The most important chemical features and biological behavioral Characteristics of the carrier molecules exploited for drug targeting purposes will be addressed in this review article. Targeted drug delivery is also known as smart drug delivery. This is self-contained, discrete Dosage form which is applied to intact skin, at a controlled rate to the systemic circulation. In this System medicament given to a patient in a manner that increases the concentration of the Medication in some parts of the body relative to others. System involves nanoparticles-mediated Drug delivery in order to reduce drawback of conventional drug delivery. Nanoparticles (NPs) are the unique tiny materials which exist on a nanometer scale ranging from 1 to 100 nm. These nanoparticles exist in variety of forms. The nanoparticles exhibit Enhanced physical and chemical properties such as high surface area, reactivity, stability, sensitivity etc. due to their small size. These NPs can be synthesized by various methods.This review article highlights the classification, method of preparation and applications of nanoparticles.
Nanoparticles, Classification of nanoparticles, methods of preparation and application of nanoparticles.
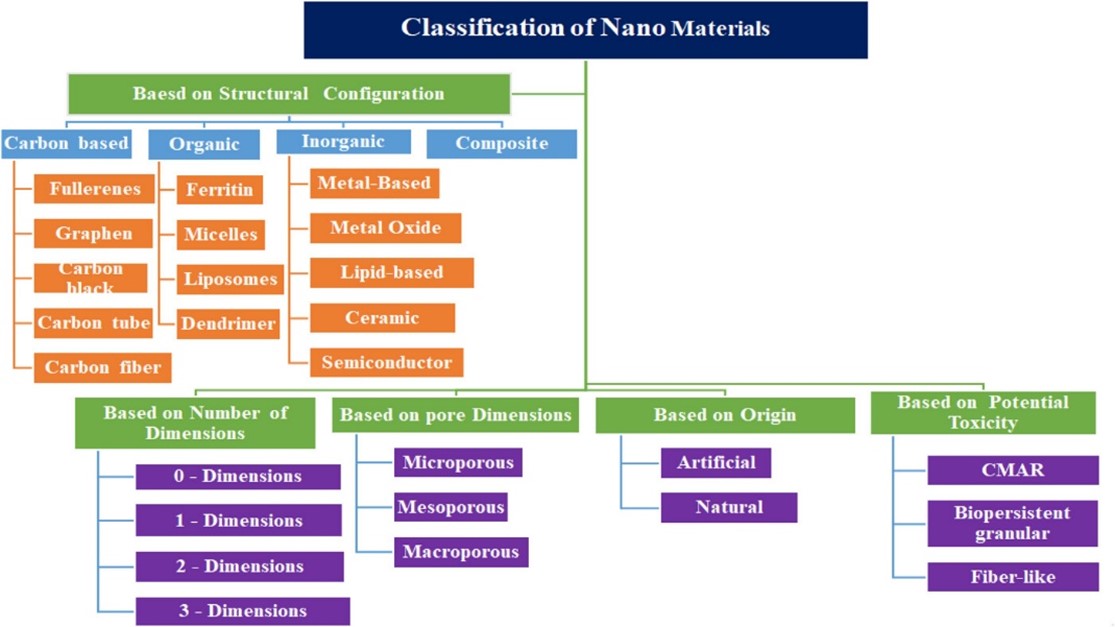
Nanomaterials can be divided into five categories depending on their size, place of origin, structural configuration/composition, pore size, and potential toxicity.
Classification of nanoparticles based on their origin:
Natural nanoparticles are generated in different environmental compartments by various physical, chemical, and biological processes, such as (bio)chemical weathering of minerals, photo-oxidation, redox and precipitation reactions, (bio)mineralization, physical fragmentation, gas-solid nucleation in the atmosphere, etc Natural nanomaterials can be found in a variety of forms in nature, including viruses, protein molecules, minerals like clay, natural colloids like milk and blood (liquid colloids), fog (aerosol type), gelatin (gel type), mineralized natural materials like shells, corals, and bones, insect wings and opals, spider silk, lotus leaves, gecko feet, volcanic ash, and ocean spray.
- Artificial nanoparticles:
Carbon nanotubes and semiconductor nanoparticles like quantum dots (QDs) are examples of artificial nanomaterials that are made consciously using precise mechanical and manufacturing procedures. Nanomaterials are categorized as metal-based materials, dendrimers, or composites depending on their structural makeup.
Classification of nanoparticles based on the structural composition:
Micelles, dendrimers, liposomes, nanogels, polymeric NPs, and layered biopolymer. Certain organic nanoparticles, such as micelles and liposomes, have a hollow sphere, and they are non-toxic and biodegradable. Organic nanoparticles can also be broken down naturally.
