Abstract
Although monkeypox was first discovered in Africa, the majority of cases in the current outbreak are being reported from numerous nations. The origin of this outbreak is still unknown, and as the COVID-19 pandemic starts to fade, several countries are now dealing with a new outbreak. A virus that may infect both humans and animals, monkeypox belongs to the Orthopoxvirus genus within the Poxviridae family. The international community took notice when, in the 1970s, the smallpox immunization program was discontinued and cases of monkeypox started to rise. Since macaque monkeys were the first to contract the virus, it was given the moniker “monkeypox.” It is believed that a variety of rodents and small mammals can spread it, while the virus’s origin is unknown. In this article, we present a thorough and current review of monkeypox, covering its epidemiology, aetiology, pathophysiology, clinical manifestations, diagnosis, and treatment. The present overview also covers prospective research fields, vaccine advancements, and preventive and control methods for this reemerging viral disease that was designated as a public health emergency of international significance.
BACKGROUND
Mpox is endemic in Central and Western Africa, where it is thought that the virus naturally exists in a number of mammal species. IN 1970, the Democratic Republic of the Congo’s Basankusu saw the diagnosis of the first human cases.Since then, there have been noticeably more outbreaks, both in terms of frequency and intensity, perhaps due to diminishing immunity following the end of routine smallpox vaccination. In 2022–2023, a clade II outbreak occurred worldwide, which was the first instance of broad community transmission beyond Africa. The epidemic was classified as a Public Health Emergency of International Concern (PHEIC) by the World Health Organization in July 2022. As the outbreak began to be contained, the WHO changed its designation in May 2023, noting that vaccination and public health awareness campaigns had been effective control efforts.[1]
Keywords
Mpox, Orthopox disease, Zoonotic ,Public health ,Transmission,Pathophysiology, Epidemiology ,Vaccine.
Introduction
An infectious viral disease called mpox, formerly known as monkeypox, can affect both people and other animals. Fever, swollen lymph nodes, and a rash that boils and then crusts over are among the symptoms. Most infected persons recover within a few weeks without therapy, as the sickness is usually minor. The duration of symptoms varies between two to four weeks, with a time span of three to seventeen days between exposure and symptom start. Severe cases, however, are possible, particularly in young children, expectant mothers, and those with weakened immune systems.[2] The zoonotic virus belonging to the genus Orthopoxvirus, sometimes known as monkeypoxvirus or "monkeypox virus," is the cause of the disease. This genus also includes the variola virus, which causes smallpox.Human-to-human transmission can happen when bodily fluids or skin come into direct touch with one another, especially during sexual activity. Individuals are contagious from the moment symptoms appear until all lesions have healed and crusted over. Animals that are infected can spread the virus by handling contaminated meat or by biting or scratching one another.Polymerase chain reaction (PCR) testing for the presence of the virus's DNA in a lesion helps confirm the diagnosis. Mpox can be treated with antiviral medications such tecovirimat, although their efficacy has not been established.[1]
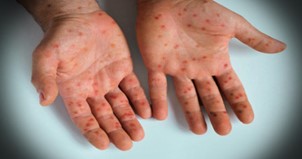
CAUSES
- Direct Veterinary Contact with Diseased Animals
- Animal bites or scratches caused by infection
- Transmission from Human to Human
- Making Touches with Lesions
- Coming into contact with tainted materials or surfaces.
- Contact with Contaminated Animal Fluids
- Inadequate Cleaning Procedures
- Not Vaccinated: Not getting vaccinated can make you more susceptible.
- Living in endemic area
- Eating Contaminated Meat
- Customs and Social Behavior
- Cross-Species Transmission: When there is a high level of wildlife-human interaction, the virus can spread from animals to people.
- A weak health infrastructure, with scant medical resources and inadequate illness surveillance.
- Uncontrolled Outbreaks: Quick dispersal in regions lacking containment strategies.
