Abstract
The worldwide predominance of neurological disarranges is rising and however we are still incapable to convey most sedate particles, in restorative amounts, to the brain. The blood brain obstruction, comprises of a tight layer of endothelial cells encompassed by astrocyte foot forms and these anatomical highlights constitute a noteworthy obstruction to medicate transport from the blood to the brain. One way to bypass the BBB and in this way treat infections of the brain is to utilize the nasal course of organization and store drugs at the olfactory locale of the nares; from where they travel to the brain by means of components that are still not clearly caught on; with travel over nerve strands and travel by means of a perivascular pathway both being hypothesized. The nose to brain course has been illustrated more than once in preclinical models, with both arrangement and particulate definitions. The nose to brain course has too been illustrated in human considers with arrangement and molecule definitions. The section of gadget producers into the field will empower the benefits of this conveyance course to ended up interpreted into affirmed items. The key components which decide the viability of conveyance by means of this course incorporate: conveyance to the olfactory range of the nares as restricted to the respiratory locale, a longer maintenance time at the nasal mucosal surface, entrance upgrade of the dynamic through the nasal epithelia and a lessening in sedate digestion system in the nasal depression. Signs where nose to brain items are likely to rise to begin with incorporate: neurodegeneration.
Keywords
Introduction, Nasal, Brain, Nose To Brain, Mechanism, New Devices
Introduction
Neurological disorders are the largest cause of disability adjusted life years (DALYS) and the second leading cause of death globally – representing 16.8% of global deaths (GBD Neurological Disorders Collaborator Group, 2017). The burden of neurological diseases is rising, with unipolar and depressive disorders predicted to become the second largest cause of morbidity by 2030 (Mathers and Loncar, 2006). In Europe, the societal cost of neurological disorders was estimated at €798 billion in 2010, a figure comprising direct medical as well as non-medical costs (60%) and productivity losses (40%) (Gustavsson et al., 2011). Conditions such as dementia, anxiety and addiction inflict the greatest costs on European health budgets. There is thus a pressing need for new central nervous system (CNS) medicines. The development of CNS drugs is currently hampered by the fact that these drugs have to cross the blood brain barrier (BBB) in therapeutic quantities. The BBB is a formidable barrier which prevents the passage of most compounds from the blood to the brain and comprises tight endothelial capillary cell junctions, with the capillaries surrounded by astrocyte foot processes, endothelial cells with low transcytotic capacity, efflux pumps on the endothelial cells and degradative enzymes close to the abluminal surface (Daneman and Prat, 2015). For drugs to cross the BBB, they must be less than 400 Da in molecular weight, be largely apolar and not multicyclic (Ghose et al., 2012). However a large number of compounds do not fit within these parameters, imparting serious constraint to the development of CNS actives. In actual fact 98% of drug molecules do not cross the BBB in therapeutic quantities (Pardridge, 2005) An alternative method of delivering molecules to the brain is the nose to brain route (Uchegbu et al., 2014; Godfrey et al., 2017). This route bypasses the BBB. The nose to brain route is gaining in popularity, as demonstrated by both preclinical (Godfrey et al., 2017) and human (Craft et al., 2012) studies. This route of delivery is the subject of this review and papers quoted are confined to publications which actually demonstrate delivery to the brain via established quantification techniques. We have also highlighted clinical studies, where nose to brain delivery was the intended outcome.