They are typically classified as those composed of metal-based or metal oxide-based or lipid-based and semiconductor nanomaterials. Inorganic nanoparticles do not contain carbon. Inorganic nanoparticles have the advantages of being hydrophilic, non-toxic, and biocompatible with living systems. The stability of inorganic nanoparticles is superior to that of organic nanoparticles.
- Metal based nanoparticles:
Metal-based nanoparticles can be synthesized through destructive or constructive processes. Aluminum (Al), cadmium (Cd), cobalt (Co), copper (Cu), gold (Au), iron (Fe), lead (Pb), silver (Ag), and zinc (Zn) are metal materials that are frequently used in nanoparticle synthesis.
- Metal oxide nanoparticles:
They also known as metal oxide nanomaterials, are composed of positive metallic ions and negative oxygen ions. Examples of metal oxide nanoparticles that are frequently synthesized include silicon dioxide (SiO2), titanium oxide (TiO2), zinc oxide (ZnO), and aluminium oxide (Al2O3).
- Semiconductor nanoparticles:
They are classified into three groups;
-
- Concentrated magnetic semiconductor nanomaterials
- Non-magnetic semiconductor nanomaterials
- Diluted magnetic semiconductor nanomaterials
- Lipid based nanoparticles:
Lipid-based nanoparticles are generally spherical, with diameters ranging between 10 and 100 nm. It consists of a solid core made of lipids and a matrix containing soluble lipophilic molecules. Lipid-based nanoparticles have applications in the biomedical field as a drug carrier and RNA release therapy in cancer therapy
Carbon-based nanomaterials:
They are composed of carbon include five main materials, namely, carbon nanotubes, Graphene, fullerenes, Carbon Nano fiber and Carbon black. Spherical and ellipsoidal nature configured of carbon nanomaterials are referred as fullerenes are called Bucky balls. Fullerenes are the spherical structure with diameters up to 8.2 nm for a single layer and from 4 to 36 nm for multi-layered fullerenes, which form from 28 to 1500 carbon atoms.
Composites nanomaterials:
A nanocomposite is a matrix to which nanoparticles have been added to improve a particular property of the material. The properties of nanocomposites have caused researchers and companies to consider using this material in several fields. The nanoparticles if added to the polymers matrix in the concentration of around .5 to 2%, the properties of the composites like mechanical, physical, electrical, optical shows a dramatic change in their magnitude.
Classification of nanoparticles according to the number of dimensions:
- Zero-Dimensional Nanoparticles:
These nanomaterials have all three dimensions (x, y, and z) within the nanoscale range or are not dimensional outside the Nano metric range (>10 nm). The few examples are QDs, Fullerenes, Atomic clusters, Organic molecules and nanoparticles.
- One-Dimensional Nanoparticles:
A one-dimensional nanostructure is one in which two dimensions are in the nanoscale. These are linear structures with diameter less than 100 nm, including nanowires, nanotubes and nanorods.
- Two-Dimensional Nanoparticles :
A two-dimensional nanostructure is one in which one dimension is in the nanoscale. For example : Thin films
- Three-Dimensional Nanoparticles:
3D nanomaterials can be synthesized in various shapes like spheres, tubes, gyroids, etc.Due to their high specific surface area, interconnected porous structure, light weight, and high mechanical strength, CNF-based three-dimensional (3D) nanomaterials have attracted more and more attention in many fields.
IDEAL CHARACTERISIC OF NANOPARTICLES:
- They have unique physical and chemical properties due to their small size, high surface area-to-volume-ratio, and ability to absorb.
- Nanoparticles will be able to deliver a concentrated dose of drug in the vicinity of the tumor targets via the enhanced permeability and retention effect or active targeting by ligands on the surface of nanoparticles.
- Nanoparticles will reduce the drug exposure of healthy tissues by limiting drug distribution to target organ.
- They are highly mobile in the free state.
ADVANTAGES OF NANOPARTICLES:
- The Potential Advantages of Nanoparticle Drug Delivery Systems in Chemotherapy of Tuberculosis.
- They have high stability and high carrier capacity.
- They have feasibility of incorporation of both hydrophilic and hydrophobic substances.
- Nanoparticles can also be designed to allow controlled (sustained) drug release from the matrix.
- They may resolve the problem of nonadherence to prescribed therapy, which is one of the major obstacles in the control of TB epidemics.
