Abstract
Nanoparticle-based targeted drug delivery systems have transformed therapeutic strategies by offering precise and controlled drug delivery to specific sites within the body, thereby enhancing treatment efficacy while reducing systemic side effects. Due to their unique properties—such as small size, high surface area, and the ability to be functionalized with targeting ligands—nanoparticles enable both passive and active targeting. Passive targeting utilizes the enhanced permeability and retention (EPR) effect, while active targeting leverages ligand-receptor interactions for precise localization. This review focuses on key types of nanoparticles used in drug delivery, including polymeric nanoparticles, liposomes, solid lipid nanoparticles, and metallic nanoparticles, each offering unique advantages for targeted delivery. Recent advancements in responsive nanoparticles—designed to release drugs in response to specific stimuli, such as pH or temperature—have further optimized therapeutic delivery, particularly in cancer treatment. While nanoparticle-based delivery systems hold immense potential, challenges such as biocompatibility, toxicity, stability, and regulatory hurdles remain. Addressing these issues through advanced research and clinical trials is essential for translating this technology into safe, effective therapies. In summary, nanoparticle-mediated targeted drug delivery presents a promising future for precision medicine, offering tailored treatments across a spectrum of diseases.
Keywords
Targeted drug delivery, Precision medicine, Bioavailability, Drug release control, Cancer therapy.
Introduction
Nanoparticles are defined as solid particles or dispersions ranging from 10 to 1000 nm in size, with drugs either dissolved, encapsulated, or attached to their matrix. Depending on the preparation method, these can be classified as nanoparticles, nanospheres, or nanocapsules. Nanocapsules contain the drug within a cavity surrounded by a specific polymer membrane, while nanospheres uniformly disperse the drug throughout a matrix. In recent years, biodegradable polymeric nanoparticles, especially those coated with hydrophilic polymers like polyethylene glycol (PEG), have emerged as promising drug delivery systems. These long-circulating particles can persist in the bloodstream for extended periods, target specific organs, and serve as carriers for DNA in gene therapy, as well as deliver proteins and peptides.
Polymeric nanoparticles have been developed for various high-performance applications, including impact-resistant polymers and specialty coatings. Advanced analytical techniques and computer simulations enable us to understand the processes involved in particle formation, allowing for precise measurement of structure and the development of control strategies. By modifying factors such as carrier shape, chemical composition, internal structure, and nanoparticle morphology, we can enhance the performance of targeted drug delivery systems. (1)
Targeted Drug Delivery System
Targeted drug delivery is a specialized system that directs medication specifically to the site of action, avoiding non-targeted organs, tissues, or cells. This approach involves administering a precise amount of a therapeutic agent over an extended period to a targeted diseased area, enhancing efficacy while minimizing side effects. By maintaining appropriate plasma and tissue drug levels, it helps prevent harm to healthy tissues from the medication. Carriers should be biodegradable or easily eliminated from the body. The development of the targeted drug delivery system should be straightforward, reproducible, and cost-effective. Targeted drug delivery offers better solubility and enhanced drug stability compared to traditional methods. Conventional drugs often suffer from poor absorption, short half-lives, and necessitate large distribution volumes, issues that are minimized with targeted delivery. (2)

Figure.1 Targeted Drug Delivery System
Targeted drug delivery is a technique that focuses on delivering medication to specific areas of the body, enhancing its concentration where it's needed while minimizing exposure in other areas. This approach not only boosts the effectiveness of the treatment but also reduces side effects. Essentially, drug targeting aims to direct medications to specific receptors, organs, or tissues, ensuring that they act primarily where desired. The therapeutic index of a drug, determined by its pharmacological effects and safety, depends on the precise delivery of the drug to its intended receptor while reducing exposure to non-target tissues. (3)
