Abstract
Anaphylaxis is the most severe type of allergic reaction that can happen minutes after being exposed to an allergen, usually from food, medicine, or insect bites. It can be fatal and is nearly invariably unplanned. Airway blockage or cardiovascular collapse can be deadly outcomes of delayed clinical diagnosis and treatment. Anaphylactic reactions were defined as IgE-mediated responses. Anaphylaxis can manifest physically as moderate skin flushing and pruritus or as severe respiratory symptoms. The only first-line drug available for treating anaphylaxis is epinephrine, which can save lives. It quickly reverses almost all anaphylactic symptoms at therapeutic levels and stabilizes mast cells. After various studies and clinical trials, FDA approved the first epinephrine product for the treatment of anaphylaxis that is not administered by injection. The FDA approved the first nasal epinephrine for the emergency treatment of type I allergic reactions, including those that are life-threatening, like anaphylactic shock. This nasal epinephrine represents an innovative approach to the treatment of anaphylaxis, a severe and potentially life-threatening allergic reaction. Traditionally, the administration of epinephrine, the first-line treatment for anaphylaxis, has been through intramuscular injection, usually with an auto-injector like the EpiPen. However, the development of nasal epinephrine formulations offers a potentially easier and quicker method of administration, which could be particularly beneficial in emergency situations where rapid treatment is critical. This review explores novel nasal epinephrine sprays for anaphylaxis emergency care.
Keywords
Anaphylaxis, Epinephrine, Fatal, FDA, Nasal, Food allergy.
Introduction
The body naturally produces epinephrine, which accounts for almost 80% of the catecholamines produced in the adrenal medulla.[1] In situations of sudden fear or life-threatening danger, this endogenous epinephrine is released and impacts various organs throughout the body that are influenced by the sympathetic nervous system. It causes an increase in heart rate and strengthens the force of heart contractions, leading to a rise in blood pressure.[2-3] Blood flow is redirected from the skin and subcutaneous tissues to the skeletal muscles, the splanchnic circulation, and the brain. The bronchi and pupils expand, oxygen levels in the blood increase, and blood glucose levels rise, preparing the body for a "fight or flight" response.[4] The onset of anaphylaxis can happen suddenly and without warning, and it frequently happens outside of a medical environment, such as upon food consumption or an insect bite. Even with the same stimulus in the same subject, the severity changes from episode to episode.[5] Healthcare providers and non-medical individuals may encounter challenges in identifying and diagnosing anaphylaxis. Epinephrine has been recognized as a necessary drug for the treatment of anaphylaxis by the World Health Organization and the World Allergy Organization.[6] The most widely utilized product for out-of-hospital care is an epinephrine autoinjector; yet, injectable product use is unpopular with patients and caregivers. Even if they are aware that they are experiencing a severe allergic reaction, up to 83% of patients and caregivers have reported forgetting to use or postponing using an epidural epinephrine autoinjector.[7] Delays in receiving treatment may raise the risk of death from vascular collapse or airway blockage. A nasal spray containing epinephrine is being developed as a potential alternative to intramuscular injections.[8] Intranasal (IN) administration is becoming more popular for delivering a range of treatments, especially for use outside of hospitals. In addition to being far less intrusive, IN administration has other benefits over other delivery methods, such as simplicity of usage, quick absorption, and avoidance of the discomfort commonly associated with intravenous (IV) or intramuscular (IM) injection.[9] Children, who are more likely to be "needle-phobic," benefit greatly from needle-free delivery choices.[10] A range of medications, including midazolam, diazepam, fentanyl, naloxone, ketamine, and dexmedetomidine are commonly administered intranasally for various medical purposes.[11] Antihistamines and corticosteroids are considered secondary treatments for anaphylaxis because they act slowly, cannot stabilize or prevent mast cell degranulation, and cannot target other mediators of anaphylaxis.[12]