Nose to brain mechanism of delivery
For the purposes of drug delivery, the nasal cavity is divided into the respiratory area and the olfactory area; with the latter situated high up in the nares and the former closer to the nostrils (Sahin-Yilmaz and Naclerio, 2011). The nasal epithelium is well vascularised (Sahin-Yilmaz and Naclerio, 2011) and within the olfactory area, olfactory neurons are exposed (Purves et al., 2001) enabling the transport of drug compounds directly into the brain via the olfactory neurons. The exact mechanism by which compounds transfer from the nasal mucosa to the brain is not fully understood. However, it is known that absorption of molecules takes place at the olfactory and respiratory epithelia (Lochhead and Thorne, 2012). The routes of compound transfer through the olfactory area, of the nares, to the olfactory bulb are transcellular through either the sustentacular cells or the exposed olfactory sensory neurons (Thorne et al., 2008; Lochhead and Thorne, 2012). The route of transfer of compounds through the nasal respiratory epithelium to the brain is via the trigeminal nerves (Thorne et al., 2008; Lochhead and Thorne, 2012). Transport to other brain areas after entry to the brain (e.g. to the mid brain from the olfactory bulb or to the brain stem from the trigeminal nerve) is thought to be mainly by either extracellular convective bulk flow (Lochhead and Thorne, 2012) or via perivascular routes (Lochhead et al., 2015). The paracellular route is not thought to be significant. Intranasally dosed nanoparticles have been observed in the olfactory bulb just 5 minutes after dosing (Godfrey et al., 2017) indicating this to be the route of entry for nanoparticle delivery systems. Drug compounds, having crossed the olfactory epithelium, may also be taken up into the general circulation via the nasal vasculature; however the nasal vasculature is devoid of fenestrations and expresses the tight junction proteins (e.g. zonula occludens 1, occludin and claudin 5) (Lochhead and Thorne, 2012), thus significant transport to the general circulation via this route will be limited to low molecular weight apolar compounds. A key advantage of the nose to brain route is the possibility of reducing plasma exposure, as has been demonstrated (Godfrey et al., 2017; Hamidovic et al., 2017), and thus eliminating peripheral side effects. The average volume of the human nasal cavity has been measured using magnetic resonance imaging as 16,449.81 mm3 ± 4288.42 mm3 with the area of the nostril opening being 357.83 mm2 ± 108.09 mm2 (Schriever et al., 2013). Nostril opening correlates positively with nasal cavity volume (Schriever et al., 2013). No difference between the average volume of the nasal cavity was observed between men and women. In human studies intranasal insulin has been located within the cerebrospinal fluid of human subjects (Born et al., 2002) and found to improve cognitive performance in Alzheimer’s Disease patients (Craft et al., 2012). Studies with intranasal insulin show that there is no increase in blood insulin levels (Hamidovic et al., 2017), indicating that preferential brain delivery of peptides in humans is possible in via this route. These studies demonstrate the utility of the nose to brain route in humans, especially if peripheral drug activity should be avoided.
Limitations
There are limitations to the use of the nose to brain route and these must be acknowledged when developing new therapeutics to be administered via this route. There is a limitation on the dose volume for liquids of (100 - 250 µL) (Davis, 1999; Djupesland et al., 2014; Santos-Morales et al., 2017) and powders (20 – 50 mg depending on the bulk density of the powder) (Davis, 1999; Tepper and Johnstone, 2018; Shrewsbury et al., 2019), making the route only possible for potent drugs. Drugs that are metabolised by nasal cavity enzymes will also need to be protected from degradation and drug formulations must be non-irritant to the nasal cavity. Furthermore, from a drug development point of view, a nasal delivery device is required to deliver drugs via the nose to brain route.
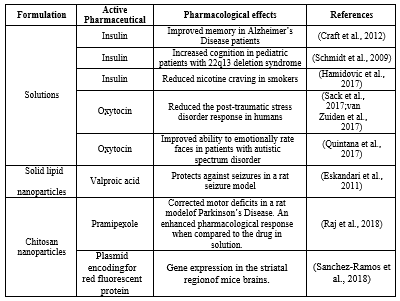
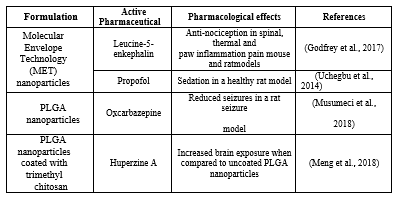
While clinical studies have predominantly involved the use of drugs in solution (Craft et al., 2012), in preclinical studies a variety of formulation types have been tested (Figure 1 and Table 1), such as both solutions (Thorne et al., 2008) and particulate dispersions (Godfrey et al., 2017). Most animal studies have been conducted in rodents and clinical studies have usually involved the use of a nasal drug delivery device.
