Abstract
Nano-suspension, are nano-sized colloidal particle systems. Nano-suspension can be understood as the sub-micron colloidal dispersions of p’ceutical active ingredient particles in a liquid phase, with a size less than 1 micrometre, without any matrix material. These nano-suspensions are stabilized by the use of various surfactants and polymers. Nano-suspensions are distinguishable from nanoparticles and solid lipid nanoparticles. Nanoparticles are polymeric colloidal carriers of the drug, while solid lipid nanoparticles are lipid-based carriers of drugs. They have recently become one of the most engaging substances in nano-pharmaceuticals. Nano-suspension have prominent commercial potential since they provide the enhanced solubility and dissolution of poor water soluble drugs by means of their small particle sizes and large surface areas. Moreover, they can alter the p'kinetics of the drug improving its efficacy and safety. These benefits can be used to enhance the bioavailability of poorly soluble drugs in dermal, oral, or systemic administration routes. The selection of stabilizer types, including surfactants and/or polymers, and their precise ratio are the most crucial factors in the formulation of nano-suspension. The delicate balance of stabilizers and the choice of fabrication method are paramount in determining the success and performance of nano-suspensions. This review article helps us gain further detailed insight on nano-suspensions as a novel drug delivery system. [1]
Keywords
Nano-suspension, formulation approaches, administration routes.
Introduction
Drug candidates with poor water solubility pose significant challenges in the pharmaceutical industry and research. One major issue is the potential loss of dose-response linearity, which can result in unexpected drug breakdown after administration. This can negatively impact patient adherence and reduce bioavailability. Additionally, because these substances are not easily soluble in water, their absorption can vary between fed and fasted states, leading to further complications. [2, 3] To improve solubility and bioavailability, a variety of drug delivery systems are employed, including nanomedicines, liposomes, lipid-based solid nanoparticles, polymeric micelles, quantum dots, nano-suspensions, and nano-emulsions. Nano-suspensions are colloidal dispersions that contain drug particles smaller than 1 micrometer (µm) and are characterized by their fine dispersion and biphasic nature. These systems can enhance drug adsorption and bioavailability, potentially reducing the conventional oral dosage required. According to the modified Noyes-Whitney equation, reducing drug particle size increases the surface area, thereby accelerating the dissolution rate. Additionally, the Ostwald-Freundlich equation suggests that smaller particle sizes lead to increased saturated solubility due to higher dissolution pressure. Nano-suspensions are particularly useful for drugs in Biopharmaceutical Classification System (BCS) classes II and IV, as they improve solubility. They offer notable benefits in drug delivery, especially for hydrophobic drugs, by increasing drug loading and therapeutic efficiency while decreasing the necessary dose. These systems also address stability issues and allow for passive drug targeting. They are favored for their ease of manufacturing and scalability, making them viable for large-scale production. Stabilizers are often included to maintain long-term stability. For oral administration, nano-suspensions can lead to faster onset of action and enhanced bioavailability. When used intravenously, they provide rapid dissolution and targeted delivery to specific tissues. Injections, whether subcutaneous or intramuscular, may experience reduced tissue irritation with nano-suspensions. They also improve bioavailability via ocular and pulmonary routes, and are particularly advantageous for drugs with high log P values, enhancing their solubility and effectiveness. Furthermore, nano-suspensions can be integrated into a range of dosage forms, including tablets, pellets, hydrogels, and suppositories, providing versatility for various therapeutic applications. [4-6]
Need for Nano-suspension
Over 40% of drugs suffer from poor water solubility, which complicates their formulation into conventional dosage forms. This issue is even more challenging for Class II drugs, which are poorly soluble in both aqueous and organic media. For such compounds, particularly those that are water-insoluble but oil-soluble with high log P values, preparing nano-suspensions is often a preferred approach. To address the problems of low solubility and bioavailability, various techniques can be employed, including micronization, co-solvency, oily solutions, salt formation, liposomes, emulsions, micro-emulsions, solid dispersions, and ?-cyclodextrin inclusion complexes. However, many of these methods are not universally applicable to all drug formulations. In situations where medications are insoluble in both water and inorganic media, nano-suspensions emerge as a preferred method over lipid-based systems. They are particularly suitable for compounds with high log P values, high melting points, and large doses. Nano-suspensions enhance the solubility of drugs that are poorly soluble in both aqueous and lipid media. This results in an increased rate of drug dissolution and a faster peak plasma concentration, whether the nano-suspension is administered orally or intravenously. This rapid onset of action is a key advantage of nano-suspensions compared to other solubility enhancement techniques. Nano-suspensions offer significant benefits for drugs with poor solubility and low permeability, which are major challenges in formulation. [9-12]
Common issues associated with poorly soluble compounds include:
- Low bioavailability
- Difficulty in optimizing lead compounds based on efficacy and safety
- Variability in bioavailability between fed and fasted states
- Lack of proportionality between dose and response
- Suboptimal dosing strategies
- Dependence on harsh excipients, such as excessive co-solvents and other additives
- Requirement for extreme pH conditions to enhance solubilisation. [13,14]
By addressing these challenges, nano-suspensions provide a promising solution for improving drug solubility and therapeutic effectiveness. [15]
Nano-suspensions differ from traditional suspension formulations in that the particle length distribution of solid debris is typically less than 1 µm (0.1nm-1000nm), with an average particle size range of 200-600nm. Pharmaceutical suspensions typically require particle sizes ranging from 1 to 50 µm. Nano-suspensions improve total bioavailability by increasing surface location and saturation solubility through particle length discounting. This machine cannot be executed using normal milling techniques. [16-18]
Benefits of Nano-suspension [19-21]
- Improves the drug's dissolution velocity & saturation solubility.
