variability in drug products. One of the key components in obtaining and preserving the final product's quality is validation. The accuracy, sensitivity, specificity, and repeatability of the test procedures used by the companies are provided by the validation research, which will also be established and recorded. The method used in the pharmaceutical business to increase the dosage form's quality and safety is called process validation. According to cGMP, process validation is a crucial component of quality assurance. Together, validation and quality assurance will guarantee the comprehensive quality of the final product. Process validation is essential to the pharmaceutical manufacturing process because it provides a high level of assurance and proof that the procedure being followed produces consistent results, meaning the necessary specifications have been met. This paper aims to provide an overview and broad analysis of validation in the pharmaceutical sector.
Validation, Pharmaceutical Industry, Quality Assurance, GMP, Consistent
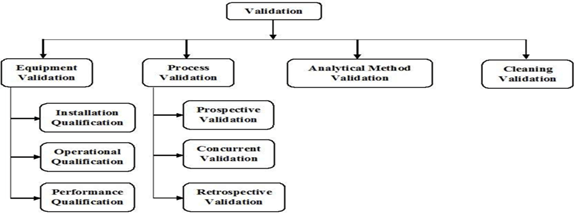
EQUIPMENT VALIDATION: [11,12]
A documented procedure known as "equipment validation" has been developed to show that any piece of equipment is reliable and functionally sound. Equipment validation is based on the idea that it has to be constructed, maintained, and customized in order to perform the necessary functions.
There are four types of equipment validation mentioned below
- Installation qualification (IQ)
- Design qualification (DQ)
- Performance qualification (PQ)
- Operational qualification (OQ)
Installation qualification (IQ)
Installation qualification confirms that the précised equipment has been received and installed as per target and agreement in exact design or format in the undamaged form with parts, spares, services gauges, and other required compounds. It is documental verification of that the equipment has been installed and calibrated appropriately. The purpose of IQ is to ensure that all the aspects of the equipment are installed correctly match with the original (URS) design. As per the manufacture’s recommendations for installation, the working sites working environmental conditions are documented and confirmed that they are suitable for the operation of the instrument .[13]
The documentation of installation includes:
- Details of supplier and manufacture.
- Equipment name, color, model and serial number
- Date of installation and calibration
Design qualification (DQ)
written confirmation that the recommended facilities, systems, and equipment are suitable for the intended application. GMP design compliance ought to be demonstrated in this certification. The design ideas employed ought to ensure that the apparatus fulfills the GMP objectives. Examining the mechanical drawings and design components that the the manufacturer of the equipment supplied.
Operational qualification (OQ):
An assessment of the equipment's performance capability is conducted through a series of tests. Operational qualification emphasizes equipment performance capabilities above performance demonstration. connected to producing a certain good.
Performance Qualification (PQ):
establishing by objective evidence that the process, under anticipated conditions, consistently produces a product which meets all predetermined requirements.
PQ considerations include:
? Actual product and process parameters and procedures established in OQ.
? Acceptability of the product.
? Assurance of process capability as established in OQ.
? Process repeatability, long term process stability.
PROCESS VALIDATION
"Establishing documented evidence which provides high degree of assurance that a specific process will consistently produce a product meeting its pre-determined specifications and quality characteristics" is how the USFDA described process validation. Process validation offers the manufacturing process controls the flexibility and limitations needed to achieve desired medicinal product quality while preventing undesired traits.[14, 15]
Types of Process Validation: [16]
A) Prospective process validation:
It can be characterized as the documented proof that a system operates as planned and follows a predefined protocol. Before a new product or one made utilizing a modified manufacturing process is released, this validation is typically carried out. Based on the procedures now in use in the commercial domain, the validation method is conducted using validation in the future.
B) Concurrent Validation:
It is related to prospective with the exception that the operational firm will sell the product to the general public during the qualification runs at its market price, and it is also related to retrospective validation. This validation includes product testing and in-process observation of crucial processing steps. This makes it easier to produce and document evidence that the production process is under control. This documentation of the critical processing validation in process is evidence that the production process is under control.