Other Delivery Systems
Physical interventions aimed at increasing drug localization in particular areas is an emerging area. Focused ultrasound with the administration of microbubbles has been used to deliver gold nanoclusters to specific brain regions (Ye et al., 2018) 64Cu labelled or Texas Red labelled gold nanoclusters were delivered to the brain stem using focused ultrasound and microbubbles to localize the nanoclusters to the brain stem. The focused ultrasound causes localized microbubble cavitation at the target region and thus enables cellular uptake, with minimal delivery to the peripheral circulation (Ye et al., 2018). No histological-level tissue damage was detected in the nose, trigeminal nerve, and brain.
Clinical use of nose to brain delivery
It is clear from the foregoing account that utilizing the nose to brain route is a suitable method of achieving brain delivery of actives. As such a variety of clinical trials have been reported which utilize this route. The first report of nose to brain delivery was made in 2002 by Born et al, in which insulin along with melanocortin(4-10) and vasopressin were administered as intranasal solutions to humans and elevated levels of all three drugs detected in the cerebrospinal fluid 10 minutes after dosing (Born et al., 2002) with peak levels observed 80 minutes after dosing. This breakthrough study has paved the way for a variety of clinical studies using the nose to brain route (Chapman et al., 2013) for various disease indications
Nasal Delivery Devices
For nose to brain delivery the dose must be deposited in the olfactory region and thus a special delivery device is required (Lochhead and Thorne, 2012). These devices are either propellant activated in the case of Kurve Technologies’ Vianase (Craft et al., 2017), Impel Neuropharma’s Precision Olfactory Device (Shrewsbury et al., 2019) and Alchemy Pharmatech’s Naltos Device (AlchemyPharmatech, 2008) or breath activated in the case of the Optinose device (Quintana et al., 2017). While nose to brain delivery is well established in the clinical trial space, it appears that devices which offer nose to brain delivery are still not associated with licensed products. Optinose’s sumatriptan product – OnzetraÔ is not specifically designated as a nose to brain product but as a nasal product (AvanairPharmaceuticals, 2016). The Vianase device is an electronic atomiser which delivers liquid droplets of 15 – 20 µm in size to the entire nasal cavity, including the olfactory region (Craft et al., 2012; Craft et al., 2017; KurveTechnology, 2017). The Precision Olfactory Device delivers liquids and powders to the olfactory region of the nasal cavity using an inert liquid (hydrofluoalkane) that forms a gas propellant (ImpelNeuropharma, 2018). Alchemy Pharmatech’s Naltos device (Figure 3) works by means of an inert gas which is actuated by the device to propel the powder through the nares (AlchemyPharmatech, 2008). Finally Optinose exploits the patient’s own exhalation, which propels the dose deep into the nose while simultaneously isolating the oral cavity from the nasal cavity (Djupesland, 2018). Only the Optinose, Precision Olfactory Delivery and Vianase devices have been used in human nose to brain studies so far.
SUMMARY
While the BBB limits the delivery of certain drugs to the brain and, as such hampers the treatment of certain CNS disorders, accessing the brain via the nose to brain route has been demonstrated by scores of preclinical studies and about a dozen clinical trial results. Solution forms of the active have been found to be effective clinically, while both nanoparticulate formulations and solutions have been used in animal experiments. The use of nanoparticles and solution penetration enhancers improves the delivery to the brain via the nose to brain route and since there are limitations in dose volume these technologies are likely to be very important in the future. A device is needed for human studies and a number of device manufacturers have now entered the market. The route may become important for indications such as pain, AD, PTSD and intracranial tumours.
REFERENCES
- Abdou EM, Kandil SM and Miniawy H (2017) Brain targeting efficiency of antimigrain drug loaded mucoadhesive intranasal nanoemulsion. Int J Pharm 529:667-677. Ahmad E, Feng Y, Qi J, Fan W, Ma Y, He H, Xia F, Dong X, Zhao W, Lu Y and Wu W (2017) Evidence of nose-to-brain delivery of nanoemulsions: cargoes but not vehicles. Nanoscale 9:1174 1183.