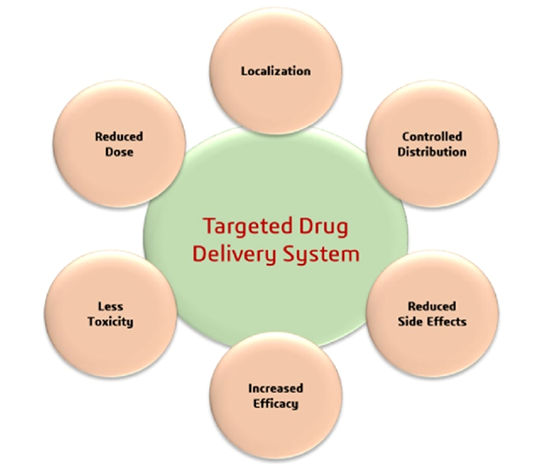
Figure.2 Benefits of Targeted Drug Delivery Systems
Advantages of targeted drug delivery system
1. Enhanced Efficacy: Delivers medication directly to the target site, improving treatment effectiveness.
2. Reduced Side Effects: Minimizes exposure to healthy tissues, decreasing adverse reactions.
3. Improved Patient Compliance: Simplifies treatment regimens, making it easier for patients to follow.
4. Lower Dosage Requirements: Allows for smaller amounts of medication, reducing costs.
5. Optimized Treatment Outcomes: Increases overall success rates by ensuring precise drug action. (4)
Disadvantages of Targeted Drug Delivery:
1. Complexity and Expense: The creation and production of targeted delivery systems can be intricate and expensive.
2. Risk of Toxicity: Certain materials used in these systems might trigger immune responses or other negative effects
3. Restricted Applicability: Not every drug or medical condition can benefit from targeted delivery methods.
4. Challenges in Release Control: Maintaining controlled and sustained release at the intended site can be difficult.
5. Regulatory Hurdles: Due to their complexity, these delivery systems may undergo stringent regulatory evaluations. (5)
Nanotechnology
Nanotechnology, originating from the Greek word ‘Nano’ meaning dwarf, integrates concepts from engineering, electronics, physical science, material science, and manufacturing at the molecular and supra-micron scale. It involves the intentional manipulation of matter into structures ranging from 1 nm to 100 nm, allowing for the creation of nano-systems with enhanced functionalities. In the past decade, the rise of nanotechnology has positioned Ireland at the forefront of scientific research.
Nanoparticles are the product of modifying matter technologically and are slightly larger than atoms due to molecular processing. They exhibit improved characteristics such as stability and self-reassembly, making them adaptable and capable of being tailored for specific properties, including a higher surface area compared to traditional substances. As a relatively new field, nanotechnology has gained significant attention over the last twenty years, transitioning rapidly from academic research to industrial applications. It is estimated that nanotechnology could contribute around three trillion dollars to the global economy by 2020, highlighting its economic potential. This impact is largely due to the unique physicochemical properties of nanoparticles at the intersection of chemistry, medicine, physics, and engineering
Nanotechnology is among the fastest-growing fields in scientific research and development, with major advancements across various applications. Currently, its cutting-edge work spans multiple areas, including electronics, energy, materials science, and biomedicine. In electronics, researchers are investigating nanoscale transistors and components to produce smaller, faster, and more energy-efficient devices. In the energy sector, nanotechnology is being applied to create innovative materials and devices for solar energy conversion and energy storage. In biomedicine, it is advancing the development of new diagnostic tools, therapies, and strategies for tissue engineering. (6)
Advantages and Disadvantages of nanoparticle
Advantages
1. It enhances the solubility of poorly water-soluble drugs, extends the drug's half-life in the system, reduces circulation by minimizing immunogenicity, releases the drug at a sustained rate, and decreases the frequency of administration.
2. It offers greater comfort and compliance for patients while also improving the therapeutic effectiveness of the drug compared to conventional systems.
Disadvantages
1. There are bioacceptability limitations to consider.
2. Large-scale manufacturing poses challenges.
3. The small particle size and large surface area can cause aggregation of particles, complicating the handling of nanoparticles in both liquid and dry forms.
4. The small size and large surface area often lead to restricted drug loading and rapid release. These practical issues must be addressed before nanoparticles can be used in clinical or commercial applications. (7)
Mechanisms of Drug Delivery via Nanoparticles
The process of drug delivery through nanoparticles involves several mechanisms. Nanoparticles can enhance drug solubility, facilitate cellular uptake, and enable targeted delivery to specific tissues or cells. By using nanoparticles, drugs can be released in a controlled manner, improving therapeutic efficacy and minimizing side effects.