ETIOLOGY
ORTHOPOXVIRUS MONKEYPOX (MONKEYPOX VIRUS)
A type of double-stranded DNA virus known as the monkeypox virus (MPV, MPXV, or hMPXV) is responsible for causing mpox disease in humans and other mammals. Being a zoonotic virus, it is closely related to the vaccinia, cowpox, and variola viruses as it belongs to the Orthopoxvirus genus. The lipoprotein outer membrane of MPV is oval in shape. The genome has a kilobyte or so. Both the smallpox and monkeypox viruses are Orthopoxvirus, and if the smallpox vaccination is administered three to five years before to the onset of the disease, it will protect against monkeypox.[3]
PATHOGENESIS

The life cycle of the monkeypox virus inside a human cell is shown in this diagram. Notably, the host cell's cytoplasm is where the monkeypox virus replicates. After the virus attaches itself, the virion fuses and bonds to the membrane of the host cell, releasing the viral core into the host cell's cytoplasm. When cells lyse, the formed viral particles become intracellular mature viruses (MV), which remain in the cytoplasm as intracellular mature virions until being discharged as extracellular wrapped viruses. Moreover, MV is capable of wrapping a second envelope, attaching it to the cell membrane, and releasing it through exocytosis. During viral DNA replication, the prodrug brincidofovir and its parent drug cidofovir inhibit the viral DNA polymerase. Tecovirimat stops the virus from exiting an infected cell, preventing the virus from spreading throughout the body. The VP37 protein is targeted by Tecovirimat and is essential for the envelopment of intracellular mature virus with Golgi-derived membrane to produce enveloped virus (EV).[4]
SIGN AND SYMPTOMS
- Fever
- Rash
- Swollen lymph nodes
- Chills
- Headache
- Muscle aches
- Fatigue

MPOX : A visual review on five stages [5]
COMPLICATIONS
Pneumonia, corneal infection resulting in blindness, dysphagia, vomiting, and diarrhea leading to severe dehydration or malnourishment, sepsis (blood infection causing a systemic inflammatory response), brain inflammation (encephalitis), heart disease (myocarditis), proctitis (rectal inflammation), balanitis (genital organ inflammation), urethritis (urinary tract inflammation), or even death are among the complications.Children, pregnant people and people with weak immune systems are at risk for complications from mpox.
DIAGNOSIS

Since monkeypox often strikes isolated communities in the tropical African rain forests, the patient's location is crucial to the identification of the illness. It's critical to distinguish this from chickenpox, which manifests as lesions at different stages of growth that are always visible in succeeding crops. Unlike smallpox, which is 'centripetal' in spread, chickenpox primarily affects the trunk rather than the face and extremities. Scratches can be sent to a reference lab for a definite diagnosis, where electron microscopy can distinguish between varicella and orthopoxviruses and confirm the presence of the latter. DNA restriction analysis can be used to identify the virus and it can be cultivated in tissue culture. It is necessary to obtain a viral culture from an oropharyngeal or nasopharyngeal swab. Analysis should be done on a skin biopsy specimen of the vesiculopustular rash or a sample taken from the ceiling of an intact vesiculopustule. It is possible to get tissue for the PCR of the monkeypox virus's DNA sequence. Analysis of paired sera for acute and convalescent titers is possible. The most effective serum for detecting the monkeypox virus infection was serum obtained more than 5 days after the rash began for IgM detection or more than 8 days after the rash started for IgG detection [7]. When making a differential diagnosis, a Tzanck smear can be used to distinguish monkeypox from other nonviral illnesses. Nevertheless, a Tzanck smear cannot distinguish between smallpox or herpes infections and monkeypox infections. Cases of monkey pox were verified by means of virus isolation or polymerase chain reaction (PCR) identification of the virus from a clinical specimen (skin biopsy or throat culture). People were considered to have a likely case of infection if they developed fever and rash within 21 days of being exposed to monkey pox and if their serum tested positive for orthopox immunoglobulin M (IgM). However, their clinical specimens did not show positive results from PCR or culture [7, 8]. The most accurate clinical indicator that distinguishes monkeypox from chickenpox and smallpox is the presence of swollen lymph nodes, particularly in the inguinal, cervical, submental, and submandibular regions. Nonspecific lesions and inflammation of the vaginal, conjunctival, and throat mucosae have been reported in relation to exanthema [6].