- Alchemy Pharmatech (2008) Alchemy Pharmatech http://wwwalchemypharmatechcom/indexhtml. Artusson P, Lindmark T, Davis SS and Illum L (1994) Effects of chitosan on the permeability of monolayers of intestinal epithelial cells (Caco-2). Pharm Res 11:1358-1361.
- Avanair Pharmaceuticals (2016) Onzentra Product Insert. https://wwwonzetracom/sites/default/files/onzetra_xsail_prescribing_informationpdf. Benedict C, Hallschmid M, Schmitz K, Schultes B, Ratter F, Fehm HL, Born J and Kern W (2007) Intranasal insulin improves memory in humans: superiority of insulin aspart. Neuropsychopharmacology 32:239-243.
- Bonaccorso A, Musumeci T, Serapide MF, Pellitteri R, Uchegbu IF and Puglisi G (2017) Nose to brain delivery in rats: Effect of surface charge of rhodamine B labeled nanocarriers on brain subregion localization. Colloids Surf B Biointerfaces 154:297-306.
- Born J, Lange T, Kern W, McGregor GP, Bickel U and Fehm HL (2002) Sniffing neuropeptides: a transnasal approach to the human brain. Nat Neurosci 5:514-516.
- Buchthal B, Weiss U and Bading H (2018) Post-injury Nose-to-Brain Delivery of Activin A and SerpinB2 Reduces Brain Damage in a Mouse Stroke Model. Mol Ther 26:2357-2365.
- Chapman CD, Frey WH, 2nd, Craft S, Danielyan L, Hallschmid M, Schioth HB and Benedict C (2013) Intranasal treatment of central nervous system dysfunction in humans. Pharm Res 30:2475-2484.
- Craft S, Baker LD, Montine TJ, Minoshima S, Watson GS, Claxton A, Arbuckle M, Callaghan M, Tsai E, Plymate SR, Green PS, Leverenz J, Cross D and Gerton B (2012) Intranasal insulin therapy for Alzheimer disease and amnestic mild cognitive impairment: a pilot clinical trial. Arch Neurol 69:29-38.
- Craft S, Claxton A, Baker LD, Hanson AJ, Cholerton B, Trittschuh EH, Dahl D, Caulder E, Neth B, Montine TJ, Jung Y, Maldjian J, Whitlow C and Friedman S (2017) Effects of Regular and Long-Acting Insulin on Cognition and Alzheimer's Disease Biomarkers: A Pilot Clinical Trial. J Alzheimers Dis 57:1325-1334.
- Craft S, Peskind E, Schwartz MW, Schellenberg GD, Raskind M and Porte D, Jr. (1998) Cerebrospinal fluid and plasma insulin levels in Alzheimer's disease: relationship to severity of dementia and apolipoprotein E genotype. Neurology 50:164-168.
- Daneman R and Prat A (2015) The blood-brain barrier. Cold Spring Harbor perspectives in biology 7:a020412.
- Danhier F, Ansorena E, Silva JM, Coco R, Le Breton A and Preat V (2012) PLGA-based nanoparticles: an overview of biomedical applications. J Control Release 161:505-522.Davis SS (1999) Delivery of peptide and non-peptide drugs through the respiratory tract. Pharm Sci Tech Today 2:450-456.
- Djupesland PG (2018) Looking to the future of nasal drug delivery - an interview with Per Gisle Djupesland. Ther Deliv 9:163-168. Djupesland PG, Messina JC and Mahmoud RA (2014) The nasal approach to delivering treatment for brain diseases: an anatomic, physiologic, and delivery technology overview. Ther Deliv 5:709-733.
- Dobrovoljac M, Hengartner H, Boltshauser E and Grotzer MA (2002) Delay in the diagnosis of paediatric brain tumours. Eur J Pediatr 161:663-667.
- Eskandari S, Varshosaz J, Minaiyan M and Tabbakhian M (2011) Brain delivery of valproic acid via intranasal administration of nanostructured lipid carriers: in vivo pharmacodynamic studies using rat electroshock model. Int J Nanomedicine 6:363-371.
