Abstract
Transdermal drug delivery systems (TDDS) represent a feasible choice for the non-invasive, long-term delivery of medication. Herbal permeation enhancers, which capitalise on the innate properties of natural substances to promote drug penetration across the skin barrier, have emerged as significant TDDS adjuncts. By studying the mechanisms behind the activity of herbal permeation enhancers, these abstracts demonstrate how these agents may improve medication bioavailability and therapeutic efficacy. Herbal components that exhibit permeation-enhancing properties include terpenes, flavonoids, and essential oils. These components modulate skin permeability and promote medication solubility. Concerns regarding synthetic enhancers are alleviated by the high safety profiles and biocompatibility of herbal permeation enhancers. Moreover, the synergistic interactions between herbal boosters and pharmaceutical molecules improve effective transdermal dispersion. This talk reviews recent advancements in the use of herbal permeation enhancers in a variety of therapeutic categories, highlighting the significance of these agents in enhancing patient compliance and medication delivery efficiency.
Keywords
TDDS, permeation enhancer, skin, herbal permeation enhancers.
Introduction
The topical administration of drugs to healthy, undamaged skin, either for systemic therapy or for the localised treatment of tissues beneath the skin, is referred to as transdermal drug administration. When designing a dose for transdermal drugs, the goal is to minimise drug metabolism and retention in the skin while also maximising the quantity of medication that gets through the skin and into the bloodstream. [1] A drug or passive polymer matrix that has been dissolved or distributed in the reservoir, an external backing sheet made of paper, plastic, or foil, and pressure-sensitive adhesive that attaches patches to the skin are the essential components of any transdermal distribution system. [2] When orientated medication delivery is paired with the two most important drug delivery criteria rate and extent the efficacy of the therapy is significantly increased. Among the many uses for herbal NDDS, the noteworthy are:
- Make the components more soluble Diminish toxicological consequences.
- Strengthen the medicinal impact.
- The components' absorption by tissue macrophages might also be enhanced because
- the lipoidal substance.
- Targeted, regulated, and sustained discharges are accomplished.
- Stopping the deterioration of the ecosystem. It can occur inside or externally, and related physical and chemical deteriorations can be prevented. [3]
A few of the many variables that influence the rate of drug transport across the skin include the drug's thermodynamic activity in the formulation, the drug and formulation's interaction with the skin, and changes in the skin with age, race, anatomical location, and illness. [4] To sustain the intended drug level for an extended period of time, drug release from the transdermal drug delivery system may follow first order, zero (or pseudo-zero order), or both dynamics. [5] The use of herbal treatments has attracted attention from people all over the world because to its noteworthy medicinal advantages, attractive appearance, and improved patient compliance. Knowledge of the ethnobotanical use of these plants by indigenous societies is beneficial for maintaining customary medicinal procedures, protecting biodiversity, and promoting health sector. These plants are widely accessible all over the world and number in the thousands. These plants include, among others, Allium sativum, Carica papaya, Murraya Koenigii, Azadirachta indica, Withania somnifera, and Aloe vera. [6] Due to their less optimal excipient selection as compared to synthetic therapies, the use of herbal drugs is now restricted.[7] However, the efficacy of the treatment has been diminished due to the outdated and antiquated technique of drug administration used to administer the herbal cure to the patient. The use of cutting-edge medication delivery technology in herbal therapy has promise for improving efficacy and reducing the negative effects of various herbal constituents and herbs. [8]
Advantages of Transdermal Drug Delivery System
- It is often recognised that for certain conditions, herbal medicines work better than conventional forms of medication. Unless they are mixed with other chemical components, it is acknowledged that they are completely natural.[8]
- One of the key benefits of taking herbal medication is the lack of side effects. They typically offer enduring benefits for overall health as well. [8]