Nanoparticles facilitate site-specific drug delivery by bypassing the reticuloendothelial system, leveraging the enhanced permeability and retention effect, and employing targeted approaches. Two primary methods are used for drug delivery with nanoparticles as carriers:
a. Surface bound: Drug molecules are attached to the surface of the nanoparticles.
b. Core bound: In this method, drug particles are concentrated within the nanoparticle matrix and transported to the target site in the body.
Drugs can be loaded onto nanoparticles by mixing them into a solution containing pre-prepared nanoparticles or by incorporating them into the reaction mixture during the polymerization process. The interaction between the nanoparticles and the drug can vary, involving chemical bonding, surface adsorption, or no interaction at all. The quantity of drug bound and the nature of the interaction depend on the chemical structures of both the drug and the polymer, as well as the conditions during drug loading. (8)
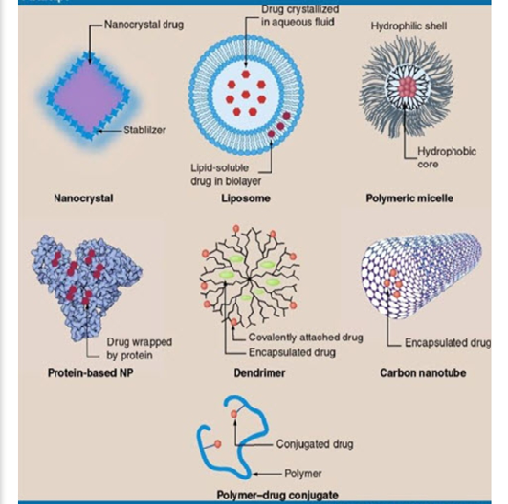
Figure.3 Types of Nanoparticles
Nanoparticles (NPs) are categorized into several groups based on their morphology, size, and chemical properties. Some of the most recognized classes of nanoparticles, classified according to their physical and chemical characteristics, include:
1. Solid lipid nanoparticles (SLNs)
2. Liposomes
3. Nanostructured lipid carriers (NLC)
4. Fullerenes
5. Nanoshells
6. Quantum dots (QD)
7. Superparamagnetic nanoparticles
1) Solid Lipid Nanoparticles (SLNs)
Introduced in 1991, Solid Lipid Nanoparticles (SLNs) offer an improved carrier system compared to traditional colloidal carriers like emulsions, liposomes, and polymeric micro and nanoparticles. SLNs are colloidal systems made up of a high-melting-point lipid that forms a solid core, which is coated with an aqueous surfactant. They are particularly suitable for drugs classified as BCS Class II and IV. In SLNs, solid lipids replace the liquid lipids typically found in other colloidal carriers. The use of solid lipids as a matrix for drug delivery is well established, as seen in lipid pellets for oral administration, such as Mucosolvan® retard capsules. (9) Solid lipid nanoparticles represent a promising novel colloidal transport system, offering an alternative to polymers and resembling oil-in-water emulsions for parenteral nutrition. In this system, liquid lipids in emulsions are replaced by solid lipids. This substitution is advantageous, as solid lipids enhance control over the release kinetics of encapsulated substances and improve the stability of chemically sensitive lipophilic compounds. (10)
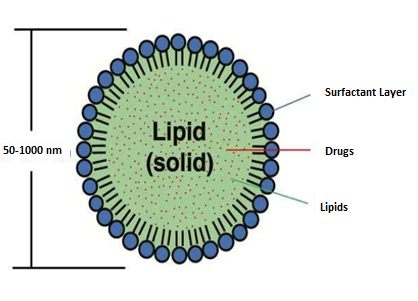