Epinephrine
Epinephrine has been utilized for treating allergic reactions for over a century and remains the only universally recommended first-line therapy for Type I allergic reactions. The use of epinephrine to treat anaphylaxis was first documented in the 1960s.[13] There is global consensus that epinephrine is the most effective treatment for anaphylaxis, supported by extensive clinical experience, case reports, and a limited number of clinical trials.[14]
Pharmacology of Epinephrine
The mechanism of action (MOA) of epinephrine in treating Type I allergic reactions, such as anaphylaxis, is widely known. It is based on the direct systemic agonism of ?- and ?-adrenergic receptors, which reverses the pathological response caused by exposure to an antigen and a stabilized mast cell to stop allergic reactions from proceeding.[15] Epinephrine functions as a nonselective agonist at G-protein-coupled ?- and ?-adrenergic receptors. By directly blocking almost all end-organ effects of immune mediators of anaphylaxis and stabilizing mast cells, epinephrine stops additional degranulation and the release of allergy mediators in a matter of minutes.[16] The primary therapeutic impact of epinephrine stems from its direct agonism of ?2-adrenergic receptors, which causes an increase in intracellular cyclic AMP synthesis and the activation of adenylyl cyclase. ?-adrenergic receptors enhance vascular permeability and decrease vasodilation, whereas anaphylaxis causes hypotension and a loss of intravascular fluid volume.[17] The relaxation of bronchial smooth muscle by ?-adrenergic receptors helps relieve dyspnea, wheezing, and bronchospasm that can happen during anaphylaxis.[18] To maintain blood pressure (BP), ?-adrenergic receptors cause an increase in heart rate and contractility. Heart rate and contractility are enhanced through ?-adrenergic receptors to help regulate blood pressure.[19] Epinephrine, known for its capacity to relax smooth muscles in the stomach, intestines, uterus, and bladder, can alleviate symptoms like itching, hives, and swelling, and may also ease gastrointestinal and genitourinary symptoms related to anaphylaxis.[20] Anaphylaxis is most commonly triggered by food, insect stings, and medications, but it can also result from exercise or occur without a known cause. Since anaphylaxis can happen outside the home, it's crucial for patients to be educated on avoiding allergens and the necessity of having epinephrine on hand.[21] The prompt administration of epinephrine by either the patient or a caregiver is key to effectively resolving symptoms. Although the risk of overdose and severe cardiac side effects is present with any injection method, it is lower with intramuscular (IM) administration compared to intravenous (IV) administration.[22]
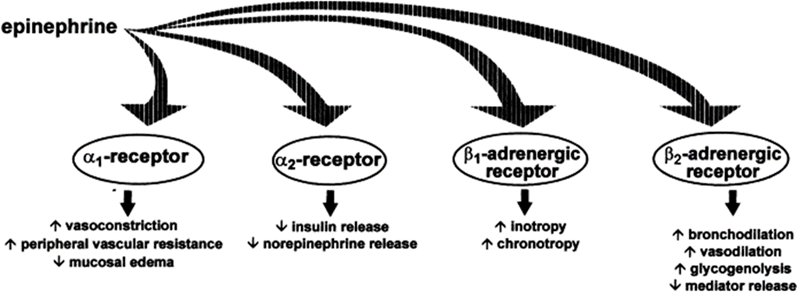
Fig 1: Adrenergic effects of epinephrine. Adapted from Simons6
Beneficial aspects
In patients experiencing anaphylaxis, epinephrine plays a critical role in saving lives through its powerful alpha-1 adrenergic vasoconstrictor effects on small arterioles and precapillary sphincters across various body organ systems. This vasoconstriction reduces mucosal oedema, helping to prevent and alleviate upper airway obstruction, and raises blood pressure, thereby preventing and treating shock.[23] Its beta-1 adrenergic effects result in an increased heart rate and stronger cardiac contractions. Additionally, its beta-2 effects promote bronchodilation and reduce the release of histamine, tryptase, and other inflammatory mediators from mast cells and basophils.[24]
Advantages of Nasal Administration
Nasal sprays are generally easier to use than injections, especially for individuals who are not trained in administering injections or are in a panicked state during an anaphylactic reaction.
The nasal mucosa is highly vascular, allowing for rapid absorption of the drug directly into the bloodstream.
This method avoids the pain and potential complications associated with needle use, making it more acceptable to some patients, particularly children and needle-phobic individuals.
