Abstract
Emulsions are liquid–liquid dispersions with one liquid phase dispersed in the other liquid phase as small droplets. Nano-emulsions consist of fine oil-in-water dispersions, having droplets covering the size range of 100–600nm. Nano-emulsions are also referred to as mini-emulsions. Nanoemulsions have great potential applications in pharmaceutics, foods and cosmetics due to their attractive properties. Nanoemulsion is a dispersion consisting of oil, surfactant and an aqueous phase, which is an isotropically clear and thermo-dynamically or kinetically stable liquid solution. One often most promising technology is the nanoemulsion drug delivery system, which is being applied to enhance the solubility and bioavailability of lipophilic drugs. The nanosized droplets leading to an enormous increase in interfacial areas associated with nanoemulsion would influence the transport properties of the drug. Correspondingly, an over view of characterisation technologies to differentiate between the micro and nanoemulsions alongside their benchmarks in terms of their physical and thermodynamic stabilities, is also described in this review.
Keywords
Nanoemulsion, Surfactants, Nanomedicine, Thermodynamic stability; Physical stability, Lipid based drug delivery.
Introduction
Emulsions are biphasic liquid systems where one liquid phase known as the internal or dispersed phase is dispersed as small droplets through the second liquid phase known as the external or continuous phase. Emulsions are thermodynamically unstable systems and will rapidly separate into two discrete phases unless surface active molecules, also called emulsifiers, are added to the mixture to stabilize the droplets.[1] NEs are metastable systems with internal phase droplets smaller than 1000nm. They are also referred to in the literature as mini emulsions, ultrafine emulsions and submicron emulsions. NEs are obtained by dispersion of oil in water (O/W) or water in oil (W/O) and stabilization with surfactants.[2] Nanoencapsulation is generally employed to improve solubility, stability and bioactivity of various oil-soluble phytochemicals due to their small droplet size and high kinetic stability. Among the nanometric encapsulation systems, nano emulsions are particularly suitable and widely accepted.[3] Nanoemulsions can be classified based on their morphology. A ‘water-based’, or oil-in-water (O/W), emulsion has water as the continuous phase and oil as the dispersed phase, whereas the inversed condition yields an ‘oil-based’ or water-in-oil (W/O) emulsion.[4] Nanoemulsions exhibit better penetration efficacy of the ingredients due to the large surface area and low surface tension of the whole emulsion system, thus requiring only 3–10% of surfactants during preparation.[5] The most popular approaches are the incorporation of the active lipophilic component into inert lipid vehicles such as oils, surfactant dispersions, self-nano or micro emulsifying formulations, solid emulsions, and liposomes.[6] The drug can be loaded into the inner phase of these systems and delivered by lymphatic route, bypassing the enzymes in the gastrointestinal tract (GIT) and reducing the pre-systemic clearance and hepatic first pass metabolism.[7] Low Dissolution rate and partial absorption will be generally seen in drugs with low permeability and low water solubility. Pharmaceutical researches focus on two fields to improve the oral bio-availability, which are: (i) enhance the dissolution rate as well as the solubility for drugs with low water-solubility, (ii) enhance the permeability related to drugs with low permeability.[8] Solubility can be defined as a major parameter used for the purpose of achieving the required drug’s concentration in a systemic circulation for pharmacological response.[9] Nanoemulsions are highly biocompatible, and isotropic dispersed systems which may be formulated by various processes of high or low energy. Low energy approaches are very attractive since they are effective at producing fine droplets, require low equipment, are simple and low costly to use, and are easy to implement.[10]