- Upon oral administration nano-suspensions display relatively fast onset of action, lower fast\fed ratio with subsequent optimized bioavailability.
- Decreased particle size leads to faster absorption.
- Production and scale-up is easier and also provides long term physical stability.
- Higher bioavailability and consistent dosing for ocular and inhalation routes is achieved.
- Poorly water soluble drugs formulation can be beneficial by using nano-suspension.
- Passive targeting is possible due to nanometre size.
- They are simple and inexpensive formulations.
- Hydrophilic drugs can be formulated by the use of nano-suspension.
- Nano-suspensions can make possible high drug loading.
- Surface modification of nano-suspension API particles enables targeted delivery to specific organs.
Methods to prepare Nano-suspension
Nowadays, top-down and bottoms-up techniques are preferred in formulation of nano-suspensions. Micronization, a key principle in the preparation of nano-suspensions, can be effectively employed through techniques such as media milling or jet milling. This process offers several advantages but also comes with certain disadvantages. For instance, while micronization improves the dissolution rate of a drug, it may not significantly increase its saturation solubility. [22-24]
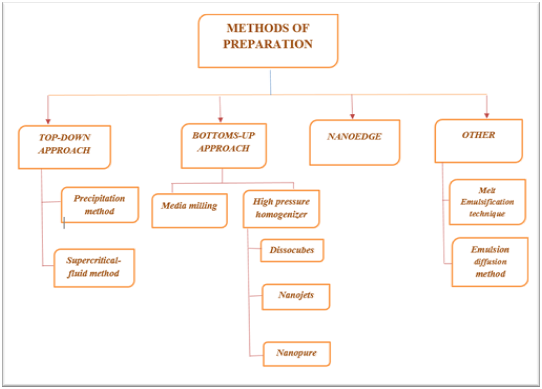
Figure: 1 Methods of Preparation [21
- Bottom-up technique
A method known as "bottom-up technology" starts at the molecular level and progresses through molecular connection to solid particles. This method employs traditional precipitation techniques, such as increasing the temperature or adding a non-solvent, to alter the solvent's quality. In pharmaceutical chemistry and technology, precipitation is a well-known process. [25]
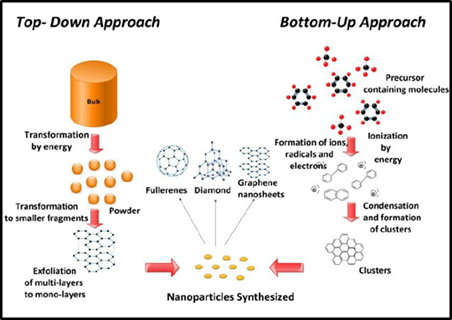
Figure: 2 Bottom up Approach representation [25]

Top-down technique
There are two methods encompassed in the top-down technique:
- Media Milling Method