- Potential for Wider Availability:
Nasal epinephrine could be more easily integrated into public spaces, such as schools and airports, where trained personnel might not always be available.[25-28]
Clinical Efficacy and Safety
Preliminary studies and clinical trials suggest that nasal epinephrine is effective in reversing the symptoms of anaphylaxis, with a similar pharmacokinetic profile to intramuscular injections.[29] However, ongoing research is necessary to establish its efficacy fully and to determine the optimal dosing and delivery mechanisms. These trials often measure outcomes such as the time it takes for symptoms to begin resolving, improvements in vital signs (e.g., blood pressure, heart rate), and the overall survival rate.[30] The effectiveness of nasal epinephrine has been found to be comparable to that of intramuscular (IM) epinephrine, which was the gold standard for anaphylaxis treatment. This similarity is crucial because IM epinephrine has a well-established track record for rapidly resolving life-threatening allergic reactions.[31] FDA recently approved the first nasal epinephrine for anaphylaxis. Approval was based on four studies conducted in 175 healthy adults, without anaphylaxis, that measured the epinephrine concentrations in the blood following administration of nasal epinephrine or approved epinephrine injection products. Results from these studies showed comparable epinephrine blood concentrations between nasal epinephrine and approved epinephrine injection products.[32]
Safety Profile:
Adverse Events:
The most frequently reported side effects of nasal epinephrine include throat irritation, tingling in the nose (intranasal paresthesia), headache, nasal discomfort, feelings of jitteriness, tingling sensations (paresthesia), fatigue, tremors, runny nose (rhinorrhea), itching inside the nose (nasal pruritus), sneezing, abdominal pain, gum pain (gingival pain), mouth numbness (oral hypoesthesia), nasal congestion, dizziness, nausea, and vomiting.[33]
Local Effects:
Special attention is given to any local side effects related to nasal administration, such as nasal irritation or congestion. Clinical trials typically report that these local effects are mild and transient.[34]
Challenges and Considerations
Dosing Consistency:
Ensuring a consistent and effective dose with each spray is a challenge, particularly given the variability in nasal anatomy and the presence of nasal congestion or other conditions.
Regulatory Approval:
As with any new drug formulation, nasal epinephrine must undergo rigorous testing and regulatory review to ensure it meets safety and efficacy standards.
Public and Medical Acceptance:
The adoption of nasal epinephrine will depend on its acceptance by both the medical community and the general public. Education and training will be essential in ensuring that patients and caregivers understand how and when to use it.[35]
The future role of nasal epinephrine in anaphylaxis
The development of nasal epinephrine is a promising step forward in the treatment of anaphylaxis. Future research should focus on optimizing the formulation, improving delivery mechanisms, and conducting large-scale studies to confirm its safety and effectiveness. If successful, nasal epinephrine could become a valuable tool in managing anaphylaxis, offering a faster, easier, and more accessible option for patients in need.[36-37]
CONCLUSION
The introduction of a new, convenient option that eliminates the need for a needle in the self-administration of epinephrine during acute anaphylaxis is a promising development. Intranasal administration, as a delivery method, has consistently proven to be both user-friendly and effective in delivering medications. Nasal epinephrine represents a significant innovation in the treatment of anaphylaxis. Its ease of use, rapid absorption, and non-invasive nature make it a compelling alternative to traditional intramuscular injections. With further research and development, nasal epinephrine could potentially revolutionize the management of anaphylaxis, making life-saving treatment more accessible and effective for individuals at risk of severe allergic reactions.
ACKNOWLEDGEMENT
We would like to acknowledge Wellcare Pharmacy for the technical support.
CONFLICT OF INTEREST
There is no conflict of interest.
REFERENCES
- Julie CB, Elinor S, Susan AR. Epinephrine in the Management of Anaphylaxis. Volume 8. J Allergy Clin Immunol;2020, 1186-1195.
- Anne K, Thomas BC, Michael K, John O, Jonathan M, Spergel, David M, et al. Development of neffy, an Epinephrine Nasal Spray, for Severe Allergic Reactions. 2nd ed. USA: Pharmaceutics; 2024.
- Simons FER. First-aid treatment of anaphylaxis to food: focus on epinephrine. J Allergy Clin Immunol. 2004;113:837Y844. IV
- Rech, MA, Barbas B, Chaney W, Greenhalgh E, Turck C. When to pick the nose: Out-of-hospital and emergency department intranasal administration of medications. Ann. Emerg. Med;2017, 70, 203–211.