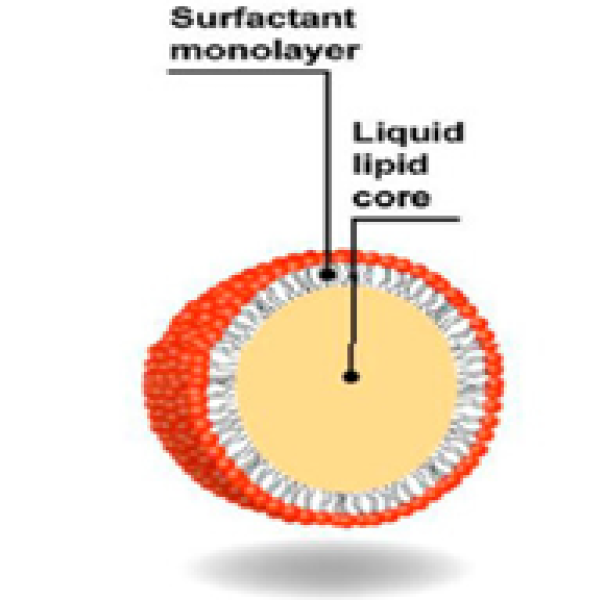
Figure 1: Nanoemulsion droplets
Advantages of nanoemulsion:
- It may be used as substitute for liposomes and vesicles
- It improves the bioavailability and physical stability of drug
- It helps to solubilize lipophilic drug
- Helpful in taste masking
- Nanoemulsions have small-sized droplets having greater surface area providing greater absorption
Components of nanoemulsion:
The main components of nanoemulsion are oil, emulsifying agents, and aqueous phases. Oils can be of any type like castor oil, corn oil, coconut oil, evening primrose oil, linseed oil, mineral oil, olive oil, peanut oil, etc. A mixture of oil and water may yield a crude temporary emulsion, which upon standing, will separate in two dis tinct phases due to the coalescence of the dispersed glob ules. Emulgents or emulsifying agents can impart stability to such systems. An emulgent, in addition to its emulsifying proper ties, should be nontoxic and its taste, odour and chemical stability should be compatible with the product.[11]
- Oil/lipids
Nanoemulsions generally contain 5–20% oil/lipid droplets in case of O/W emulsions, though it may sometimes be significantly larger. Lipids/oils to be used in nanoemulsions are generally propositioned on solubility of drug. Type of oil used in formulation of nanoemulsion sometimes determines bioavailable fraction of active constituent.[10]
- Surfactants and co-surfactants
Surfactants are amphiphilic molecules which stabilize nanoemulsions by reducing interfacial tension, and prevent droplet aggregation. They tend to rapidly adsorb at oil water interface and provide steric or electrostatic or dual electro-steric stabilization. Sometimes, co-surfactants are used to complement surfactants, as they fit suitably in between structurally weaker areas, fortifying the interfacial film.[12]
- Preservatives, antioxidants and chemoprotectants
Preservatives employed in nanoemulsions should meet criteria like low toxicity, stability to heat and storage, physical and chemical compatibility, reasonable cost, ease of availability. Emulsified oil and lipids are subject to autoxidation upon exposure to air; many drugs used in nanoemulsion are also highly susceptible to oxidative degradation.[12]
Construction of Pseudoternary Phase Diagram
The initial concentration of the constituents is determined using the water titration method at room temperature by building pseudoternary phase diagrams in the nano emulsion system. Different ratios of the weight of the emulsifier and coemulsifier are used to produce various phase diagrams. These ratios are selected with increasing concentrations of coemulsifier relative to emulsifier and emulsifier relative to coemulsifier in order to thoroughly examine the phase diagrams.[13]
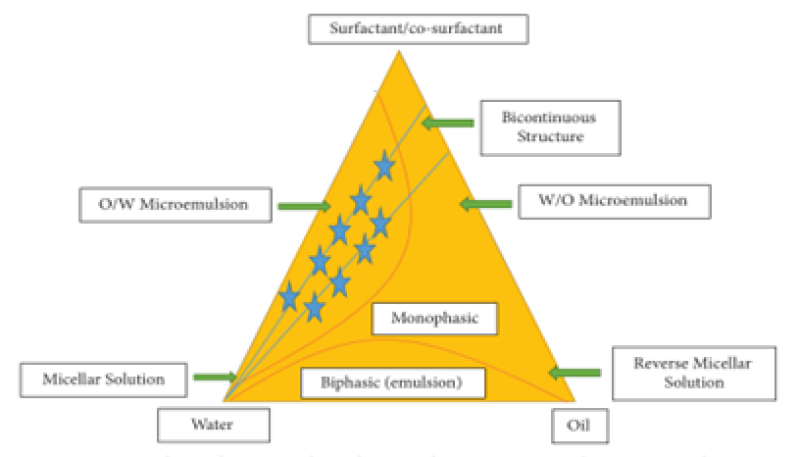
Figure 2: Constructed pseudoternary phase diagram showing microemulsion/nanoemulsion region
METHOD OF PREPARATION:
Low-Energy Methods:
Low-energy emulsification techniques use less power to produce nanoemulsion particles and are more energy-efficient since they utilize the systems’ intrinsic chemical energy and only require gentle stirring. Phase inversion emulsification and self-emulsification, also known as spontaneous emulsification, are two low-energy emulsification techniques.[13]
Spontaneous Emulsification:
There are three steps to it: a homogenous organic solution encompassing oil, a lipophilic emulsifier, a water-soluble cosolvent, and hydrophilic emulsifiers are first prepared as the process’s initial step. Additionally, a continuous magnetic stirring process is used to produce an o/w nanoemulsion, and the aqueous phase is removed with a reduced evaporation pressure process.[14]
Phase Inversion Temperature Method (Self Nanoemulfcation Method):