Nucleic Acid Amplification Testing (NAAT)
For the purpose of confirming MPXV infections, real-time and conventional PCR methods are included in nucleic acid amplification testing, or NAAT. These techniques are essential for identifying the distinct viral DNA sequences that define MPXV [10]. PCR is a crucial tool for the identification of viral clades, since it can distinguish between the strains of the Congo Basin (Clade I) and West African (Clade II) viruses [11]. It can be used independently or in combination with sequencing. The development of validated PCR procedures has been the outcome of collaborative work amongst multiple research organizations, producing methods that are able to distinguish between its clades and detect both OPXV and MPXV. The scholarly community has made significant contributions to this sector, offering a multitude of primer and probe sequence sets that facilitate the creation of in-house assays in well-equipped labs. Some techniques use a split approach in which OPXV is detected by a first PCR reaction that does not identify the species, and MPXV clades or lineages are then identified with precision by a second PCR reaction or sequencing effort [9]. Commercial PCR test kits targeting MPXV and OPXV have been observed to be emerging; performance reviews highlight the kits with the best sensitivity and specificity. To confirm the assay’s analytical performance, the use of positive control material obtained through specialized initiatives is advised. It is critical to implement suitable controls in order to maintain specimen and assay integrity, which will reduce false negatives and strengthen the diagnostic process’s dependability.
Electron Microscopy
One method for visually identifying possible poxviruses in specimens is electron microscopy. However, its use in MPXV diagnosis is not common due to the requirement for specialized technical knowledge, necessary equipment, and the development of more approachable molecular assays [12,13]. Consequently, conventional diagnostic evaluation of poxviruses rarely use electron microscopy.
Virus Isolation and Culture
The established techniques of virus isolation and culture are essential for the diagnosis of viral illnesses, such as MPXV. The creation of vaccines, clinical applications, research methodology, and antiviral drug testing are all made possible by this approach, which is essential for comprehensive characterisation through sequencing [14]. By locating the virus's origin, identifying mutations, and reconstructing transmission chains through genomic and phenotypic comparisons among isolates, isolating viruses from important cases supports outbreak investigation and containment efforts. Mammalian cell lines like HeLa, Vero, BSC-1, and RK-13, as well as chicken embryos, which are particularly vulnerable to poxviruses, show vigorous proliferation for MPXV [15]. The virus causes cytopathic consequences in chicken embryos' chorioallantois membranes (CAMs), which become visible 1-4 days after inoculation and include granulation, cell rounding, cytoplasmic bridging, and the formation of syncytium [16].On the other hand, in Vero cell culture, characteristic detached and rounded cells become visible in around 24 hours, which makes it possible to identify viral particles using certain antibodies and immunofluorescence. Even if this method is accurate, its general implementation is severely limited by its lengthy detection time, high-level biosafety lab requirements (level 3 or higher), skilled personnel requirements, and the possibility of infection even with complete personal protection.
Serology
It is not recommended to employ serology in isolation for the clinical diagnosis of MPXV, especially when looking for antibodies in serum or plasma [18]. The possibility of cross-reactivity with antibodies against other orthopoxviruses and those induced by vaccination, whether current or past, reduces the effectiveness of MPXV-specific serological testing. Until more evidence is presented to support the use of serological or antibody-detecting point-of-care (POC) tests outside of these settings, it is advised that serological testing be limited to reference laboratories [17]. The identification of IgM in recently acutely unwell patients or IgG in matched serum specimens—collected at least 21 days apart from the initial sample obtained—if a reference laboratory has a serologically approved test available when other test results are inconclusive—can improve diagnosis accuracy during the first week of sickness [19]. This method highlights the complex function of serology within the diagnostic framework, highlighting its potential usefulness in certain situations and within the bounds of strict application and validation guidelines.