- Obesity is known to provide major health concerns to an individual. This is becoming a bigger problem. Herbal medication can be a very efficient solution to handle the obesity problem with very little time and effort. [8]
- TDDS can stop issues with gastrointestinal drug absorption by avoiding stomach pH, enzymatic activity, and drug interactions with food, drink, and other oral drugs.[2]
- TDDS may inhibit the chemical's deactivation by liver and digestive enzymes by preventing drug compounds from entering the systemic and portal circulation during the first pass, which happens after gastrointestinal absorption. [2]
- Because of continuous advancements in the industry and the capacity to transfer the medication to the site of action without creating any changes to the skin, transdermal delivery is quickly becoming one of the most generally recognised ways of drug administration.[9]
- Giving drugs to people who are not aware.[9]
Disadvantages of Transdermal Drug Delivery System
- The drug must have a few advantageous physiochemical properties in order to cross the stratum corneum. [10]
- Transdermal distribution will be very difficult if the medicine's total daily dosage for therapeutic purposes is more than 10 mg. [10]
- TDDS may be used with drugs less than 500 Dalton. [11]
- Poisoning can occur when herbal treatments are utilised improperly, that is, when the wrong part of the plant is employed.[12]
- Because herbal products are not strictly regulated, consumers could buy inferior herbs. The quality of herbal products might vary between producers, brands, or batches. This might make figuring out how much of a herb to take even more difficult.
- In comparison to traditional treatment, the healing process usually takes longer. Taking herbal medication requires a great deal of patience. [12]
- People occasionally switch to herbal therapy without realising that there may be a completely other cause for their problems. As opposed to conventional medication, which necessitates continuous health monitoring, herbal medications can be taken without a prescription. Because of this, people who use herbal medications may go through a trial-and-error phase. [8]
Ideal Properties of Transdermal Drug Delivery System [10]
- The drug has a two-year shelf life, a smaller patch size (less than 40 cm2), and a controlled dosing frequency (once a day to once a week).
- Sufficient for cosmetic purposes (i.e., a transparent, white hue). Simple packaging (i.e., the least amount of pouches and steps required to put the system in place). Simple to remove (ideal for younger patients and older adults).
- Enough skin adhesion (i.e., no peeling off during the dosing interval and simple removal without causing harm to the skin).
- No residue refers to the absence of cold flow around the patch's edge during storage, after it has been applied to the skin or underneath it after removal.
Skin Anatomy and Skin as a Barrier to Drug Permeation
A] Skin Anatomy and Physiology:
Anatomically, skin has four different layers of tissue:
- Stratum corneum (SC)
- Epidermis
- Dermis
- Hypodermis
Stratum Corneum
It is also known as the nonviable epidermis, the outermost layer of skin. The stratum corneum serves as the actual skin barrier to the majority of dangerous substances. The SC has an overall thickness of 10–20 cell layers. Every cell has a length of -34–44. The dimensions of each cell are 0.5–0.20 micrometres in thickness and 25–36 micrometres in breadth. The surface area of a cell is between 750 and 1200 micrometres. Proteins make about 2.7585% of SC, while lipids make up 5- 15% of it. Lipids include phospholipids, glycosphingolipids, cholesterol sulphate, and neutral lipids. Keratin is the most common kind of protein.
Viable Epidermis
Viable epidermis lies between the SC's skin layers and dermis. The thickness is between 50 and 100 micrometres. The cell structures in this layer are similar to those of other living tissues in terms of physiochemistry. The cells are joined by tonsils. The densities of water and viable epidermis are almost comparable. Water makes up ninety percent of the sample.
Dermis
The dermis is the layer that lies underneath the viable epidermis. The structure of dermal fibrils is structural. a fibrous protein matrix that is fixed in an unclear foundation. Subcutaneous Connective Tissue: The hypodermis is the layer of tissue underneath the skin. Its composition is a layer of loose, connective, white fibrous tissue. The only layer with sweat-secreting pores, lymphatic and blood vessels is the hypodermis. A lot of scientists think that drugs go through the skin, get into the bloodstream, and then end up in the hypodermis. [14]