It involves the natural bending of the emulsifier, which uses a heating process during emulsification that transforms the dispersed phase into the dispersion phase and vice versa. Changes in temperature and composition are two factors that affect spontaneous curvature. In this, phase transitions occur along the emulsify cation path, resulting in the production of fine dispersions through the application of chemical energy. Variations in temperature at constant composition can cause phase transitions. The effectiveness of this approach depends on how non-ionic emulsifiers change solubility as a function of temperature.[15]
Phase Inverse Composition Method (Self Nanoemusilfication Method):
By gradually adding water to an oil-emulsifier solution while gently stirring and maintaining a steady temperature, it is possible to produce kinetically stable nanoemulsions having droplets size 50nm.[16]
High-Energy Method:
In order to provide strong, disruptive forces for size reduction during high-energy emulsification, mechanical equipment is required. Microfluidizers, homogenizers, and ultrasonicators can provide these forces, but they are expensive and produce high working temperatures, which are inappropriate for drugs that are thermolabile.[17]
High-Pressure Homogenization Methods:
The process produces NEs from a high-pressure homogenizer/piston homogenizer with very fine particle sizes (up to 1nm). A high-pressure homogenizer forces two liquids (oily phase and aqueous phase) through a tiny inlet hole at an incredibly high pressure to create dispersion.[17]
Microfludization:
Microfludization is a unique mixing technique that simultaneously reduces particle size by at trition, impact, hydraulic shear, impingement, severe cavitation, and turbulence. Tis utilizes a microfluidizer device. Using a high-pressure positive displacement pump (500 to 20000 psi), the formulation is driven into the interaction chamber, which is made up of minuscule, repeated “microchannels,” producing dispersity and incredibly thin particles in the submicron range. To manufacture homogenous NEs, the procedure is done numerous times to get the required particle size.[18]
Piston Pump Homogenizer:
A high-pressure homogenizer/piston homogenizer used in the process generates NEs with extremely small particle sizes (up to 1nm). To achieve dispersion, an extremely high-pressure homogenizer pushes two liquids (oily phase and aqueous phase) via a minute inlet hole.[18]
Ultrasonication Method:
An emulsion of microscale droplets that have been premixed is agitated by ultrasonic waves to produce NEs. This technique uses sonotrodes known as sonicator probes to deliver energy. It contains piezoelectric quartz crystal, which responds to an alternating electric voltage by contracting and expanding. Cavitation takes place as the sonicator’s tip makes contact with the liquid, causing mechanical vibration. The collapse of vapor holes in a liquid is known as cavitation. Since emulsion may be made directly using ultrasound, it is typically employed in laboratories to make emulsion in droplets as thin as 0.2 micrometres.[19]
EVALUATION OF NANOEMULSION
FTIR Spectroscopy
The FTIR spectral analysis was carried out by pressed pellet technique. IR spectrum of any substance gives information about the group present in a specific substance. An IR spectrum of drug was taken using (KBr potassium bromide) pellets. Small quantities of drug sample were mixed with oil, and a drop was placed between KBr pellets and spread uniformly. The pellets were placed in the holder, and an infrared spectrum was taken. The range of scanning was 400 4000 cm?1, Different peaks in the infrared spectrum were interpreted for presence of various group in the structure of the drug.[20]
Differential Scanning Calorimeter (DSC)