Pearl mills can produce nano-suspensions. This technique uses a recirculation chamber, milling shaft, and milling chamber. To begin, add a medication suspension and an aqueous medium to the mill, then add pearls or small (grinding) balls. At high shear rates, rotating balls cause friction and impact within the grinding jar, reducing particle size. Milling media, comprised of robust materials like zirconium oxide, are very resistant to wear and strain. [26, 27]
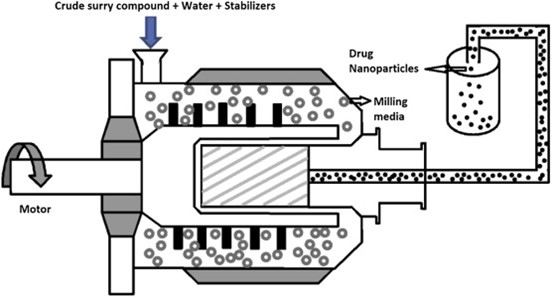
Figure: 3 Schematic diagram of Media Milling Process [26]
High Pressure Homogenizer
This technique includes sending a drug suspension via a narrow valve under pressure. This method uses cavitation and gas bubbles to reduce particle size. To get larger solid concentrations, it is recommended to pre-mill tiny medication particles. High-pressure homogenisation offers advantages such as suitability for diluted and concentrated solutions, as well as the ability to manufacture aseptically. [28]
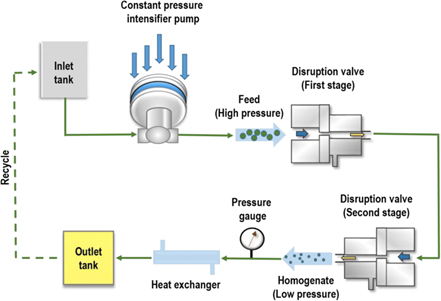
Figure: 4 Schematic diagram of High Pressure Homogenizer [27]


- Micro-emulsion as a template
To create an emulsion, the medication is dispersed in a mixture of organic or inorganic solvents, followed by an aqueous phase containing surfactants. The drug particles precipitate at low pressure as the organic phase evaporates, resulting in nano-suspension. Drug particles precipitate and form nano-suspensions by rapidly evaporating the organic phase at low pressure. Surfactants ensure the stability of the nano-suspension. Alternative solvents for the dispersion phase include triacetin, benzyl alcohol, and butyl lactate. [26, 29, 30]
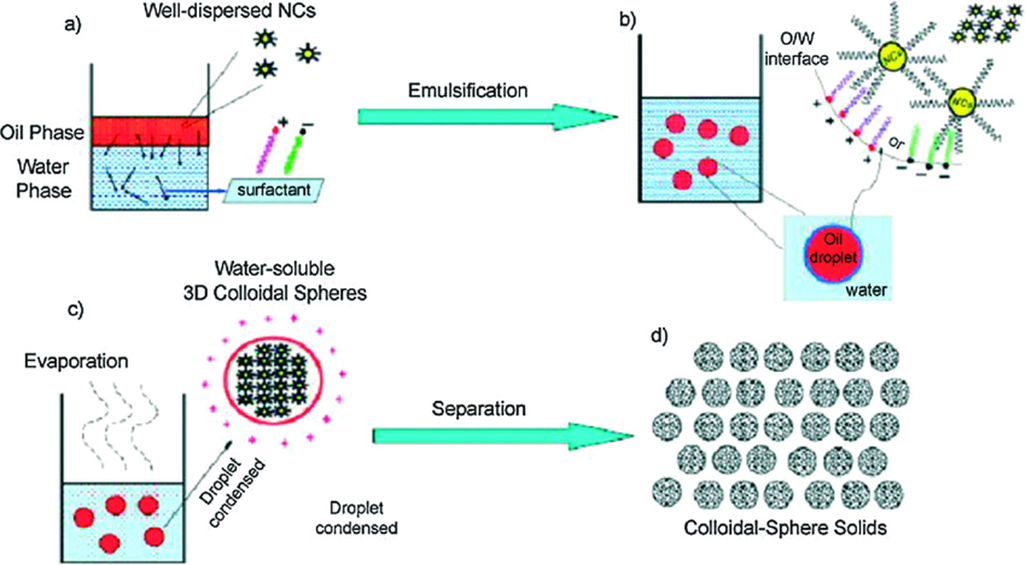
Figure: 5 Schematic of Micro-emulsion-template [29]

Administration Routes for Nano-suspensions
Nano-suspensions can usually be administered through ocular, parenteral, dermal, pulmonary and oral route. The most commonly used route for administration of nano suspension however is, oral route.