- Fonseca DA, Teixeira RM, Silva JC, J DESDGF, Meirelles OC, Landeiro JA and Quirico-Santos T (2013) Long-term outcome in patients with recurrent malignant glioma treated with Perillyl alcohol inhalation. Anticancer Res 33:5625-5631.
- GBD Neurological Disorders Collaborator Group (2017) Global, regional, and national burden of neurological disorders during 1990-2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet Neurol 16:877-897.
- Garrett NL, Lalatsa A, Uchegbu I, Schatzlein A and Moger J (2012) Exploring uptake mechanisms of oral nanomedicines using multimodal nonlinear optical microscopy. J Biophotonics 5:458-468.
- Ghose AK, Herbertz T, Hudkins RL, Dorsey BD and Mallamo JP (2012) Knowledge-Based, Central Nervous System (CNS) Lead Selection and Lead Optimization for CNS Drug Discovery. ACS Chem Neurosci 3:50-68.
- Godfrey L, Iannitelli A, Garrett NL, Moger J, Imbert I, King T, Porreca F, Soundararajan R, Lalatsa A, Schatzlein AG and Uchegbu IF (2017) Nanoparticulate peptide delivery exclusively to the brain produces tolerance free analgesia. J Control Release 270:135-144.
- Groothuis DR (2000) The blood-brain and blood-tumor barriers: A review of strategies for increasing drug delivery. Neuro-Oncology 2:45-59.
- Gustavsson A, Svensson M, Jacobi F, Allgulander C, Alonso J, Beghi E, Dodel R, Ekman M, Faravelli C, Fratiglioni L, Gannon B, Jones DH, Jennum P, Jordanova A, Jonsson L, Karampampa K, Knapp M, Kobelt G, Kurth T, Lieb R, Linde M, Ljungcrantz C, Maercker A, Melin B, Moscarelli M, Musayev A, Norwood F, Preisig M, Pugliatti M, Rehm J, Salvador-Carulla L, Schlehofer B, Simon R, Steinhausen HC, Stovner LJ, Vallat JM, Van den Bergh P, van Os J, Vos P, Xu W, Wittchen HU, Jonsson B, Olesen J and Group CD (2011) Cost of disorders of the brain in Europe 2010. Eur Neuropsychopharmacol 21:718-779.
- Hamidovic A, Khafaja M, Brandon V, Anderson J, Ray G, Allan AM and Burge MR (2017) Reduction of smoking urges with intranasal insulin: a randomized, crossover, placebo-controlled clinical trial. Mol Psychiatry 22:1413-1421.
- Herculano-Houzel S, Ribeiro P, Campos L, Valotta da Silva A, Torres LB, Catania KC and Kaas JH (2011) Updated neuronal scaling rules for the brains of Glires (rodents/lagomorphs). Brain Behav Evol 78:302-314.
- ImpelNeuropharma (2018) Impel Neuropharma. http://impelnpcom/pod-technology/.
- Kahn CR (1985) The molecular mechanism of insulin action. Annu Rev Med 36:429-451.
- Kamei N, Okada N, Ikeda T, Choi H, Fujiwara Y, Okumura H and Takeda-Morishita M (2018) Effective nose-to-brain delivery of exendin-4 via coadministration with cell-penetrating peptides for improving progressive cognitive dysfunction. Sci Rep 8:17641.
- KurveTechnology (2017) Kurve Technology. http://www.kurvetech.com/nasaltechnology.asp.
- Lane CA, Hardy J and Schott JM (2018) Alzheimer's disease. Eur J Neurol 25:59-70.
- Lin W, Xie X, Deng J, Liu H, Chen Y, Fu X, Liu H and Yang Y (2016) Cell-penetrating peptide doxorubicin conjugate loaded NGR-modified nanobubbles for ultrasound triggered drug delivery. J Drug Target 24:134-146.
- Lochhead JJ and Thorne RG (2012) Intranasal delivery of biologics to the central nervous system. Adv Drug Deliv Rev 64:614-628.
- Lochhead JJ, Wolak DJ, Pizzo ME and Thorne RG (2015) Rapid transport within cerebral perivascular spaces underlies widespread tracer distribution in the brain after intranasal administration. J Cereb Blood Flow Metab 35:371-381.