The sample of diclofenac sodium (about 5 mg) was loaded and sealed into DSC pan with a DSC loading puncher. The sample was scanned between 30 350°C at a heating rate of 10°C/ min, under nitrogen atmosphere (60 ml/min flow rate), using a differential scanning calorimeter. An empty pan was used as a reference.[20]
Particle charge (zeta potential)
Particle charge determines the physical stability of the nanoemulsion. Particle charge is quantified as zeta potential value which is measured via electrophoretic mobility of particles in an electrical field. Zeta potential of optimized formulation was measured using Beckman coulter Delsa-Nano C particle analyser, USA.[21]
Particle size
Globule size and size distribution (PDI) of optimized formulation was determined by photon correlation spectroscopy by using Delsa Nano C Zeta Sizer (Beckman coulter DelsaTM Nano C, USA). For the measurement of globule size and PDI, 2 ml of nanoemulsion was placed into cuvettes of Beckman coulter and measurements were recorded.[22]
Transmittance percentage (%T)
The translucence of the prepared nanoemulsions was checked by the turbidity test. By taking 2 ml of each nanoemulsion formula and measuring absorbance at 650 nm (light wavelength) using UV/Vis spectrophotometer and distilled water was used as a blank.[22]
SEM
SEM was used for the size analysis, topographical and elemental information by using magnifications up to 10X to 100,000X. A concentrated aqueous dispersion of nanoparticles was finely spread over a slab and dried under vacuum. The sample was shadowed in a cathodic evaporator with a gold layer (20 nm thick). The surface morphology of the nanoparticles was observed by SEM using a JSM-6400 scanning electron microscope.[23]
Dilution test
Aqueous dilution test was done, 1 mL of each nanoemulsion formula from (F1-F8) diluted to 50 mL, 100 mL and 500 mL with distilled water at 37° C with constant stirring and was maintained at 50 rpm. turbidity, clarity and the phase separation for each formula was observed visually.[24]
Dye solubility test (o/w test)
Few drops of water-soluble dye (Eosin yellow) were added to 1 ml of nanoemulsion in an eppendrof and mixed properly. The formulation was observed under fluorescent inverted microscope (Olympus CK147, USA). [24]
Emulsifying time
Emulsification time was determined as per the method described by Khoo et al. (1998) with slight modification. This was done by adding 0.3 ml of self-emulsifying oil formulations into a beaker containing 200 ml milli-Q water at 37°C. The sample was stirred and visually monitored to determine the time for complete emulsification.[24]
Drug content
Development and characterization of nanoemulsion. Drug content of the nanoemulsion formulation was carried out by dissolving 1 ml of the formulation in 10 ml of methanol. This formulation was then placed in shaking incubator (LSI-2005 RL, Lab Tech Co., Korea) (50 rpm at 37) for 30 min. After 30 min supernatant was collected and analysed using UV spectrophotometer (UV-1700 Pharma Spec, Shimadzu, Japan) at 210 nm against methanol as blank.[25]
Viscosity
Viscosity of nanoemulsion was determined using 1 ml of the formulation and speed of the spindle was adjusted to 100 rpm and a shear rate of 100 s±1 was applied at 37 ± 0.5°C for 10 min. Viscosity of the optimized nanoemulsion was determined using R/S CPS plus Rheometer.[25]
In vitro drug dissolution study
The in vitro release of NE occurs using USP dissolution apparatus type–II. Dialysis bag (Molecular cut off 12000Da) was utilized. 10 ml of each formula which contain 2mg of NE was put in the bag, and this bag was immersed in 500 ml of dissolution medium. The rotation speed was 50 rpm, and the dissolution medium was 0.1N HCl with 1% tween 20 at 37 ± 0.5 °C (30). Samples (5 ml) were withdrawn at a regular time intervals (5,10,15, 20,30, 40,50 and 60 min) from the dissolution medium and the samples then filtered by using through a 0.45 ?m filter syringe and were analysed by UV/Vis spectrophotometer at the ? max. of the drug.[26]
Entrapment efficiency determination