TREATMENT
ANTIVIRAL DRUGS
- Tecovirimat
- Cidofovir
- Brincidofovir

Tecovirimat
In 2018, the FDA authorized tecovirimat for smallpox . The Centers for Disease Control and Prevention (CDC) used an expanded-access investigational new drug application to make tecovirimat available for mpox. Additional treatments could be necessary to totally eliminate mpox in patients with severe immunocompromised states since tecovirimat targets the viral protein p37, which is crucial in cell-to-cell viral transmission, but it does not stop viral replication. Moreover, a single mutation may result in resistance to tecovirimat. Tecovirimat can be administered intravenously or orally; for best absorption, the latter method needs a high-fat meal.[20]
- Cidofovir
The original indication for cidofovir was CMV retinitis. Cidofovir is integrated into viral DNA upon phosphorylation, which results in chain termination. Poxviruses are active against cidofovir [21]. However, cidofovir accumulates in the proximal tubules and is linked to severe nephrotoxicity. In order to address this, probenecid, which blocks organic anion transporters in the nephron, and cidofovir are given intravenously together with fluids. Another treatment for smallpox was discovered using cidofovir's oral prodrug, brincidofovir [22]. However, during the 2022–2023 mpox outbreak, it was not widely employed because to logistical difficulties and medicine scarcity [21].

- Brincidofovir
Adults, children, and newborns who have smallpox due to the virola virus can be treated with brincidofovir (Tembexa). Cidofovir diphosphate’s prodrug is called brincidofovir. The production of viral DNA by the orthopoxvirus DNA polymerase is specifically inhibited by cidofovir diphosphate. Compared to cidofovir, brincidofovir has not been associated with severe renal damage or other adverse effects during the treatment of cytomegalovirus infections, suggesting that brincidofovir may have a better safety profile. In order to help expedite the use of brincidofovir as a treatment for monkeypox, the CDC is now working on an EA-IND.[23]
Vaccination
- MVA-BN (Bavarian Nordic)
- ACAM 2000.

There are two vaccinations available to prevent MPX: ACAM 2000 and MVA-BN (Bavarian Nordic). The Food and Drug Administration (FDA) and the European Medicine Agency (EMA) approved the first, whereas the FDA alone approved the second. Since animal models could be used to determine the efficiency of the vaccine, emergency procedures were conducted and vaccine authorizations for MPX prevention were granted in response to the outbreak’s heightened demand for vaccinations.Additionally, the administration method was changed to enhance the quantity of accessible doses. Specifically, a low dose regimen was approved for MVA-BN, consisting of two intradermal injections of 0.1 ml each spaced four weeks apart. Those who identify as homosexual, bisexual, or other men, or as transgender persons who have sex with multiple men, are at higher risk of exposure to certain vaccinations. These individuals should receive primary preventive (pre-exposure) vaccinations. Those who have been in close proximity to affected individuals should receive post-exposure preventive vaccinations . An observational case-control research revealed that the MVA-BN vaccine was efficacious in 66.6 percent of fully vaccinated participants and 35.6 percent of partially vaccinated subjects, respectively [24].
ADVERSE DRUG REACTIONS (ADR)
Some common ADR of Antiviral drugs and vaccine respectively
Oral:
headache, osteoarthritis, and hidradenitis
Intravenous:
infusion site pain, swelling, erythema, extravasation; headache
PREVENTIONS
- Avoid contact with infected people and animals
- Avoid close contact with people who have rash looks like mpox
- Avoid sharing items
- Avoid sharing clothes,bedlinens,etc
- Practice safe sex
- Wear a mask
MPOX AFFECTED CONTRIES
DEMOCRATIC REPUBLIC OF THE CONGO
1970 saw the discovery of the first human mpox case in history in the Democratic Republic of the Congo (then known as Zaire), in a nine-month-old child[27]. two years subsequent to the announcement of its final smallpox case.[28] On August 24, their rash started.[29] Four other children that year, including three playmates from Liberia, were found to have the illness.[30] At the time, non-human primates in Liberia and Sierra Leone had viral evidence.[30] The World Health Organization (WHO) detected 338 cases with a 28% human-to-human transmission rate through active surveillance between 1981 and 1986.[31][32] Up until 1986, the DRC was responsible for 95% of cases globally.[33]. People above the age of 15 were not frequently affected by cases, and animal interaction in the rainforests was the cause of over two thirds of illnesses.