Oral Administration
When it comes to safety, non-invasiveness, convenience of use, dose flexibility, and patient compliance, the oral route is the recommended method of medication administration. Oral dosage forms offer benefits for patients as well as cost-effectiveness, practicality, and adaptability for large-scale production. The reason to use nano-suspension mainly is, Enhancing solubility and dissolution of drugs significantly improves their bioavailability and absorption, leading to better therapeutic outcomes. This enhancement also contributes to increased drug stability and allows for proportional dosing, facilitating a rapid formulation development process. Moreover, it eliminates variability caused by fed or fasted states and reduces inter-subject variability, ensuring more consistent drug efficacy. Improved solubility allows drugs to be used directly in liquid dosage forms and can be easily converted into various solid dosage forms such as tablets, capsules, pellets, oral films, powders, and granules. These advancements simplify large-scale production and enhance the drug's mucoadhesive properties, improving its attachment to cell membranes. Ultimately, these improvements can lead to a reduction in the required drug dose, minimizing side effects and optimizing overall drug utilization. [31, 32]
Parenteral Administration
The parenteral route of drug administration, which involves giving medication through injections or infusions, is commonly used in clinical practice. This method offers several benefits: it ensures that nearly all of the drug is available for use by the body (100% bioavailability), works quickly to produce effects, doesn't rely on the digestive system, and avoids the liver's first-pass metabolism, which can break down the drug before it reaches the bloodstream. These advantages make parenteral administration a highly effective and efficient option for delivering medications. Due to their low volume of administration, high drug loading capacity, and ease of permeability, nano-suspensions for parenteral administration are commonly manufactured at the nanometer scale. Additionally, because these formulations contain a small number of excipients, there is less chance of toxicity and allergic responses. It is important to remember that Kupffer cells in the liver and reticuloendothelial system should not phagocytose the delivery system while creating a novel drug delivery system for parenteral administration. Therefore, for parenteral nano-suspensions, a size range of ?100 nm is essential. [32, 33]
Ocular Administration
Since the eye is the most specialized organ in the body, different drug delivery techniques have been used to get the medications into the eye. In the pharmaceutical industry, creating drug delivery systems for ocular administration has grown more difficult. Certain disorders include glaucoma, dry eyes, diabetes retinopathy, proliferative vitreoretinopathy, keratoconus, macular degeneration, conjunctivitis, blepharitis, and uveitis require the administration of drugs via the eyes. When treating these disorders systemically, the effects of the drug may be restricted due to blood-aqueous and blood-retinal barriers that form following ocular delivery. One particularly useful method for getting medications across the ocular mucosa that are both hydrophilic and extremely hydrophobic is the ocular application of nano-suspension. The primary process by which nano-suspension increases ocular bioavailability is by increasing the saturation solubility and dissolution velocity of poorly water-soluble medications. [34]
Because nano-sized particles are used in nano-suspension, there is less chance of ocular irritation, and the charge on the surface of NS promotes the particles' adherence to the cornea. With all these benefits, the NSs can address significant problems such low contact time and poor ocular bioavailability that affect drug drainage, tear turnover, and dilution or lacrimation. [35]
Dermal Administration
Dermal drug application has numerous benefits, including a decrease in adverse effects, ensuring drug accumulation in the targeted location, regulated drug administration to the organism, patient self-administration, high patient compliance, and a targeted effect. [33] Drugs can be applied topically using either of two fundamental methods: transdermal or dermal. In a dermal application, the applied formulation is localized in the dermal layers; in a transdermal application, it travels via the carrier to the skin's lower layer before entering the bloodstream. Superiority in terms of dermal usage is provided by using nano suspension technology to reduce the active substance's particle size to nano-size, improving its surface area and solubility and, consequently, its bioavailability. The concentration of the active ingredient on the skin surface rises with an increase in saturation solubility, and the pace at which the active material passes through the skin by passive diffusion quickens based on an increase in the concentration gradient. Increased penetration into the negatively charged stratum corneum layer can be achieved by selecting positively charged polymers for the NSs structure. As a result, creating NS formulations has become more crucial in recent years in order to boost the cutaneous bioavailability of active ingredients that have a low to medium water solubility. [36, 37]
Drug Selection Criteria for Nano-suspensions [39]
Nano-suspension can be used to build APIs (Active Pharmaceutical Ingredients) that have the following characteristics:
- Soluble in oil but insoluble in water.
- API is insoluble in both oil and water. -
- API which requires larger dosage.
- Drugs with limited crystal solubility in any solvent.