- Bailey AM, Baum RA, Horn K., Lewis T, Morizio K, Schultz A, Weant, K, Justice SN. Review of intranasally administered medications for use in the emergency department. J. Emerg. Med;2017, 53, 38–48.
- Misra SN, Sperling MR, Rao V.R, Peters JM, Penovich, P, Wheless, Hogan, R.E, Davis CS, Carrazana E, Rabinowicz AL. Analyses of patients who self-administered diazepam nasal spray for acute treatment of seizure clusters. Epilepsy Behav. Rep; 2024, 25.
- Abdelal R, Raja BA, Carlberg-RS, Darwaza, N, Ito D, Shoaff J, Epstein J. Real-world study of multiple naloxone administration for opioid overdose reversal among bystanders. Harm Reduct. J; 2022, 19, 49.
- Cornett EM, Nemomsa MA, Turbeville B, Busby MA, Kaye JS, Kaye AJ, Choi J, Ramírez GF, Varrassi G, Kaye AM, et al. Midazolam nasal spray to treat intermittent, stereotypic episodes of frequent seizure activity: Pharmacology and clinical
- role, a comprehensive review. Health Psychol. Res; 2022, 10, 38536.
- Rabinowicz AL, Carrazana E, Maggio ET. Improvement of intranasal drug delivery with Intravail alkylsaccharide excipient as a mucosal absorption enhancer aiding in the treatment of conditions of the central nervous system. Drugs R D; 2021, 21, 361–369.
- Harris E. FDA approves Nalmefene, a longer-lasting opioid reversal nasal spray. JAMA; 2023, 329.
- Martirosov AL, Giuliano C, Shupp M, Channey S, Kale-Pradhan PB. Zavegepant intranasal spray for migraines. Ann. Pharmacother; 2023.
- Munjal S, Gautam A, Offman E, Brand-Schieber E, Allenby K, Fisher. D.M. A randomized trial comparing the pharmacokinetics, safety, and tolerability of DFN-02, an intranasal sumatriptan spray containing a permeation enhancer, with intranasal and subcutaneous sumatriptan in healthy adults. Headache; 2016, 56, 1455–1465.
- Shaker MS, Wallace DV, Golden D.B.K, Oppenheimer J, Bernstein JA, Campbell RL, Dinakar C, Ellis A, Greenhawt M, Khan DA, et al. Anaphylaxis-a 2020 practice parameter, and grading of recommendations, assessment, development and evaluation (GRADE) analysis. J. Allergy Clin. Immunol; 2020, 145, 1082–1123.
- Panel NS. Guidelines for the diagnosis and management of food allergy in the United States: Report of the NIAID-sponsored expert panel. J. Allergy Clin. Immunol; 2010, 12, S1–S58.
- Golden DBK, Wang J, Waserman S, Akin C, Campbell RL, Ellis AK, Greenhawt M, Lang DM, Ledford DK, Lieberman J, et al. Anaphylaxis: A 2023 practice parameter update. Ann. Allergy Asthma Immunol; 2024, 132, 124–176.
- Brooks C, Coffman A, Erwin E, Mikhail I. Diagnosis and treatment of food allergic reactions in pediatric emergency settings. Ann. Allergy Asthma Immunol; 2017, 119, 467–468.
- Fleming JT, Clark S, Camargo CA, Rudders SA. Early treatment of food-induced anaphylaxis with epinephrine is associated with a lower risk of hospitalization. J. Allergy Clin. Immunol. Pract; 2015, 3, 57–62.
- Patel N, Chong KW, Yip AYG, Ierodiakonou D, Bartra J, Boyle RJ, Turner PJ. Use of multiple epinephrine doses in anaphylaxis: A systematic review and meta-analysis. J. Allergy Clin. Immunol; 2021, 148, 1307–1315.
- Hochstadter E, Clarke A, De Schryver S, La Vieille S, Alizadehfar R, Joseph L, Eisman H, Ben-Shoshan M. Increasing visits for anaphylaxis and the benefits of early epinephrine administration: A 4-year study at a pediatric emergency department in Montreal, Canada. J. Allergy Clin. Immunol; 2016, 137, 1888–1890.E4.