C) Retrospective validation:
In this case, historical data from records of manufacturing batches that have been completed is used to offer the documented evidence that the process has been in a controlled state before the request for such evidence
Analytical Method Validation
Method validation verifies that the analytical process used for a particular test is appropriate for its intended use. The verification of an analytical technique is the means by which it is demonstrated through experimental research that the The method's performance attributes
\fulfill the prerequisite for the intended usage. This suggests that the legitimacy of
A technique can only be shown through research in laboratories [17]. Techniques ought to be confirmed or reaffirmed[18, 19].
- before their introduction and routine use;
- whenever the conditions change for which the method has been validated, e.g., instrument with different characteristics; and
- wherever the method is changed and the change is outside the original scope of the method.
Cleaning Validation : [20]
The cleaning process is validated to make sure that production facility residues are effectively reduced to below a predetermined threshold. Cleaning validation is mostly utilized in the pharmaceutical industry for cleaning process equipment. Cleaning cycles or techniques are analyzed through cleaning validation. Additionally, it should describe how acceptability standards, like Developments were made to the chemical and microbiological parameters, detection limits, and sampling technique selection.
Objective of Cleaning Validation:
Reduction of solvents.
- Increased cleaning equipment and shorter cleaning times.
- Equipment utilization, equipment life extension, and multiproduct.
- Infrastructure, worker safety, and cost-effectiveness are few other objectives.
Benefits of Cleaning Validation:
- Operator safety: Validation enhances operator security. To reduce accidents and boost safety, equipment that has been properly calibrated and approved is used.
- Better Customer Quality: Proper validation helps to decrease market recalls, which leads to better customer service and product quality
Validation Protocol:[21]
The validation protocol should be numbered, signed and dated, and should contain as a minimum the following information
Title
- Objective & Scope
- Responsibility
- Protocol Approval
- Validation Team
- Product Composition
- Process Flow Chart
- Manufacturing Process
- Review of Equipments / Utilities
- Review of Raw Materials and Packing Materials Review of Analytical and Batch Manufacturing Records
- Review of Batch Quantities for Validation (Raw Materials)
- Review of Batch Quantities for Validation (Packing Materials)
- HSE Requirements
- Review of Process Parameters Validation Procedure
- Sampling Location
- Documentation
- Acceptance Criteria
- Summary
- Conclusion
ADVANTAGES OF VALIDATION
- Enhanced reporting capability.
- Improved ability to set target parameters and Control limits for routine production, correlating With validation results.
- Enhanced data and evaluation capabilities and Increased confidence about process Reproducibility and product quality.
- Enhanced ability to statistically evaluate process Performance and product variables e.g. Individuals, mean, range, control limits.
Disadvantages of validation:
- Validation is time consuming process.
- The process for manufacture is often complex and costly.
- Validation also has practical limit and related cost.
- Application of validation[22]
- Reduction of quality cost:
The cost of the subsequent procedures can be reduced with proper validation. a) Preventive expenses may be incurred to lower appraisal costs and avoid failure. b) Inspection, testing, and quality assessment appraisal charges. d) The cost of internal failures
- Safety:
Validation can also result in increased worker safety. Properly standardized, validated instruments and devices are used to reduce accidents and results in safety.
Validation can also result in the increase in operation safety. e.g. instruments used on equipment that intended to operate at certain temperature and pressures must be
dependable i.e. They need to be calibrated.
- Better consumer quality:
Through proper validation, market recall is evaded which results in better consumer care and quality of the
product.
CONCLUSION
Validation is a fully functional quality-accounting instrument that has been demonstrated to provide assurance for the robustness and efficiency of the process. pharmaceutical sectors. Verification is the most often used term in the drug creation, production, and specification of completed goods. It also produces a decrease.in the price associated with process observation, testing and sampling . Aside from everything else, an industry's need for a proven process that consistently produces high-quality products is crucial.


 Prajakta K. Ugalmugale*
Prajakta K. Ugalmugale*
 Vinayak M. Gaware
Vinayak M. Gaware
 10.5281/zenodo.13286695
10.5281/zenodo.13286695