Figure. 4 Solid lipid nanoparticles
Solid lipid nanoparticles (SLNs) are nanoscale carriers composed of solid lipids that are used for delivering drugs and other bioactive compounds. They provide benefits like improved stability, controlled release, and enhanced bioavailability, making them valuable in pharmaceutical and cosmetic applications. (11)
2) Liposomes
Liposomes were among the first drug delivery vehicles to be studied. These vesicles consist of an aqueous core surrounded by a hydrophobic lipid bilayer. While solutes within the core, such as drugs, cannot cross the hydrophobic barrier, the bilayer allows for the absorption of hydrophobic molecules, making liposomes effective amphiphilic carriers. Liposomes vary in their composition, size, and number of layers. They can be classified as "unilamellar," which have a single bilayer, or "multilamellar," which contain multiple bilayers. Unilamellar vesicles are further categorized into small unilamellar vesicles (SUVs) and large unilamellar vesicles (LUVs) based on size. Drugs encapsulated in liposomes demonstrate significantly improved pharmacokinetic properties, including enhanced therapeutic indices. (12)
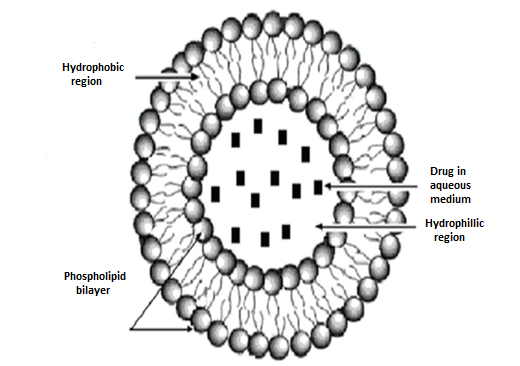
Figure. 5 Liposomes
Liposomes are tiny spherical structures made up of lipid layers that can encapsulate various substances. They are used in drug delivery and other applications because they can enhance the absorption and effectiveness of medications while reducing side effects. (13)
3) Nanostructured lipid carriers
Newly created nanostructured lipid carriers (NLCs) contain a unique lipid matrix developed by Müller. This specific nanostructure enhances the drug's bioavailability, loading capacity, and solubility under various conditions. Various methods, such as high-pressure homogenization, can be employed to prepare or formulate these NLCs.

Figure. 6 Nanostructured lipid carriers.
NLCs consist of a hybrid mixture of solid lipid and liquid lipid (oil), with an average size ranging from 10 to 500 nm. This mixture typically features a long-chain liquid lipid to solid lipid ratio of 99.9:0.1, and a short-chain solid lipid to liquid lipid ratio of 70:30. (14)
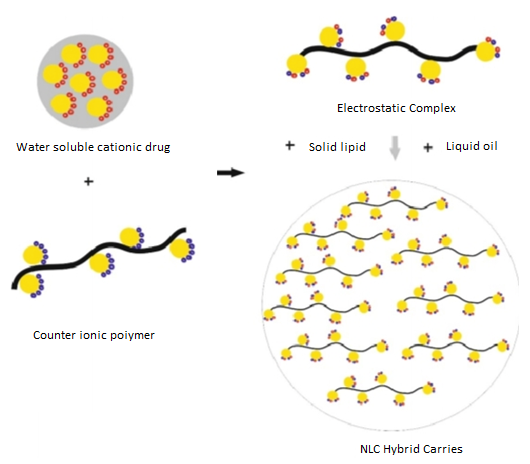
Figure. 7 hybrid nano-lipid carrier
The figure 7 illustrates a hybrid nano-lipid carrier composed of a solid lipid matrix combined with a liquid lipid phase. This mixture enhances the stability and bioavailability of the carrier, allowing for improved delivery of active compounds. The solid lipid provides structural integrity, while the liquid lipid offers flexibility and solubility advantages, making this system effective for various pharmaceutical applications.