Figure 1. Diagrammatic representation of the cross-section of the human skin.
B] Skin as a Barrier:
The SC, viable epidermis, and dermis work together to prevent molecules from entering. In SC, the drug that enters the skin faces the most resistance. It is made up of the keratinised, flat remains of epidermal cells that are actively growing. It functions as a robust, pliable, hygroscopic, yet water-impermeable membrane. [15] Situated in a lipophilic matrix and forming a lamella of keratin-filled corneocytes, the SC is a distinct bio-membrane in terms of both composition and structure. The lipids are arranged in tightly packed bilayers due to the high degree of hydrogen bonding. There exist several distinctions among the lipids within this extracellular matrix. [16]
- They provide the only continuous phase and diffusion conduit from the skin's surface to the SC base.
- In the absence of phospholipids, the characteristic composition of bio-membranes is composed of cholesterol, free fatty acids, and ceramides.
- Despite the lack of the polar bilayer that produces lipids, SC lipids are still present as multilamellar sheets.
- Saturated long-chain hydrocarbon tails primarily generate highly organised interdisciplinary structures.
- The emergence of gel-phase membrane domains as an alternative to the more prevalent, fluid, and permeable liquid crystalline membrane systems.
Routes for Drug Absorption through Skin
There are several ways to absorb a medication via the skin, depending on its physicochemical properties. Different methods apply to the absorption of drugs that are lipophilic or hydrophilic. Drugs cannot be absorbed through the skin's top stratum corneum, but they can enter the body and travel down many absorption routes to reach the systemic circulation more easily.
- Trans-follicular Route
The trans-follicular pathway is the quickest way for a medication to enter the systemic circulation and provides a large area for drug dispersion. Numerous sweat, oil, and hair follicles as well as pores are found on skin, and they open via ducts to the skin's outside surface. Drug transport by these ducts offers a continuous route across the stratum corneum; nevertheless, the effectiveness of this route depends on several factors, such as gland secretion and the amount and makeup of it. However, the trans-appendageal route contributes very little, accounting for only 0.1% of the skin's surface area. [17]
- Transcellular Route
Although corneocytes have highly hydrated keratin that forms a hydrophilic route, the medication is administered through this channel. The cells surrounding the corneocytes are connected by lipids. A medication must thus pass through many diffusion and partitioning processes. It is the pathway that many types of drugs most commonly use. The transcellular method allows the medication to enter the cell through the cytoplasm, or matrix. This is one technique to take hydrophilic drugs. The drug penetrates and leaves the corneocytes that line the stratum corneum. The highly hydrated keratin provides an aqueous channel for the hydrophilic medications. For the medication to get through the cell matrix, it must go through many phases of diffusion and partitioning. [18]
- Intercellular Route
As the name implies, the medication diffuses throughout the continuous lipid matrix that is present in the intercellular channel, which separates the cells. The convoluted structure formed by corneocytes is responsible for the barrier quality of this pathway; for the drug to pass through the alternating lipid and aqueous domain, it must diffuse to the inner side of the lipid bilayer. This route requires water to travel 50 times further than it does, hence it is best suited for uncharged lipophilic medications. [18]
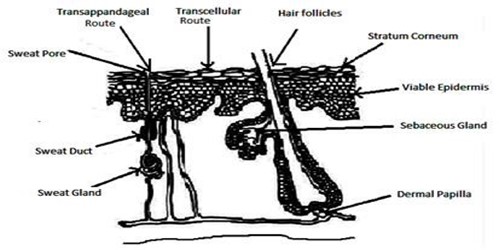
Figure 2: Skin showing route of absorption
Permeation Enhancers
Compounds known as "permeation enhancers" work with the elements of the stratum corneum (SC), the skin's outermost and rate-lim
iting layer, to make the SC more permeable. Researchers in the pharmaceutical sector are highly motivated to develop physical, chemical, and organic approaches to enhance the medicinal medications' percutaneous absorption. There are two main types of penetration enhancers available: [19]
a. Artificial Permeation Inhibitors
b. Enhancers of Natural Permeation
Synthetic Permeation Enhancers:
Chemicals referred to as sorption promoters or accelerants can temporarily compromise the skin's barrier, hence increasing drug flow. Many kinds of chemical permeation enhancers are used, including fatty acids, pyrrolidones, oxazolidinones, sulfoxides, Azon analogues, and surfactants. [20]
Natural Permeation Enhancers:
Natural permeability enhancers (NPE) are a helpful class of transdermal drug delivery systems (TDDS) in the pharmaceutical industry. NPEs are a unique type that the pharmaceutical industry is still getting used to. Further investigation is required in order to implement the commercial manufacture of final dosage forms and expand NPE systems. [21]
- Menthol:

(1R,2S,5R) -2-isopropyl-5-methylcyclohexanol

Figure 3: Peppermint Leaves [30]
A terpene alcohol with a distinct minty flavour and aroma, menthol is cooling. It is manufactured synthetically by hydrogenating thymol, or it can be derived from peppermint oil. Ointments containing menthol are used medicinally. Menthol is an organic compound that is synthetically generated from peppermint or other mint oils. When menthol is administered topically, ingested, or breathed, it can generate the well-known cooling feeling because it can chemically activate the skin's cold-sensitive TRPM8 receptors. as a topical analgesic for minor aches and pains such sprains and muscular cramps. Because of the way that menthol affects the skin's barrier properties, there is an eight-fold increase in skin flux throughout the process of enhancing skin permeation. [22]
- Citrus Fruit Peel:

1-methyl-4(1-methylethenyl)-cyclohexane

Figure 4: Citrus Fruit Peel [31]
Limonene, a colourless liquid aliphatic hydrocarbon classified as a cyclic monoterpene, is the main component of the volatile oil recovered from citrus fruit peels. The bioavailability of nicardipine hydrochloride from membrane-moderated transdermal therapy devices was shown to be impacted by limonene in human volunteers. [23]
- Tulsi:

Figure 5: Tulsi Plant [32]
The goal of the current study is to develop a transdermal naproxen gel that incorporates tulsi oil, a natural penetration enhancer, to increase naproxen penetration. Tulsi oil may enhance percutaneous absorption by momentarily changing the stratum corneum's barrier properties, while its precise mode of action is uncertain. This research investigates the effectiveness of basil oil, a volatile oil containing alcoholic terpenes, as a potential penetration enhancer for improved skin permeation of labetalol hydrochloride (LHCl), in contrast to camphor, geraniol, thymol, and clove oil. Basil oil is recommended as a possibly useful penetration enhancer for improved transdermal drug delivery of labetalol. [24]
- Papaya:

Figure 5: Raw Papaya [33]
Papain is an enzyme found in the white fluid (latex) that surrounds raw papaya fruit. Since it is a protease, proteins are broken down by it. The influence of the proteolytic enzyme papain was investigated in this work by examining the penetration of low molecular weight heparin (LMWH) both in vitro and in vivo. An innovative method to improve the oral heparin's absorption and bioavailability is to co-administer papain with heparin. [25]
- Capsaicin:

Figure 6: Chili Pepper [34]
Capsaicin is an active component of chili peppers, which are plants belonging to the genus Capsicum. One of the main alkaloids is capsaicin. in capsaicinoids; Capsaicin is only found in the fruits of the genus Capsicum, which is a member of the Solanaceae family. [26] Capsaicin's ability to boost naproxen absorption was investigated by contrasting it with azone, the traditional enhancer. The skin was treated with varying concentrations of the chosen enhancer before to the experiment. We also looked at and compared the results of two commercially available naproxen gel formulations and a 3?psaicin formulation. It was found that after applying azone and capsaicin to the skin, penetration increased and that capsaicin changed the SC layer. Thus, capsaicin was discovered to augment naproxen's penetration via SC, indicating that it is a somewhat efficient skin enhancer akin to the well-known enhancer azone. [27]
- Eucalyptus Oil:
The general term for distilled oil obtained from the leaves of the Eucalyptus genus, which is farmed globally and native to Australia, under the Myrtaceae plant family, is Eucalyptus oil. Eucalyptus citriodora, Eucalyptus dives, Eucalyptus globulus, Eucalyptus polybractea, and Eucalyptus radiata are among the plants in the Myrtaceae family that produce eucalyptus oil. The process of steam distilling the leaves yields eucalyptus oil. In comparison to the solution of chlorhexidine/isopropyl alcohol alone, the oil enhanced the penetration of chlorhexidine (2% [w/v]) into the dermis and the lower layer of the epidermis when coupled with 70% (w/v) isopropyl alcohol and 10% (v/v) eucalyptus oil. Studies on human skin that was fully thick were done on penetration. [28]
Natural Polymers Used in the Transdermal Drug Delivery System:
Transdermal drug delivery systems (TDDS) primarily consist of polymers, which control the release of medicine from the device. Often referred to as a macromolecule, a polymer is a large molecule composed of repeated structural units. These subunits are often joined by covalent chemical bonds. Due to their ability to localise at the site of action, polymers are used to distribute medications uniformly, reduce dosage frequency, and increase therapeutic efficacy. Three types of polymers may be distinguished among them: natural, semi-synthetic, and synthetic. Specifically, solid monolithic matrix systems, implants, films, beads, microparticles, injectable and inhalable systems, and viscous liquid formulations are all made with plant-derived polymers in pharmaceutical formulations. Polymeric polymers have found application in a wide range of dosage forms. They can be used as gelling agents, suspending agents, binders, film coating formers, thickeners or viscosity enhancers, stabilisers, disintegrants, solubilizers, emulsifiers, and bioadhesives. The majority of transdermal drug delivery methods are made of polymers. The polymers used in TDDS should have strong stability, good compatibility with the drug and other system components, and the capacity to distribute a medication throughout the device safely. [29]
- Gum Arabica/Gum Acacia:

Figure 8: Gum Arabica/Gum Acacia [36]
The sticky, dried exudates from the stems and branches of Acacia Arabica (Combretaceae) or Acacia Senegal (Leguminosae) are referred to as "Indian gum," also known as "acacia gum." Gum Arabic, gummy arabicum, gummi africanum, gum acacia, gummi mimosae, and gummi are synonyms. Acacia gum is composed of potassium, magnesium, calcium, and arabic acid, a high molecular weight glycosidal acid. Gum arabic is a structurally branching molecule consisting of 1, 3-linked ?-D galactopyranosyl units in its main chain. Monosaccharides such as arabinose, glucuronic acid, and rhamnose make up its composition. [29]
- Agar:
Gelidium amansii (Gelidaceae) and a few other red algae species, such as Pterocladia (Gelidaceae) and Grailaria (Gracilariaceae), are the sources of the dried gelatinous substance known as agar, or agar-agar. The two primary polysaccharides that may be isolated from agar are agarose and agaropectin. The components of agarose are 3,6-anhydro-(-)-galactose and (+)-galactose. Agarose is sometimes referred to as the neutral gelling fraction. Agarose is responsible for the gel strength of agar. It comprises around 3.5?llulose and 6% nitrogen-containing substance. Agaropectin, a sulphated non-gelling fraction consisting of sulphonated polysaccharide with partially esterified galactose and uronic acid moieties, is responsible for the viscosity of agar solutions. In short, it is believed to have a complex range of polysaccharide chains with alternating alpha-(1_3) and beta-(1_4) connections and a variable overall charge content. [29]
- Xanthan gum

Figure 9: Xanthan gum powder [37]
The fermentation process of the gram-negative bacteria Xanthomonas campestris produces the high-molecular-weight extracellular polysaccharide xanthan gum. The basic structure of this naturally occurring cellulose derivative is made up of alternating main chain glucose residues connected to a side chain of trisaccharides consisting of alpha-D-glucose, beta-D-glucuronic acid, and beta-D-mannose. Xanthan gum is used in dosage forms of oral controlled-release tablets as a matrix-forming agent and potential excipient. Xanthan gum is an extracellular polysaccharide with a high molecular weight that is produced by the pure-culture fermentation of a carbohydrate using bacteria of Xanthomonas campestris. With its cream colour and high viscosity, this granular gum dissolves easily in hot or cold water, even at low quantities. [29]
- Tragacanth:

Figure 10: Tragacanth Gum [38]
Dividing into This gum comes from the Leguminosae plant Astragalus gummifer. Tragacanthin, the water-soluble part of tragacanth, is composed of arabinogalactan and tragacanthic acid, which together account for 20% to 30% of the plant. Between 60% and 70% of the other sugars that make up tragacanthic acid, such as D-xylose, L-fructose, D-galactose, and D-galacturonic acid, are composed of the water-insoluble component bassorin. The mixture of arabinose and uronic acid that is tragacanthin swells to create a thick gel, whereas bassorin dissolves in water to form a viscous colloidal solution. [29]
- Jackfruit Mucilage:

Figure 11: Jackfruit with white mucilage [39]
Artocarpus heterophyllus, often known as jackfruit, is a member of the Moraceae family. In Hindi, it is called Kathal. This enormous evergreen tree, which may reach heights of up to 1200 meters, is indigenous to the Western Ghats' evergreen forests. The bark covering the straight, cylindrical stem is either smooth or somewhat rough in green or black. The leaves are obovate-elliptic to elliptic, decurrent, glabrous, entire, and measure 5–25 cm x 3.5–12 cm. Fruits are firm, meaty, and have a thicker stem. One of the most popular fruits in South India is jackfruit. Given that jackfruit is largely composed of carbohydrates, it may be the most effective energy source. Fruit pulp hydrolyses to produce glucose, galactose, rhamnose, xylose, arabinose, and galacturonic acid. Due to its special qualities, which include good wettability, water uptake, and swelling property, the polysaccharide from jackfruit has been reported as a pharmaceutical excipient in mucoadhesive formulations; it may be used as a binder in tablets, sustaining agent in matrix tablets, or mucoadhesive material in buccal tablets. Iron, lipids, calcium, phosphorus, and proteins are also present. The seeds are higher in pectin and primarily starchy, with decent levels of proteins, calcium, and thiamine. [29]
CONCLUSION
Herbal permeation enhancers provide an efficient way of increasing the skin-level medication penetration and consequently the effectiveness of transdermal patches. These organic substances offer a useful substitute for artificial boosters in minimising systemic negative effects and boosting absorption. The study emphasises the necessity of continued investigation to tackle issues including herbal enhancer compatibility and stability in patch formulations. Moreover, thorough clinical trials are essential to guarantee their efficacy and safety. Transdermal drug delivery systems have a great deal of potential to improve via the integration of modern pharmaceutical procedures with traditional herbal knowledge, offering more natural and effective treatment options. Future developments in this area may result in better treatment results and more satisfied patients.
REFERENCE
- Misra, A. N. (1997). Controlled and novel drug delivery. In N. K. Jain (Ed.), Transdermal drug delivery (pp. 100-101). CBS Publishers.
- Gaikwad, A. K. (2013). Transdermal drug delivery system: Formulation aspects and evaluation. Knowledgebase Publishers.
- He, Z. F., Liu, D. Y., Zeng, S., & Ye, J. T. (2008). Study on preparation of ampelopsin liposomes. Journal of Chinese Medicine and Materials, 33, 27–30.
- Bhowmik, D., Chiranjib, C., Chandira, M., Jayakar, B., & Sampath, K. P. (2010). Recent advances in transdermal drug delivery system. International Journal of PharmTech Research, 2(1), 68-77.
- Ramteke, K. H., Dhole, S. N., & Patil, S. V. (2012). Transdermal drug delivery system: A review. Journal of Advanced Scientific Research, 3(1), 22-35.
- Dongare, P. N., Motule, A. S., Dubey, M. R., More, M. P., Patinge, P. A., Bakal, R. L., & Manwar, J. V. (2021). Recent development in novel drug delivery systems for delivery of herbal drugs: An update. GSC Advanced Research and Reviews, 8(2), 008–018.
- Marwick, C. (1995). Growing use of medicinal botanicals forces assessment by drug regulators. JAMA, 273(8), 607–610.
- Akki, R., Sri, K. N., Govardhani, K. L., & Ramya, M. G. (2019). Phytosomes: A novel drug delivery for herbal extracts. RJLBPCS, Life Science Informatics Publications.
- Hafeez, A., Singh, J., Maurya, A., Rana, L., & Jain, U. (2013). Recent advances in transdermal drug delivery system (TDDS): An overview. Journal of Scientific Innovation Research, 2(3), 733-744.
- Hardainiyan, S., Nandy, B. C., Jasuja, N. D., Vyas, P., & Raghav, P. K. (2014). The recent innovations in transdermal drug delivery for herbal therapy. Journal of Biomedical and Pharmaceutical Research, 3(3), 88-101.
- Jhawat, V. C., Saini, V., Kamboj, S., & Maggon, N. (2013). Transdermal drug delivery systems: Approaches and advancements in drug absorption through skin. International Journal of Pharmaceutical Sciences Review and Research, 21(1), 1-9.