Formulation considerations
- Stabilizer
A stabilizer prevents Ostwald's maturation and clustering of nano-suspensions, resulting in a physically stable formulation with a steric barrier. The amount of stabilizer added determines the physical stability and performance of nano-suspension in vivo. Stabilizers used nowadays include poloxomers, polysorbates, cellulosics, povidones, and lecithin. Lecithin is the ideal stabilizer for auto-clavable and parenteral nano-suspensions. [40, 41]
- Organic solvent
Organic solvents are employed in nano-suspension formulations that use an emulsion system (micro- or nano-) as a template. Pharmaceuticals prefer water miscible solvents (e.g., methanol, isopropanol and ethanol) and partially miscible solvents (e.g., ethyl formate, triacetin, propylene carbonate) for nano-suspension formulation due to their lower toxicity compared to traditional hazardous solvents like dichloromethane. [42, 43]
- Co-surfactant
When creating nano-suspensions with micro-emulsions, selecting a suitable co-surfactant is crucial. Co-surfactants have a substantial impact on phase behaviour, hence it's crucial to study their effect on internal phase uptake for certain micro-emulsion compositions and drug loadings. Bile salts and dipotassium glycerrhizinate are commonly employed as co-surfactants in micro-emulsions, but other solubilizers, such as transcutol-p and ethanol, can also be used safely. [44]
- Post-production processing
Chemical breakdown or cleavage of drug molecules requires post-production processing of nano suspensions. Processing may be required if the formulation cannot be stabilised using the simplest stabiliser or if the specified path has acceptability limits. Lyophilisation, or spray drying, can create dry powder from nano-scaled medication particles. Fair selection of drugs is crucial in unit operations, taking into account their qualities and cost impact. Lyophilisation is more expensive and less efficient than spray drying. [45, 46]
Characterization of Nano-suspensions [39, 41]
Chemical
- Active ingredient.
- Degradation products.
- Moisture (for lyophilized and solid dosage forms).
- Preservatives.
- pH (Potential of Hydrogen)
Physical
- Particle-size distribution.
- Particle-size distribution in response to accelerated ageing and shipping (freeze/thaw, mechanical agitation, and centrifugation).
- Drainability (from sides of container).
- Syringeability, and injectability.
- Re-suspendability.
- Dissolution in water or bio-relevant medium.
- Compatibility after admixture.
- Zeta potential (electrostatic self-repulsion of particles).
Biological
- Sterility.
- Pyrogenecity.
- In-vivo pharmacokinetics.
Mean-particle size and size distribution
Nano suspensions' saturation solubility, dissolving velocity, physical stability, and biological performance are all influenced by their mean particle size and particle-size distribution width. A PI number between 0.1-0.25 implies a limited size distribution, whereas a PI value beyond 0.5 indicates a relatively broad distribution. A logarithmic normal distribution cannot be attributed to such a high PI value. Although PCS (Photon Correlation Spectroscopy) is a versatile approach, its low measuring range (3 nm to 3 ????m) makes it difficult to detect the likelihood of nano-suspension contamination by micro-particulate medicines (particles larger than 3 ????m). [47]
Zeta potential
The zeta potential indicates how stable the suspension is. A stable suspension stabilized by electrostatic repulsion requires a minimum zeta potential of 30 mV. However, a suspension stabilized by both electrostatic and steric stabilizers requires just a zeta potential of 20 mV.
Crystalline state and particle morphology
High pressure homogenisation can alter the crystalline structure of nano-suspensions, resulting in amorphous or polymorphic forms. Understanding the crystalline state and particle shape is crucial for understanding drug polymorphism. X-ray diffraction and DSC studies are used to monitor medication particle solidification and amorphous fraction. Scanning electron microscopy (SCM) reveals particle morphology. [48]
Stability
Particle size is an important factor in determining stability. Nano-suspensions with smaller particle sizes have a bigger surface area and higher dissolving rates. Increasing particle size increases surface energy and aggregation. Stabilisers prevent Ostwald ripening and enhance suspension stability by forming a steric or ionic barrier. Nano-suspensions typically contain stabilizers including cellulose, poloxamers, PVA, polysorbates, HPMC, lecithin, polyoleate, and povidone. Stability is evaluated over three months under various stress conditions, including temperature (15, 25, 35, 45°C), thermal cycling, and mechanical shaking. Mean particle size changes are also observed. Other in-vitro evaluation factors include pH, osmolarity, and drug content. [46.49]
In-vivo pharmacokinetic correlation
The in vivo evaluation of nano-suspensions focusses on the drug and method of delivery. HPLC-UV visible spectrophotometry was used to determine plasma drug levels after administering the formulation via the relevant route. In vivo evaluations typically include surface hydrophilicity, adhesion characteristics, and interaction with body proteins. [50]
FUTURE PERSPECTIVES AND RESEARCH CONSIDERATIONS