DISADVANTAGES OF NANOPARTICLES:
- Negative Environmental Impact
- Decreased in Employement
- Economic Imbalance
- Health Problems
- High Cost
- Practicals Problems
METHODS OF PREPARATION OF NANOPARTICLES:
For the preparation of nanoparticles , variety of materials required such as proteins, polysaccharides and synthetic polymers.
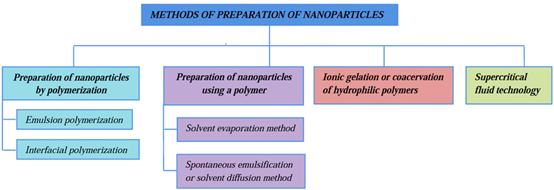
Fig.3 Applications of Nanoparticles
Making nanoparticles using polymerization:
- Polymerization by emulsion:
The preparation process involves dispersing the monomer, if it is a liquid, in an agitated continuous phase where it is immiscible. Usually, the reaction of the initiators with the monomer molecules dissolved in the emulsion's continuous phase starts the polymerization process. By adding more monomer molecules, which diffuse toward the expanding polymer chain during the continuous phase, the polymerization process continued unabated. Up until it reaches a particular molecular weight, the expanding polymer chain is soluble. After that, it becomes insoluble. Phase separation is caused by the nucleation of the polymer particles and the development of the Tyndall scattering effect. The overall system's stability characteristics are what drive the continued expansion of the nucleated particles.
Using a polymer to prepare nanoparticles:
The nanoparticles in this group of techniques are made from a polymer using an entirely different way of preparation. How to evaporate a solvent: Using an organic solvent such as dichloromethane, chloroform, or ethyl acetate—which is also used to dissolve the hydrophobic medication—the polymer is dissolved in this approach. An emulsifying agent or surfactant is used to emulsify the medication and polymer mixture in an aqueous solution to create an oil in water (o/w) emulsion. Upon the formation of a stable emulsion, the organic solvent is removed through either constant stirring or pressure reduction. The kind, concentrations of stabilizers, and speed all affect the final particle size.
- Solvent diffusion method:
It is also known as spontaneous emulsification, is a modified version of the solvent evaporation method. This approach uses the water miscible solvent as the oil phase, with a modest addition of the water immiscible organic solvent. Small particles arise as a result of an interfacial turbulence that occurs between the two phases as a result of the abrupt diffusion of solvents. Particle size can decrease as the concentration of the water miscible solvent rises. Both of the aforementioned techniques can be applied to hydrophilic or hydrophobic medications. When using a hydrophilic drug, it is necessary to create a multiple w/o/w emulsion with the drug dispersed in the internal aqueous phase.
Coacervation or ionic gelation method:
The majority of research in the last few years has focused on creating nanoparticles utilizing hydrophilic polymers that degrade naturally, such as sodium alginate, chitosan, and gelatin. Calvo and colleagues have devised a method for ionic gelation-based hydrophilic chitosan nanoparticle production. This process involves combining two aqueous phases: chitosan, a di-block co-polymer of ethylene oxide or propylene oxide, and the polyanion sodium tripolyphosphate (PEO-PPO). This process creates coacervates with a variety of nanoscale sizes by combining the negatively charged tripolyphosphate with the positively charged amino group of chitosan.
Production of nanoparticles using supercritical fluid technology:
Conventional methods like solvent extraction-evaporation, solvent diffusion and organic phase separation methods require the use of organic solvents which are hazardous to the physiological systems as well as to environment. A supercritical fluid can be generally explained as a solvent at a temperature above its critical temperature, where the fluid remains in a single phase irrespective of pressure [19]. Supercritical CO2 (SC CO2) is the universally used supercritical fluid because of its meek critical conditions (Tc = 31.10C, Pc = 73.8 bars), non-flammability, nontoxicity, and low price
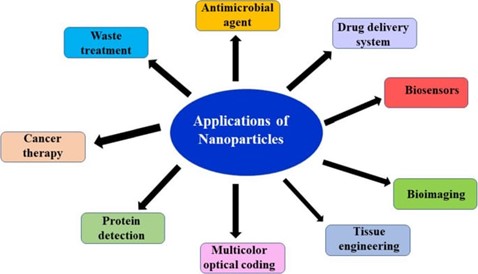
APPLICATIONS OF NANOPARTICLES:


 Dipali N. Hagir*
Dipali N. Hagir*
 Pranjal Amol Girme
Pranjal Amol Girme
 Prachi chandrakant phadtare
Prachi chandrakant phadtare


 10.5281/zenodo.10965452
10.5281/zenodo.10965452