The entrapment efficiency (EE) was calculated by a direct method in which the amount of the drug that had been encapsulated within the droplets was measured. This was investigated by filtering the samples through a 0.2 µm syringe filter to eliminate any un-encapsulated drug. The filtrate was then diluted with methanol (1/1000 (v/v)) and the concentration of olanzapine was determined by UV visible spectrophotometer.[27]

Drug-release kinetics and mechanism of drug release
The release kinetics of the drug from the nanoemulsion is described using four different kinetic models, that is, zero order, first order, Hixson–Crowell Model and Higuchi Model (Zellner et al., 1996). All of these models explain the release pattern to be dependent on some individual property. The mechanism of drug release was studied using Peppas equation.[28]
Stability of nanoemulsion
Stability of the optimized nanoemulsion was determined by keeping at accelerated conditions of 40 ± 2°C and 75 ±5% RH for 3 months. Samples were then withdrawn at the end of 0, 30, 60 and 90 days and analysed for any change in the droplet size and drug content using UV/VIS spectrophotometer at 210 nm.[28]
Thermal cycling
The physical stability of nanoemulsions was evaluated by subjecting the formulation to stress conditions of freeze thaw cycling and measuring the change in the particle size at definite time interval.
Centrifugation
The effect of centrifugation was studied to evaluate potential metastable conditions, including phase separation, creaming and/or drug precipitation. This was done by centrifuging each formulation of nanoemulsion at 2000 rpm for 30 min and visually observed for phase separation and drug precipitation. The appearance of the system was also observed microscopically. Secondly, nanoemulsion formulations were also subjected to stress conditions, temperature cycling consisting of 12 h refrigeration (4°C) followed by 12 h storage at room temperature (25°C) for a period of 1 week. Formulations were then evaluated for phase separation or drug precipitation.[28]
APPLICATION OF NANOEMULSION IN DRUG DELIVERY
Nanoemulsion and Drug Targeting.
Their submicron size makes it simple to target the tumour’s location. Aqueous insoluble drugs have historically been delivered by nano emulsions, but more recently, attention has been focused on colloidal particles as a carrier for the targeted delivery of different anticancer medications, photosensitizers, neutron capture therapy agents, or diagnostic agents.[29]
Drug Delivery via Transdermal Nanoemulsions.
There has been a lot of interest in this area since it is practical to provide medications through the skin to the systemic circulation for a variety of clinical diseases.[29]
Drug Delivery via Pulmonary Nanoemulsions.
As an alternative to liposomes as a gene transfer vector, emulsion systems have been developed. Other researches on emulsion for gene administration (non-pulmonary route) indicated that the emulsion or DNA combination had a strong affinity than liposomal carriers.[29]
Delivery of Parenteral Drugs Using Nanoemulsions.
This is one of the most common and efficient drug delivery methods, and it is typically used for active ingredients with low bioavailability and limited remedial indices. Because of the ability to dissolve large amounts of hydrophobics, mutual compatibility, and the potential to protect medicines from enzymatic degradation and hydrolysis, nanoemulsions are ideal carriers for parenteral administration.[30]
Delivery of Ophthalmic Drugs Using Nanoemulsions.
A wide range of diseases are categorized as ophthalmic ailments, including glaucoma, cataracts, dry eye syndrome, and numerous ocular infections. Due to the defensive systems of the eye, including tear film dynamics and the blood ocular barrier, it is frequently difficult to deliver medications to the eye successfully.[31]
Delivery of Intranasal Drugs Using Nanoemulsions.