- Mathers CD and Loncar D (2006) Projection of global mortality and burden of disease from 2002 to 2030. Plos Med 3:2011 - 2030.
- Meng Q, Wang A, Hua H, Jiang Y, Wang Y, Mu H, Wu Z and Sun K (2018) Intranasal delivery of Huperzine A to the brain using lactoferrin-conjugated N-trimethylated chitosan surface modified PLGA nanoparticles for treatment of Alzheimer's disease. Int J Nanomedicine 13:705-718.
- Muller RH, Mader K and Gohla S (2000) Solid lipid nanoparticles (SLN) for controlled drug delivery - a review of the state of the art. Eur J Pharm Biopharm 50:161-177.
- Musumeci T, Serapide MF, Pellitteri R, Dalpiaz A, Ferraro L, Dal Magro R, Bonaccorso A, Carbone C, Veiga F, Sancini G and Puglisi G (2018) Oxcarbazepine free or loaded PLGA nanoparticles as effective intranasal approach to control epileptic seizures in rodents. Eur J Pharm Biopharm 133:309-320.
- Pardridge WM (2005) The blood-brain barrier: bottleneck in brain drug development. NeuroRx 2:3-14.
- Parker KJ, Oztan O, Libove RA, Sumiyoshi RD, Jackson LP, Karhson DS, Summers JE, Hinman KE, Motonaga KS, Phillips JM, Carson DS, Garner JP and Hardan AY (2017) Intranasal oxytocin treatment for social deficits and biomarkers of response in children with autism. Proc Natl Acad Sci U S A 114:8119-8124.
- Purves D, Augustine GJ, Fitzpatrick D, Katz LC, LaMantia A, McNamara JO and Williams SM (2001) The Olfactory Epithelium and Olfactory Receptor Neurons, in Neuroscience 2nd Edition (Purves D, Augustine GJ, Fitzpatrick D, Katz LC, LaMantia A, McNamara JO and Williams SM eds), Sinauer Associates, Sunderland (MA) https://www.ncbi.nlm.nih.gov/books/NBK10896/.
- Quintana DS, Westlye LT, Hope S, Naerland T, Elvsashagen T, Dorum E, Rustan O, Valstad M, Rezvaya L, Lishaugen H, Stensones E, Yaqub S, Smerud KT, Mahmoud RA, Djupesland PG and Andreassen OA (2017) Dose-dependent social-cognitive effects of intranasal oxytocin delivered with novel Breath Powered device in adults with autism spectrum disorder: a randomized placebo-controlled double-blind crossover trial. Transl Psychiatry 1136.
- Raj R, Wairkar S, Sridhar V and Gaud R (2018) Pramipexole dihydrochloride loaded chitosan nanoparticles for nose to brain delivery: Development, characterization and in vivo anti Parkinson activity. Int J Biol Macromol 109:27-35.
- Saar K, Lindgren M, Hansen M, Eiriksdottir E, Jiang Y, Rosenthal-Aizman K, Sassian M and Langel U (2005) Cell-penetrating peptides: a comparative membrane toxicity study. Anal Biochem 345:55-65.
- Sack M, Spieler D, Wizelman L, Epple G, Stich J, Zaba M and Schmidt U (2017) Intranasal oxytocin reduces provoked symptoms in female patients with posttraumatic stress disorder despite exerting sympathomimetic and positive chronotropic effects in a randomized controlled trial. BMC Med 15:40.
- Sahin-Yilmaz A and Naclerio RM (2011) Anatomy and physiology of the upper airway. Proc Am Thorac Soc 8:31-39.
- Sanchez-Ramos J, Song S, Kong X, Foroutan P, Martinez G, Dominguez-Viqueria W, Mohapatra S, Mohapatra S, Haraszti RA, Khvorova A, Aronin N and Sava V (2018) Chitosan-Mangafodipir nanoparticles designed for intranasal delivery of siRNA and DNA to brain. J Drug Deliv Sci Technol 43:453-460.