Numerous research groups have used nano-suspension technology to investigate a range of formulations thus far. Only a small number of these formulations, especially for oral delivery, have been effectively introduced to the market, though. The fundamental causes of this predicament go beyond technological difficulties; replacing a well-known product with a nano-suspension is challenging due to significant market entry costs. Additionally, when introducing a novel chemical, existing and tested administration techniques are frequently chosen. In conclusion, nano-suspension technology offers a flexible framework for creating safe and efficient formulations for poorly soluble active ingredients. The use of surface-modified nano-suspensions in the development of customized formulations has become more popular in recent years. Medication nanocrystals have the potential to improve pharmacological efficacy by enabling targeted medication delivery to certain diseased tissues, such as brain tumors, infected macrophages, and tumors. The use of nano-suspensions to deliver nanoparticles to certain cells and improve their absorption within the cellular milieu is the subject of ongoing research. As a result, using nanocrystals to address issues with the existing dose formulations is a feasible solution. [18, 22]
BCS Class II comprises a large number of recently identified pharmacological compounds with extremely low water solubility. In light of the growing quantity of these low-soluble medications that cannot be synthesized using conventional methods, NSs have recently become increasingly significant. The extensive use of nano-suspensions in the production of formulations can be attributed to their advantages, which include their applicability to a wide range of pharmaceuticals, simplicity of scaling up, minimal usage of excipients, and higher solubility followed by increased dissolution rate and bioavailability. Therapeutic applications for nano-suspensions that enable drug delivery via the cutaneous, pulmonary, parenteral, and ophthalmic routes—particularly the oral route—have been demonstrated for a number of disorders. The growing number of commercial products based on nano-suspension mostly reflects these benefits. After clinical trials into different delivery methods are completed in the future, nano-suspensions will probably be commercialized in addition to the current products. Even though nano-suspension formulations have advanced greatly, there are still many issues with stabilizer selection, stability maintenance, and other elements, as well as a dearth of in vivo investigations and clinical trials. Therefore, it is necessary to conduct more clinical studies, improve the pharmacokinetic data obtained from the administration of different nano-suspensions, and build theoretical models to pinpoint the steps involved in the formulation creation and optimization of nano-suspensions. [38, 52]
REFERENCES
- P?nar, S.G.; Oktay, A.N.; Karaküçük, A.E.; Çelebi, N. Formulation Strategies of Nanosuspensions for Various Administration Routes. Pharmaceutics 2023, 15, 1520. https://doi.org/ 10.3390/pharmaceutics15051520
- R. Jayalakshmy, K.S Sreethu, 2021. Nano-suspensions: a method for solubility enhancement. Jour. of Med. P’ceutical &Alli. Sci. V 10 - I 3, 1070 P-2744-2752. DOI: 10.22270/jmpas.V10I3.1070
- Guner, G.; Yilmaz, D.; Yao, H.F.; Clancy, D.J.; Bilgili, E. Predicting the temperature evolution during nanomilling of drug suspensions via a semi-theoretical lumped parameter model. Pharmaceutics 2022, 14, 2840.
- Dong, Z., Wang, R., Wang, M., Meng, Z., Wang, X., Han, M., & Wang, X. (2022). Preparation of naringenin nanosuspension and its antitussive and expectorant effects. Molecules, 27(3), 741.
- Gera, S., Sampathi, S., Maddukuri, S., Dodoala, S., Junnuthula, V., & Dyawanapelly, S. (2022). Therapeutic potential of naringenin nanosuspension: in vitro and in vivo anti-osteoporotic studies. Pharmaceutics, 14(7), 1449.
- Khare, P., Chogale, M. M., Kakade, P., & Patravale, V. B. (2022). Gellan gum–based in situ gelling ophthalmic nanosuspension of Posaconazole. Drug Delivery and Translational Research, 12(12), 2920-2935.
- Jakubowska, E., Bielejewski, M., Milanowski, B., & Lulek, J. (2022). Freeze-drying of drug nanosuspension–study of formulation and processing factors for the optimization and characterization of redispersible cilostazol nanocrystals. Journal of Drug Delivery Science and Technology, 74, 103528.
- Ding, X., Guo, L., Du, Q., Wang, T., Zeng, Z., Wang, Y., & Cui, B. (2024). Preparation and Comprehensive Evaluation of the Efficacy and Safety of Chlorantraniliprole Nanosuspension. Toxics, 12(1), 78.
- Nsairat, H.; Khater, D.; Sayed, U.; Odeh, F.; Bawab, A.A.; Alshaer, W. Liposomes: Structure, composition, types, and clinical applications. Heliyon 2022, 8, e09394.
- Ran, Q.; Wang, M.; Kuang, W.; Ouyang, J.; Han, D.; Gao, Z.; Gong, J. Advances of combinative nanocrystal preparation technology for improving the insoluble drug solubility and bioavailability. Crystals 2022, 12, 1200.
- Abdulbaqi, M. R., Taghi, H. S., & Jaafar, Z. M. (2021). Nanosuspension as an innovative nanotechnology trend drug delivery system: A review. Benefits, 2(10), 11.
- Ran, Q.; Wang, M.; Kuang, W.; Ouyang, J.; Han, D.; Gao, Z.; Gong, J. Advances of combinative nanocrystal preparation technology for improving the insoluble drug solubility and bioavailability. Crystals 2022, 12, 1200.