- Andrew E, Nehma Z, Bernard S, Smith K. Pediatric anaphylaxis in the prehospital setting: Incidence, characteristics, and management. Prehospital Emerg. Care; 2018, 22, 445–451.
- Liu X, Lee S, Lohse CM, Hardy CT, Campbell RL. Biphasic reactions in emergency department anaphylaxis patients: A prospective cohort study. J. Allergy Clin. Immunol. Pract; 2020, 8, 1230–1238.
- Turner PJ, Jerschow E, Umasunthar T, Lin R, Campbell DE, Boyle RJ. Fatal anaphylaxis: Mortality rate and risk factors. J.Allergy Clin. Immunol. Pract; 2017, 5, 1169–1178.
- Noimark L, Du Toit G, Pastacaldi C, Haddad D, Gardner J, Hyer W, Vance G, Townshend C, Alfaham, M, Arkwright PD, et al. The use of adrenaline autoinjectors by children and teenagers. Clin. Exp. Allergy; 2012, 42, 284–292.
- Simons FE. Anaphylaxis, killer allergy: Long-term management in the community. J. Allergy Clin. Immunol; 2006, 117, 367–377.
- Upton J, Vadas P. Potential therapeutic strategies for severe anaphylaxis targeting platelet-activating factor and PAF acetylhydrolase. Curr. Treat. Options Allergy; 2014, 1, 232–246.
- Ebisawa M, Ito K, Fujisawa T. Japanese guidelines for food allergy 2020. Allergol. Int; 2020, 69, 370–386.
- Cardona V, Ansotegui IJ, Ebisawa M, El-Gamal Y, Rivas MF, Fineman S, Geller M, Gonzalez-Estrada A, Greenberger PA, Borges MS, et al. World Allergy Organization Anaphylaxis Guidance 2020. World Allergy Organ. J; 2020, 13, 100472.
- Ring J, Klimek L, Worm M. Adrenaline in the acute treatment of anaphylaxis. Medicine; 2018, 115, 528–534.
- Brown JC, Simons E, Rudders SA. Epinephrine in the management of anaphylaxis. J. Allergy Clin. Immunol. Pract; 2020, 8,1186–1195.
- Pauw EK, Stubblefield WB, Wrenn JO, Brown SK, Cosse MS, Curry ZS, Darcy TP, James TE, Koetter PE, Nicholson CE, et al. Frequency of cardiotoxicity following intramuscular administration of epinephrine in emergency department patients with anaphylaxis. J. Am. Coll. Emerg. Physicians Open; 2024, 5, e13095.
- Abramowicz M, Zuccotti G, Pflomm JM. An epinephrine prefilled syringe (Symjepi) for anaphylaxis (reprinted from Medical Letter on Drugs and Therapeutics, vol 61, pg 25-26, 2019). JAMA; 2019, 321, 1306–1307.
- Edwards ES, Gunn R, Simons ER, Carr K, Chinchilli VM, Painter G, Goldwater R. Bioavailability of epinephrine from Auvi-Q compared with EpiPen. Ann. Allergy Asthma Immunol; 2013, 111, 132–137.
- Tanimoto S, Kaliner M, Lockey RF, Ebisawa M, Koplowitz LP, Koplowitz B, Lowenthal R. Pharmacokinetic and pharmacodynamic comparison of epinephrine, administered intranasally and intramuscularly-an integrated analysis. Ann.Allergy Asthma Immunol; 2023, 130, 508–514
- Ebisawa M, Kaliner MA, Lowenthal R, Tanimoto S. Rapid increase in epinephrine concentration following presumed intra-blood vessel administration via epinephrine autoinjector. J. Allergy Clin. Immunol. Glob; 2023, 2, 3.
- Sparapani S, Authier S, Lowenthal R, Tanimoto S. The impact of anaphylaxis on the absorption of intranasal epinephrine in anaesthetized non-naïve beagle dogs. J. Allergy Clin. Immunol. Glob. 2023, 2, 4.
- FDA.gov. Available online: https://www.fda.gov/news-events/press-announcements/fda-approves-first-nasal-spray-treatment-anaphylaxis (released on 09 August 2024).


 SILPA SASIKUMAR*
SILPA SASIKUMAR*

 10.5281/zenodo.13765697
10.5281/zenodo.13765697