- Santos-Morales O, Diaz-Machado A, Jimenez-Rodriguez D, Pomares-Iturralde Y, Festary Casanovas T, Gonzalez-Delgado CA, Perez-Rodriguez S, Alfonso-Munoz E, Viada-Gonzalez C, Piedra-Sierra P, Garcia-Garcia I, Amaro-Gonzalez D, Neuro EPOSG, Garcia-Rodriguez JC, Sosa-Teste I, Lagarto-Parra A, Barrero-Viera L, David-Baldo M, Tamayo-Rodriguez M, Rivero-Vazquez I, Gonzalez-Gamiz G, Martin-Trujillo A, Rodriguez-Fernandez Y, Ledo-de la Luz AA, Alvarez-Delgado M, Howland-Alvarez I and Cruz-Gomez Y (2017) Nasal administration of the neuroprotective candidate NeuroEPO to healthy volunteers: a randomized, parallel, open-label safety study. BMC Neurol 17:129.
- Schmidt H, Kern W, Giese R, Hallschmid M and Enders A (2009) Intranasal insulin to improve developmental delay in children with 22q13 deletion syndrome: an exploratory clinical trial. J Med Genet 46:217-222.
- Schriever VA, Hummel T, Lundstrom JN and Freiherr J (2013) Size of nostril opening as a measure of intranasal volume. Physiol Behav 110-111:3-5.
- Seju U, Kumar A and Sawant KK (2011) Development and evaluation of olanzapine-loaded PLGA nanoparticles for nose-to-brain delivery: In vitro and in vivo studies. Acta Biomater 7:4169-4176.
- Serrano DR, Lalatsa A, Dea-Ayuela MA, Bilbao-Ramos PE, Garrett NL, Moger J, Guarro J, Capilla J, Ballesteros MP, Schatzlein AG, Bolas F, Torrado JJ and Uchegbu IF (2015) Oral particle uptake and organ targeting drives the activity of amphotericin B nanoparticles. Mol Pharmaceutics 12:420-431.
- Shah B, Khunt D, Misra M and Padh H (2016) "Application of Box-Behnken design for optimization and development of quetiapine fumarate loaded chitosan nanoparticles for brain delivery via intranasal route* ". Int J Biol Macromol 89:206-218.
- Shah B, Khunt D, Misra M and Padh H (2018) Formulation and In-vivo Pharmacokinetic Consideration of Intranasal Microemulsion and Mucoadhesive Microemulsion of Rivastigmine for Brain Targeting. Pharm Res 35:8.
- Shin NY, Park HY, Jung WH, Park JW, Yun JY, Jang JH, Kim SN, Han HJ, Kim SY, Kang DH and Kwon JS (2015) Effects of Oxytocin on Neural Response to Facial Expressions in Patients with Schizophrenia. Neuropsychopharmacology 40:1919-1927.
- Shingaki T, Inoue D, Furubayashi T, Sakane T, Katsumi H, Yamamoto A and Yamashita S (2010) Transnasal delivery of methotrexate to brain tumors in rats: a new strategy for brain tumor chemotherapy. Mol Pharmaceutics 7:1561-1568.
- Shrewsbury S, Swardstrom M, Satterly K, Campbell J, Tugiono N, Gillies J and Hoekman J (2019) Placebo/Active Controlled, Safety, Pharmaco-Kinetic/Dynamic Study of INP105 (POD® olanzapine) in Healthy Adults. Western Journal of Emergency Medicine: Integrating Emergency Care with Population H
- Siew A, Le H, Thiovolet M, Gellert P, Schatzlein A and Uchegbu I (2012) Enhanced oral absorption of hydrophobic and hydrophilic drugs using quaternary ammonium palmitoyl glycol chitosan nanoparticles. MolPharmaceutics 9:14-28.
- Sigurdsson P, Thorvaldsson T, Gizurarson S and Gunnarsson E (1997) Olfactory absorption of insulin to the brain. Drug Deliv 4:195-200.
- Simao Carlos MI, Zheng K, Garrett N, Arifin N, Workman DG, Kubajewska I, Halwani AA, Moger J, Zhang Q, Schatzlein AG and Uchegbu IF (2017) Limiting the level of tertiary amines on polyamines leads to biocompatible nucleic acid vectors. Int J Pharm 526:106-124.