4) Fullerenes
Fullerenes (C60) are spherical carbon molecules formed by carbon atoms connected through sp2 hybridization. These structures typically consist of 28 to 1500 carbon atoms, with diameters ranging from 8.2 nm for single-layer fullerenes to 4–36 nm for multilayer variants. Fullerenes are nanomaterials characterized by their hollow, globular cages and various allotropic forms of carbon. Their notable properties, including electrical conductivity, high strength, unique structure, electron affinity, and versatility, have garnered significant commercial interest. (15)
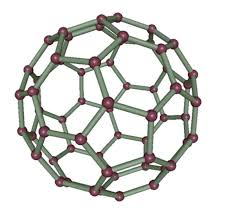
Figure. 8 Fullerenes structure
Fullerenes, specifically C60, are molecular structures composed of 60 carbon atoms arranged in a spherical shape, resembling a soccer ball. This unique configuration consists of hexagons and pentagons, creating a stable and symmetrical form. (16)
5) Nanoshells
Nanoshells are nanoparticles consisting of a dielectric core encased in a thin metallic shell, typically made of gold. These nanoshells exhibit plasmonic properties, which means they involve quasiparticles known as nanoshell plasmons. This phenomenon involves collective excitations or quantum plasma oscillations, where electrons oscillate collectively in relation to the ions. (17)
Figure.9 Nanoshells
Nanoshells are microscopic particles that consist of a core material, typically silica, surrounded by a thin metallic shell, often gold or silver. They exhibit unique optical properties due to the interaction of light with the metallic layer, allowing them to be used in applications like medical imaging, drug delivery, and photothermal therapy. By varying the size and composition of the nanoshells, their absorption and scattering characteristics can be finely tuned for specific purposes. (18)
6) Quantum dots (QD)
Quantum dots are tiny devices composed of small clusters of free electrons, classified as colloidal semiconductor nanocrystals ranging from 2 to 10 nm in size. (19) These quantum dots can be either semiconductor nanocrystals or core-shell nanocrystals that include interfaces between various semiconductor materials. (20)
Quantum dots (QDs) are nanostructures that confine electrons to zero dimensions, resembling a three-dimensional quantum well limited to this scale. They are structurally and electrostatically isolated from their environment and typically consist of hundreds to millions of atoms, while containing fewer than a hundred free electrons. (21)

Figure. 10 Quantum dots
Quantum dots are tiny semiconductor particles that exhibit unique optical and electronic properties due to their size. These nanometer-sized structures can confine electrons and holes, leading to quantized energy levels. As a result, they can emit light in various colors depending on their size, making them useful in applications such as displays, solar cells, and biological imaging.

Figure. 11 Structure Quantum dots
Figure 11 illustrates the structure of a quantum dot. It typically consists of a semiconductor material that confines electrons in three dimensions, leading to quantized energy levels. The unique properties of quantum dots arise from their size, which is on the nanoscale, allowing for applications in electronics and photonics. (22)
7)Superparamagnetic nanoparticles
Superparamagnetic nanoparticles show considerable magnetization only in the presence of a magnetic field, losing this magnetization once the field is removed. This reversible behavior enables the control of these materials using applied magnetic fields, which opens up various potential applications like microactuators, magnetic separation, and drug delivery. Importantly, the phenomenon of superparamagnetism is closely linked to the size of the particles. (23)
In the context of medical imaging, the effectiveness of Magnetic Particle Imaging (MPI) must be evaluated based on key imaging parameters, including image contrast, spatial resolution, and sensitivity. Since human tissue is diamagnetic, the tracer materials provide the sole source of the MPI signal. MPI images achieve nearly perfect contrast, making them ideal for detecting tracer materials with minimal background interference. (24)

Figure. 12 Superparamagnetic nanoparticles
Figure 12 depicts superparamagnetic nanoparticles, which are small particles that exhibit magnetic properties only in the presence of an external magnetic field. When the field is removed, these nanoparticles lose their magnetism, making them ideal for various applications, including targeted drug delivery and medical imaging. Their size and unique magnetic behavior are crucial for their functionality in these fields. (25)
Evaluation of nanoparticles
Electrophoretic mobility
Zeta potential refers to the potential difference between the surface of a solid particle submerged in a conductive liquid (such as water) and the surrounding liquid. The surface charge of nanoparticles is typically assessed using zeta potential measurements. Particles with a zeta potential greater than ±30 mV are considered stable in suspension, as their surface charge inhibits particle aggregation. (26)
Analysis of particle size
The particle size of the nanoparticles was assessed using a Scanning Electron Microscope, with sizes ranging from 350 nm to 600 nm, varying according to the polymer load. (27)
Scanning Electron Microscopy (SEM) analysis
Using scanning electron microscopy, researchers examined the surface morphology and particle structure of nanoparticles. Completely moisture-free lyophilized samples were attached to aluminum stubs with adhesive tape, coated with gold using a sputter coater, and analyzed for morphology at an acceleration voltage of 20 kV. (28)
Differential scanning calorimetry (DSC)
Differential scanning calorimetry (DSC) is a technique used to measure the heat flow associated with material transitions as a function of temperature.