- Shivraj Popat Jadhav, Santosh Kumar Singh, Himmat Singh Chawra , "Review on Nanosuspension as a Novel Method for Solubility and Bioavailability Enhancement of Poorly Soluble Drugs," Advances in Pharmacology and Pharmacy, Vol. 11, No. 2, pp. 117 - 130, 2023. DOI: 10.13189/app.2023.110204.
- Wanole, O. S. (2022). REVIEW ON: NANOSUSPENSION.
- Singh, S., & Jain, S. Nanosuspension: an emerging nanotechnology for drug delivey system.
- Sharma, P., Bose, S., Moudgil, A., Arora, D., Sushila, S., Vyas, M., & Tomar, B. (2023, September). Nanosuspension as a promising drug delivery approach for the antidiabetic drug: An inclusive review on technology and future aspects. In AIP Conference Proceedings (Vol. 2800, No. 1). AIP Publishing.
- Quadri, N., & Abdullah, M. M. (2023). Review on polyherbal nanosuspension and approaches to enhance solubility of drugs. World J. Pharm. Res, 10, 313-335.
- Jaini, P., Shah, C., & Upadhyay, U. A Review on Nanosuspension.
- Ahamed, M. I., Akiladevi, D., & Karthick, G. (2022). A Comprehensive review of a new nanosuspension for improving the oral bioavailability of poorly soluble drugs. Journal of Pharmaceutical Research International, 16-21.
- Tupe, A., & Mankar, S. D. (2023). Nanosuspension: A novel approach to improve the solubility, bioavailability and pharmacokinetics of poorly soluble drugs. Asian Journal of Pharmacy and Technology, 13(3), 194-200.
- Jadhav, S. P., Singh, S. K., & Chawra, H. S. (2023). Review on Nanosuspension as a Novel Method for Solubility and Bioavailability Enhancement of Poorly Soluble Drugs. Advances in Pharmacology and Pharmacy, 11(2), 117-30.
- Aldeeb, M. M. E., Wilar, G., Suhandi, C., Elamin, K. M., & Wathoni, N. (2024). Nanosuspension-based drug delivery systems for topical applications. International Journal of Nanomedicine, 825-844.
- P?nar, S. G., Oktay, A. N., Karaküçük, A. E., & Çelebi, N. (2023). Formulation strategies of nanosuspensions for various administration routes. Pharmaceutics, 15(5), 1520.
- Shahidulla, S. M., Miskan, R., & Sultana, S. Nanosuspensions in Pharmaceutical Sciences: A Comprehensive Review. Situations, 8, 9.
- Elsebay, M. T., Eissa, N. G., Balata, G. F., Kamal, M. A., & Elnahas, H. M. (2023). Nanosuspension: A Formulation Technology for Tackling the Poor Aqueous Solubility and Bioavailability of Poorly Soluble Drugs. Current Pharmaceutical Design, 29(29), 2297-2312.
- Mahajan, K. C., Deore, M. M. S., Gaikwad, S. S., & Dama, G. Y. (2024). A Breif Review on Nanosuspension Technology for Solubility Enhancement. Current Trends in Biotechnology and Pharmacy, 18(2).
- R. Jayalakshmy, K.S Sreethu, 2021. Nano-suspensions: a method for solubility enhancement. Jour. of Med. P’ceutical &Alli. Sci. V 10 - I 3, 1070 P-2744-2752. DOI: 10.22270/jmpas.V10I3.1070
- Sutradhar, K. B., Khatun, S., & Luna, I. P. (2013). Increasing possibilities of nanosuspension. Journal of nanotechnology, 2013(1), 346581.
- Jabar, H. E., & Abd-Alhammid, S. N. (2022). Improvement of the Solubility and Dissolution Characteristics of Risperidone via Nanosuspension Formulations. Iraqi J Pharm Sci, 31(1), 43-56.
- Hadke, A. N. K. I. T. A., Pethe, A. N. I. L., Vaidya, S. U. N. I. T. A., & Dewani, S. U. N. I. L. (2022). Formulation development and characterization of lyophilized febuxostat nanosuspension. Int J Appl Pharm, 14(6), 91-99.
- Ahirrao, S. P., Bhambere, D. S., Ahire, E. D., Dashputre, N. L., Kakad, S. P., & Laddha, U. D. (2023). Formulation and evaluation of Olmesartan Medoxomil nanosuspension. Materials Today: Proceedings.
- Andrade da Silva, L. H., Vieira, J. B., Cabral, M. R., Antunes, M. A., Lee, D., Cruz, F. F., ... & Suk, J. S. (2023). Development of nintedanib nanosuspension for inhaled treatment of experimental silicosis. Bioengineering & Translational Medicine, 8(2), e10401.