In addition to oral and parenteral administration routes, intranasal drug delivery systems are now recognized as an effective route for the administration of dosage forms. The nasal mucosa has been shown to be a therapeutically effective route for systemic medication administration and an effective strategy for circumventing barriers that prevent direct drug entry into the target-oriented site.[32]
CONCLUSION
Nanoemulsion formulations offer several advantages for the delivery of drugs, biologicals, or diagnostic agents and able to protect labile drug, control drug release, increase drug solubility, increase bioavailability and reduce patient variability.[32] Nanoemulsion was found to be one of the potential drug delivery strategies for nose-to-brain delivery. For poorly soluble and poorly permeable drugs, nanoemulsion approach increases the surface area and gives lipophilic nature to disperse the drug. The development of nanoemulsion formulation was done with ternary phase studies. Nanoformulation provides fast onset of action and helps to achieve site-specific delivery.[33]
REFERENCES
- Russell J. Wilson, Yang Li, Guangze Yang, Nanoemulsions for drug delivery, Particuology, 2022, 64, 85-97
- Afife Busra Ugur Kaplan, Meltem Cetin, Dilara Orgul, Formulation and in vitro evaluation of topical nanoemulsion and nanoemulsion-based gels containing daidzein, Journal of Drug Delivery Science and Technology, 2019, 52 189–203
- T.P. Sari, Bimlesh Mann, Rajesh Kumar, Preparation and characterization of nanoemulsion encapsulating curcumin, Food Hydrocolloids, 2015, 43, 540-546
- Nur Haziqah Che Marzuki, Roswanira Abdul Wahab and Mariani Abdul Hamid, An overview of nanoemulsion: concepts of development and cosmeceutical applications, Biotechnology & Biotechnological Equipment, 2019, 33, (1), 779–797
- Afzal Hussain, Vikas Kumar Singh, Om Prakash Singh, Formulation and optimization of nanoemulsion using antifungal lipid and surfactant for accentuated topical delivery of Amphotericin B, Drug Deliv, 2016, 23(8), 3101–3110
- Gulshan Chhabra, Krishna Chuttani, Anil K. Mishra, Design and development of nanoemulsion drug delivery system of amlodipine besilate for improvement of oral bioavailability, Drug Development and Industrial Pharmacy, 2011, 37(8), 907–916
- Samira Khani, Fariborz Keyhanfar, and Amir Amani, Design and evaluation of oral nanoemulsion drug delivery system of mebudipine, Drug Delivery, 2016, 23(6), 2035–2043
- AlishaN.Kadam, MohammadNajlah, Ka-WaiWan, Stability of parenteral nanoemulsions loaded with paclitaxel: the influence of lipid phase composition, drug concentration and storage temperature, Pharmaceutical Development and Technology, 2014; 19(8): 999–1004
- E. Gué , M. Since, S. Ropars, Evaluation of the versatile character of a nanoemulsion formulation, International Journal of Pharmaceutics, 2016, 498, 49–65
- Ahlam Zaid Alkilani, Rania Hamed, Ghaid Hussein, Nanoemulsion-based patch for the dermal delivery of ascorbic acid, Journal of Dispersion Science and Technology, 2021, 1-10
- Manjit Jaiswal, Rupesh Dudhe, P. K. Sharma, Nanoemulsion: an advanced mode of drug delivery system, Biotech, 2015, 5, 123–127
- Yuvraj Singh, Jaya Gopal Meher, Kavit Raval, Nanoemulsion: Concepts, development and applications in drug delivery, Journal of Controlled Release, 2017, 252, 28–49
- Conxita Solans, Isabel Solé, Nano-emulsions: Formation by low-energy methods, Current Opinion in Colloid & Interface Science, 2012, 17, 246–254
- K. Bouchemal, S. Briançon, E. Perrier, Nano-emulsion formulation using spontaneous emulsification: solvent, oil and surfactant optimisation, International Journal of Pharmaceutics, 2004, 280, 241–251