The thermal properties of the drug and excipients were analyzed using DSC to identify the physical state of the drug, the physical mixture, and the optimized formulation. DSC thermograms were recorded for both the drug and the optimized formulation. (29)
X-ray diffraction analysis was conducted
X-ray diffraction analysis was performed with an XRD-6000 diffractometer to assess the crystallinity of both the pure drug and the formulation. The powder samples were placed in an aluminum holder, and Cu radiation was produced at 30 mA and 40 kV. The samples were scanned from 10° to 90° at a rate of 10° per minute, as described earlier. (30).
REFERENCES
- Kannadasan, M., Bichala, P. K., Agrawal, A., & Singh, S. (2020). A review: Nano particle drug delivery system. International Journal of Pharmaceutical Sciences and Medicine (IJPSM), 5(12), 46. ISSN: 2519-9889.
- Nagoba, S. N., Warkari, R. D., Chandrawanshi, M. J., Bhalekar, R. V., & Swamy, V. S. (2018). A review on targeted drug delivery. American Journal of PharmTech Research. ISSN: 2249-3387.
- Gupta, M., & Sharma, V. (2011). Targeted drug delivery system: A review. Research Journal of Chemical Sciences, 1(2).
- Chandrakala, V., Aruna, V., & Angajala, G. (2022). Review on metal nanoparticles as nanocarriers: Current challenges and perspectives in drug delivery systems. Emergent Materials, 5, 1593–1615.
- Choudhury, H. K., Panigrahi, G., & Choudhury, P. K. (n.d.). Targeted drug delivery systems: A prospective review. Research Journal of Pharmacy and Life Sciences, 3, 5–27. ISSN: 2582-6441.
- Yusuf, A., Almotairy, A. R. Z., Henidi, H., Alshehri, O. Y., & Aldughaim, M. S. (n.d.). Nanoparticles as drug delivery systems: A review of the implication of nanoparticles’ physicochemical properties on responses in biological systems.
- Singh, S., Pandey, V. K., Tewari, R. P., & Agarwal, V. (2011). Nanoparticle-based drug delivery system: Advantages and applications. Indian Journal of Science and Technology, 4(3). ISSN: 0974-6846.
- Hnawate, R. M., & Deore, P. (2017). Nanoparticle - novel drug delivery system: A review. PharmaTutor, 5(5). Print ISSN: 2394-6679; e-ISSN: 2347-7881.
- Lingayat, V. J., Zarekar, N. S., & Shendge, R. S. (2017). Solid lipid nanoparticles: A review. Nanoscience and Nanotechnology Research, 4(2), 67–72.
- Yadav, N., Khatak, S., & Singh, U. V. (2013). Solid lipid nanoparticles: A review. International Journal of Applied Pharmaceutics, 5(2). ISSN: 0975-7058.
- Saini, A., & Gupta, P. (2017). Targeted solid lipid nanoparticles: A novel approach against malaria. Indian Research Journal of Pharmacy and Science, 12, 925–944.
- Mahaparale, S. P., & Deshmukh, J. U. (2020). Review on: Targeted drug delivery. International Journal of Creative Research Thoughts (IJCRT), 8(4). ISSN: 2320-2882.
- Rewar, S., Singh, C. J., Bansal, B. K., Pareek, R., & Sharma, A. K. (2014). A vital role of liposomes on controlled and novel drug delivery. International Journal of Pharmaceutical & Biological Archives, 5(2), 51–63.
- Sharma, A., & Baldi, A. (n.d.). Nanostructured lipid carriers: A review. Journal of Developing Drugs, 7. ISSN: 2329-6631.