- Tanaka A, Furubayashi T, Arai M, Inoue D, Kimura S, Kiriyama A, Kusamori K, Katsumi H, Yutani R, Sakane T and Yamamoto A (2018) Delivery of Oxytocin to the Brain for the Treatment of Autism Spectrum Disorder by Nasal Application. Mol Pharmaceutics 15:1105-1111.
- Tepper SJ and Johnstone MR (2018) Breath-powered sumatriptan dry nasal powder: an intranasal medication delivery system for acute treatment of migraine. Med Devices (Auckl) 11:147 156.
- Thorne RG, Hanson LR, Ross TM, Tung D and Frey WH, 2nd (2008) Delivery of interferon-beta to the monkey nervous system following intranasal administration. Neuroscience 152:785 797.
- Trotta V, Pavan B, Ferraro L, Beggiato S, Traini D, Des Reis LG, Scalia S and Dalpiaz A (2018) Brain targeting of resveratrol by nasal administration of chitosan-coated lipid microparticles. Eur J Pharm Biopharm 127:250-259.
- Uchegbu IF, Jones MC, Corrente F, Godfrey L, Laghezza D, Carafa M, Holm P and Schätzlein AG (2014) The Oral and Intranasal Delivery of Propofol Using Chitosan Amphiphile Nanoparticles. Pharm Nanotech 2:65-74.
- Van Woensel M, Mathivet T, Wauthoz N, Rosiere R, Garg AD, Agostinis P, Mathieu V, Kiss R, Lefranc F, Boon L, Belmans J, Van Gool SW, Gerhardt H, Amighi K and De Vleeschouwer S (2017) Sensitization of glioblastoma tumor micro-environment to chemo- and immunotherapy by Galectin-1 intranasal knock-down strategy. Sci Rep 7:1217.
- van Zuiden M, Frijling JL, Nawijn L, Koch SBJ, Goslings JC, Luitse JS, Biesheuvel TH, Honig A, Veltman DJ and Olff M (2017) Intranasal Oxytocin to Prevent Posttraumatic Stress Disorder Symptoms: A Randomized Controlled Trial in Emergency Department Patients. Biol Psychiatry 81:1030-1040.
- Vllasaliu D, Exposito-Harris R, Heras A, Casettari L, Garnett M, Illum L and Stolnik S (2010) Tight junction modulation by chitosan nanoparticles: comparison with chitosan solution. Int J Pharm 400:183-193.
- Wang Q, Zhang Y, Wong CH, Edwin Chan HY and Zuo Z (2017) Demonstration of Direct Nose-to Brain Transport of Unbound HIV-1 Replication Inhibitor DB213 Via Intranasal Administration by Pharmacokinetic Modeling. AAPS J 20:23.
- Yang J, Lu L, Wang HC, Zhan HQ, Hai GF, Pan YJ, Lv QQ, Wang DX, Wu YQ, Li RR, Xue L, Wang XH, Deng XM, Liu XF, Qian YN, Deng ZK, Zhang ZJ, Zhan XH, Zhou XJ, Wang GL, Zhai JX and Wang JC (2012) Effect of intranasal arginine vasopressin on human headache. Peptides 38:100-104.
- Ye DZ, Zhang XH, Yue YM, Raliya R, Biswas P, Taylor S, Tai YC, Rubin JB, Liu YJ and Chen H (2018) Focused ultrasound combined with microbubble-mediated intranasal delivery of gold nanoclusters to the brain. J Control Rel 286:145-153.
- Zhang C, Chen J, Feng C, Shao X, Liu Q, Zhang Q, Pang Z and Jiang X (2014) Intranasal nanoparticles of basic fibroblast growth factor for brain delivery to treat Alzheimer's disease. Int J Pharm 461:192-202


 Nikita N. Bagade* 1
Nikita N. Bagade* 1
 Shripad M. Dalvi 2
Shripad M. Dalvi 2
 Pranjali Ramesh Gaikwad 3
Pranjali Ramesh Gaikwad 3


 10.5281/zenodo.11183620
10.5281/zenodo.11183620