- Aher, D. S. J., & Patil, P. P. (2022). Formulation and Evaluation of ketoprofen nanosuspension. European Journal of Molecular & Clinical Medicine, 9(09).
- Hanagandi, V., Patil, A. S., Masareddy, R. S., Dandagi, P. M., & Bolmal, U. B. (2022). Development and Evaluation of Nanosuspension Incorporated in situ gel of Brimonidine Tartarate for Ocular Drug Delivery. Indian J. Pharm. Educ. Res, 56, 94-102.
- A Breif Review on Nanosuspension Technology for Solubility Enhancement. (2024). Current Trends in Biotechnology and Pharmacy, 18(2).
- Chavhan, R. (2024, June). NANOSUSPENSIONS: ENHANCING DRUG BIOAVAILABILITY THROUGH NANONIZATION. In Annales Pharmaceutiques Françaises. Elsevier Masson.
- Fathi-Karkan, S., Ramsheh, N. A., Arkaban, H., Narooie-Noori, F., Sargazi, S., Mirinejad, S., & Rahman, M. M. (2024). Nanosuspensions in ophthalmology: Overcoming challenges and enhancing drug delivery for eye diseases. International Journal of Pharmaceutics, 124226.
- Malgundkar, H. K., Pomaje, M. D., & Nemade, L. S. Breaking Barriers with Nanosuspension: A Comprehensive Review.
- Roldan, T. L., Li, S., Guillon, C., Heindel, N. D., Laskin, J. D., Lee, I. H., & Sinko, P. J. (2024). Optimizing Nanosuspension Drug Release and Wound Healing Using a Design of Experiments Approach: Improving the Drug Delivery Potential of NDH-4338 for Treating Chemical Burns. Pharmaceutics, 16(4), 471.
- Kanawade, S., Shreyash, D., Krutika, B., Mayur, N., & Pawar, H. (2024). Nanosuspensions for improved Cancer Therapy: A Comprehensive Review. Research Journal of Pharmaceutical Dosage Forms and Technology, 16(2), 157-162.
- Singh, S., & Bharati, D. (2024). Nanosuspensions: A Novel Drug Delivery System. Sch Acad J Pharm, 5, 153-162.
- Modak, A., Bhakat, S. P., Kar, B., & Roy, S. Innovative Approaches in Drug Delivery: Exploring Nanosuspension as Phytosome.
- Raza, A., Ali, T., Naeem, M., Asim, M., Hussain, F., Li, Z., & Nasir, A. (2024). Biochemical characterization of bioinspired nanosuspensions from Swertia chirayita extract and their therapeutic effects through nanotechnology approach. Plos one, 19(2), e0293116.
- Liu, X., Fan, H., Meng, Z., Wu, Z., Gu, R., Zhu, X., & Dou, G. (2023). Combined silver sulfadiazine nanosuspension with thermosensitive hydrogel: an effective antibacterial treatment for wound healing in an animal model. International Journal of Nanomedicine, 679-691.
- Huang, S., Wu, H., Jiang, Z., & Huang, H. (2021). Water-based nanosuspensions: Formulation, tribological property, lubrication mechanism, and applications. Journal of Manufacturing Processes, 71, 625-644.
- Tian, Y., Wang, S., Yu, Y., Sun, W., Fan, R., Shi, J., & Zheng, A. (2022). Review of nanosuspension formulation and process analysis in wet media milling using microhydrodynamic model and emerging characterization methods. International journal of pharmaceutics, 623, 121862.
- Jeslin, D., & Masilamani, K. (2024). Nanosuspension Of Poorly Soluble Anti-Diabetic Drug For Enhancement Of Solubility And Dissolution. Acta Scientiae, 25(1), 73-89.
- Kakad, S. P., Gangurde, T. D., Kshirsagar, S. J., & Mundhe, V. G. (2022). Nose to brain delivery of nanosuspensions with first line antiviral agents is alternative treatment option to Neuro-AIDS treatment. Heliyon, 8(7).
- Fathi-Karkan, S., Ramsheh, N. A., Arkaban, H., Narooie-Noori, F., Sargazi, S., Mirinejad, S. ... & Rahman, M. M. (2024). Nanosuspensions in ophthalmology: Overcoming challenges and enhancing drug delivery for eye diseases. International Journal of Pharmaceutics, 124226.


 Mubaraka Rampurawala *
Mubaraka Rampurawala *
 Chainesh Shah
Chainesh Shah










 10.5281/zenodo.13629942
10.5281/zenodo.13629942