- Esther Santamaría, Alicia Maestro, Susana Vilchez, Study of nanoemulsions using carvacrol/MCT-(Oleic acid-potassium oleate)/ Tween 80 ®- water system by low energy method, Heliyon, 2023, 9, e16967
- Hanmin Li, Hongsheng Lu, Ying Zhang, Oil-in-water nanoemulsion with reversible charge prepared by the phase inversion composition method, Journal of Molecular Liquids, 2021, 336, 116174
- Sonia Calligaris, Stella Plazzotta, Francesca Bot, Nanoemulsion preparation by combining high pressure homogenization and high power ultrasound at low energy densities, Food Research International, 2016, 83, 25–30
- Preeti, Sharda Sambhakar, Rohit Malik, Nanoemulsion: An Emerging Novel Technology for Improving the Bioavailability of Drugs, Hindawi Scientifica, 2023, 1-25
- S. Kentish, T.J. Wooster, M. Ashokkumar, The use of ultrasonics for nanoemulsion preparation, Innovative Food Science and Emerging Technologies, 2008, 9, 170–175
- Neha Agnihotri, Girish Chandra Soni, Sunil Kumar Prajapati, Formulation and Evaluation of Nanoemulsion for Targeting and Systemic Delivery of Diclofenac Sodium, Scholars Academic Journal of Pharmacy, 2019, 8(8), 376-393
- Osman Gul, Furkan Turker Saricaoglu, Aysegul Besir, Effect of ultrasound treatment on the properties of nano-emulsion films obtained from hazelnut meal protein and clove essential oil, Ultrasonics- Sonochemistry, 2018, 41, 466-474
- Sumaya B. Hamed and Shaimaa N. Abd Alhammid, Formulation and Characterization of Felodipine as an Oral Nanoemulsions, Iraqi J Pharm Sci, 2021, 30(1), 209-217
- Basavaraj K. Nanjwade, Pratikkumar J. Varia, Vikrant T. Kadam, Development and Evaluation of Nanoemulsion of Repaglinide, JSM Nanotechnology & Nanomedicine, 2013, 1(2), 1016
- Moksha Laxmi, Ankur Bhardwaj, Shuchi Mehta, Development and characterization of nanoemulsion as carrier for the enhancement of bioavailability of artemether, Artificial Cells, Nanomedicine, and Biotechnology, 2015, 43, 334–344
- Mrunali R. Patel, Mitali H. Patel, Rashmin B. Patel, Preparation and in vitro/ex vivo evaluation of nanoemulsion for transnasal delivery of paliperidone, Appl Nanosci, 2016, 6, 1095–1104
- Rajaa A. Dahash and Nawal A. Rajab, Formulation and Investigation of Lacidipine as a Nanoemulsions, Iraqi J Pharm Sci, 2020, 29(1), 41-54
- Zahraa Hussein Ali, Myasar Alkotaji, Nanoemulsion-based nasal in situ gel of olanzapine, Journal of Excipients and Food Chem., 2023, 14 (1), 3-21
- Sabna Kotta, Hibah Mubarak Aldawsari, Shaimaa M. Badr-Eldin, Thermosensitive Hydrogels Loaded with Resveratrol Nanoemulsion: Formulation Optimization by Central Composite Design and Evaluation in MCF-7 Human Breast Cancer Cell Lines, Gels 2022, 8, 450, 2-9
- Megumi Nishitani Yukuyama, Edna Tomiko Myiake Kato, Raimar Löbenberg, Challenges and Future Prospects of Nanoemulsion as a Drug Delivery System, Current Pharmaceutical Design, 2017, 23, 495-508
- Dalia S. Shaker, Rania A. H. Ishak, Amira Ghoneim, Nanoemulsion: A Review on Mechanisms for the Transdermal Delivery of Hydrophobic and Hydrophilic Drugs, Sci. Pharm. 2019, 87, 17, 2-34
- J.M. Gutiérrez, C. González, A. Maestro, Nano-emulsions: New applications and optimization of their preparation, Current Opinion in Colloid & Interface Science, 2008, 13, 245–251
- Navneet Sharma, Sharadendu Mishra, Suryadev Sharma, Preparation and Optimization of Nanoemulsions for targeting Drug Delivery, International Journal of Drug Development and Research, 2013, 5 (4), 37-48
- S. M. Nemade, S. P. Kakad, S. J. Kshirsagar, Development of nanoemulsion of antiviral drug for brain targeting in the treatment of neuro-AIDS, Beni-Suef University Journal of Basic and Applied Sciences, 2022, 11, 138, 1-10


 E. P. Punnya*
E. P. Punnya*
 Ranitha R.
Ranitha R.



 10.5281/zenodo.10777516
10.5281/zenodo.10777516