- Salem, S. S., Hammad, E. N., Mohamed, A. A., & El-Dougdoug, W. (2023). A comprehensive review of nanomaterials: Types, synthesis, characterization, and applications. Volume, 13(1), 41.
- Yadav, B. C., & Kumar, R. (2008). Structure, properties, and applications of fullerenes. International Journal of Nanotechnology and Applications, 2(1), 15–24. ISSN: 0973-631X.
- Lakshmi Prasanna, M., Meghana, D., Mani, N. S., Mohan Reddy, N. V. J., & Narendra, D. (2019). A review on recent advances in nanotechnology- gold nanoshell synthesis and its applications. International Journal of Scientific Development and Research (IJSDR), 4(1). ISSN: 2455-2631.
- Halas, N. J. et al. (2003). Nanoshell-enabled photonics-based imaging and therapy of cancer. Technology in Cancer Research & Treatment, 3(1), 33–40.
- Prakash, A., Raj, P. R., Daisy, A. P., & Abeena, P. B. (2020). A review on nanoparticle. International Journal of Pharmaceutical Sciences Review and Research, 64(68). ISSN: 0976–044X.
- Hnawate, R. M., & Deore, P. (2017). Nanoparticle - novel drug delivery system: A review. PharmaTutor, 5(5). Print ISSN: 2394-6679; e-ISSN: 2347-7881.
- Joglekar, P. V., Mandalkar, D. J., Nikam, M. A., Pande, N. S., & Dubal, A. (2019). Review article on quantum dots: Synthesis, properties, and application. International Journal of Research in Advent Technology, 7(1). e-ISSN: 2321-9637.
- Sathe, K. P., Garud, N. S., Bangar, V. B., & Gadakh, N. R. (2017). A review on quantum dots (QDs). Journal of Advanced Scientific Research. ISSN: 0976-9595.
- Hu, M., Butt, H. J., Landfester, K., Bannwarth, M. B., Wooh, S., & Therien-Aubin, H. (2018). Shaping the assembly of superparamagnetic nanoparticles. DOI: 10.1021/acsnano.8b07783.
- Du, Y., Lai, P. T., Leung, C. H., & Pong, P. W. T. (2013). Design of superparamagnetic nanoparticles for magnetic particle imaging (MPI). International Journal of Molecular Sciences, 14, 18682–18710. ISSN: 1422-0067.
- Li, W., & Fortner, J. D. (2020). (Super)paramagnetic nanoparticles as platform materials for environmental applications: From synthesis to demonstration. Frontiers of Environmental Science & Engineering, 14, Article 77.
- Sriharitha, S., Patil, A., & Swaroop, H. (2016). A review on nanoparticles in targeted drug delivery system. Research & Reviews: Journal of Material Science (JoMS), 4(4), November. e-ISSN: 2321-6212; p-ISSN: 2347-2278.
- Pokhriyal, A., & Tripathi, G. (2022). Evaluation parameters of nanoparticles: A review. Indian Journal of Novel Drug Delivery, 14(3), 138–142.
- Rathod, A. V., Katekar, V. A., & Deshmukh, S. P. (2023). A review: Recent advancement in the formulation and evaluation of nanoparticles and its application. GSC Biological and Pharmaceutical Sciences, 25(2), 116–122. e-ISSN: 2581-3250.
- Gautham, U., Patil, A., & Hemanth, G. (2023). Formulation and evaluation of nanoparticle drug delivery system for treatment of hypertension. International Journal of Applied Pharmaceutics, 15(6). ISSN: 0975-7058.
- Pokhriyal, A., & Tripathi, G. (2022). Evaluation parameters of nanoparticles. Der Pharmacia Lettre, 14(3), 24–29. ISSN: 0975-5071


 Gaikwad Vaishnavi *
Gaikwad Vaishnavi *
 Palghadmal Varsharani
Palghadmal Varsharani
 Throat Suvarna
Throat Suvarna












 10.5281/zenodo.14281647
10.5281/zenodo.14281647