- Dongare, P. N., Motule, A. S., Dubey, M. R., More, M. P., Patinge, P. A., Bakal, R. L., & Manwar, J. V. (2021). Recent development in novel drug delivery systems for delivery of herbal drugs: An update. GSC Advanced Research and Reviews, 8(2), 8-18
- Mbah, C. J., Uzor, P. F., & Omeje, E. O. (2011). Perspectives on transdermal drug delivery. Journal of Chemical and Pharmaceutical Research, 3(3), 680-700.
- Roy, N., Agrawal, M., Chaudhary, S., Tirkey, V., Dhwaj, A., & Mishra, N. (2017). Review article on permeation enhancers: A major breakthrough in drug delivery technology. International Journal of Pharmaceutical Sciences and Research, 8(3), 1001-1011.
- Gennaro, A. R. (2000). Remington's practice of pharmacy (20th ed., p. 836). Williams & Wilkins.
- Panchagnula, R., Bokalial, R., Sharma, P., & Khandavilli, S. (2005). Transdermal drug delivery systems. International Journal of Pharmaceutics, 293, 213-216.
- Benson, A. E. H. (2005). Current drug delivery systems. Current Drug Delivery, 2, 23-33.
- Vyas, S. P., & Khar, R. K. (2002). Controlled drug delivery: Concepts and advances (1st ed., pp. 411-445). Vallabh Prakashan.
- Morow, D. I. J., Carron, P. A. Mc, Woolfson, A. D., & Donnelly, R. F. (2007). Innovative strategies for enhancing topical and transdermal drug delivery. The Open Drug Delivery Journal, 1, 36-59.
- Loyd, A. V., Popovich, N. J. R., & Ansel, H. C. (2005). Pharmaceutical dosage forms and drug delivery systems (8th ed., pp. 300). B.I. Publications Pvt. Ltd.
- Pathan, I. B., & Setty, C. M. (2009). Chemical penetration enhancers for transdermal drug delivery system. Tropical Journal of Pharmaceutical Research, 8(2), 173-179.
- Lahor, D. W., Chaudhary, V., & Shah, K. S. (2011). Terpenes: Natural skin penetration enhancers in transdermal drug delivery systems. International Journal of Pharma Research and Development, 2(12), 39-45.
- Eccles, R. (1994). Menthol and related cooling compounds. Journal of Pharmacy and Pharmacology, 46(8), 618-630.
- Krishnaiah, Y. S. R., Satyanarayana, V., & Bhaskar, P. (2002). Influence of limonene on the bioavailability of Nicardipine hydrochloride from membrane-moderated transdermal therapeutic system in human volunteers. International Journal of Pharmaceutics, 247(1-2), 91-102.
- Jain, R., Aquil, M., Ali, A., & Khar, R. K. (2008). Basil oil as a promising skin penetration enhancer for transdermal delivery of Labetalol hydrochloride. Drug Development and Industrial Pharmacy, 34(4), 384-389.
- Gabovac, V., Schmit, Z. T., & Bernkop, S. A. (2007). Papain: An effective permeation enhancer for orally administered low molecular weight heparin. Pharmaceutical Research, 24(5), 1001-1006.
- Arora, R., Gill, N. S., Chauhan, G., & Rana, A. C. (2011). An overview about versatile molecule capsaicin. International Journal of Pharmaceutical Sciences and Drug Research, 3(4), 280-286.
- Saini, S., Chauhan, S. B., & Agarwal, S. S. (2014). Recent development in transdermal drug delivery system. Journal of Advanced Pharmacy Education and Research, 4(1), 31-40.
- Karapen, T. J., Conway, B. R., Worthington, T., Hilton, A. C., Elliott, T. S., & Lambert, P. A. (2017). Enhanced chlorhexidine skin penetration with eucalyptus oil. BioMed Central Infectious Diseases, 17, 278.
- Sonawane, P. R., & Katti, S. A. (2016). Natural polymers: Carriers for transdermal drug delivery systems. International Journal of Research in Pharmacy and Chemistry, 6(3), 534-542.
- https://images.app.goo.gl/Cq5hoGaxUYDYMSzA6
- https://images.app.goo.gl/2imrELhJfkmH9vZH7
- https://images.app.goo.gl/3CT6ykHBv5Wiu4mV9
- https://images.app.goo.gl/JGd1schhvGgQNEeS8
- https://images.app.goo.gl/NxTZ9ffWKSmU4JYD8
- https://images.app.goo.gl/uBSiq3Fosnywamxs6
- Bernstein, W. (n.d.). Gum arabic (aka acacia gum). https://www.wbernsteinco.com/portfolio/gum-arabic-aka-acacia-gum/
- Health Jade. (n.d.). Xanthan gum. Retrieved September 2, 2024, from https://healthjade.com/xanthan-gum/
- Health Benefits Times Tragacanth.m https://www.healthbenefitstimes.com/tragacanth/ngallery/slideshow
- Mag for Women. (n.d.). Health benefits of jackfruit. https://www.magforwomen.com/health-benefits-of-jackfruit/


 Abhishek R Ghule*
Abhishek R Ghule*
 Archana K Gaikwad
Archana K Gaikwad
 Vaibhav M Hiwale
Vaibhav M Hiwale
 Kuldeep S Kanadje
Kuldeep S Kanadje













 10.5281/zenodo.13819802
10.5281/zenodo.13819802