[34] At first, if a family member had a smallpox scar—a sign of past vaccination—it was rare for them to have the virus.[34] Less than 25% of cases were linked to interaction with animals in the rainforest, and close contact infections were more common in elderly individuals between 1996 and 2005.[34] The Democratic Republic of the Congo reported 2,734 instances of probable human mpox between January 2001 and December 2004.[32] Only 171 clinical specimens were retrieved from 136 suspected cases due to inadequate surveillance caused by the civil war, which accounts for less than 5% of all reported cases.[32] An mpox outbreak in the Democratic Republic of the Congo in 2023 led to the reporting of 14,626 suspected cases and 654 related deaths, resulting in a 4.5?se-fatality rate. With 3,576 probable mpox cases and 265 confirmed deaths through the first nine weeks of 2024 in the Democratic Republic of the Congo, the outbreak was still active. This translated into an estimated CFR of 7.4%.[39] Cases are being reported in places like South Kivu and Kinshasa where there is no previous history of mpox. Sexual and close family contact appears to be the main mode of virus transmission in this outbreak. Children have accounted for an estimated 64% of illnesses and 85% of mortality. The epidemic is comprised of two distinct clade I sub-variants, one of which has a new mutation that renders identification using conventional techniques questionable.[39][40] 43 cases were reported in March 2024 as the outbreak extended to the Republic of the Congo, a neighboring country.[39] Early in August, cases of clade I and clade II strains were reported in Burundi, Rwanda, Uganda, Kenya, Cote d’Ivoire, and South Africa. This further extended the outbreak across central and southern Africa.[41][42] On August 14, 2024, the WHO deemed this a Global Health Emergency. Sweden was the first non-African nation to report a case of clade I mpox the next day. In a press conference on August 19, 2024, Samuel-Roger Kamba Mulamba [fr], the Minister of Public Health for the Democratic Republic of the Congo, stated that the outbreak had affected every province in the nation, including Kinshasa. The ministry also announced that the national government would implement a response plan worth €45 million, which would include patient care, medical team deployment, and awareness campaigns, but not vaccines.[27] Kamba added that the DRC need approximately 3.5 million doses of the mpox vaccine, with Belgium expected to provide about 215,000 doses and Japan potentially donating up to three million doses.
The DRC began to record more than 1,000 suspected cases annually after 2005.[35] A total of 760 laboratory-confirmed instances of human mpox were identified between November 2005 and November 2007. These cases were primarily found in men, under-15-year-olds, residents of forested areas, and those without a prior smallpox vaccine.[36] Though there are also occasional big outbreaks, most instances occur intermittently or in tiny clusters. During the 2013 outbreak, it was found that the probability of human-to-human transmission inside DRC households ranged from 50% to 100%.[28] In the DRC, the Bokungu Health Zone experienced a 600-fold rise in cases during that year. Three,794 suspected cases and 73 deaths were recorded by the DRC in 2019.[33] It recorded more than 4,500 suspected instances of mpox in the first nine months of 2020, with 171 deaths.[33] In the DRC, where the disease is prevalent and the disease burden is still significant, mpox cases must be reported.[37] In those areas, some of the most impoverished and marginalized groups globally have been impacted by the more aggressive Clade I.[38] Every suspected case of mpox is reported to a regional surveillance system, and if feasible, an investigation may be conducted into it.

UGANDA
Due to increased reports of mpox cases in the neighboring country, Kasese District increased surveillance for the disease in June and early July of 2024 along the border with the Democratic Republic of the Congo. On July 11, six suspected cases were found after screeners at the Bwera point of entry and Bwera Hospital received instruction. Samples from the suspected cases were obtained for laboratory testing, and on July 15, two of the samples proved positive for MPXV Clade Ib. A 37-year-old woman is the first verified case, and a 22-year-old female Democratic Republic of the Congolese national is the second. These are the nation’s first cases of mpox to be discovered. The Uganda Virus Research Institute conducted a PCR test on July 15, 2024, to confirm the beginning of symptoms in both instances, which began on July 11. As of August 12, 2024, no secondary transmission has been connected to the two cases, according to investigations that found the transmission happened outside of Uganda. 39 suspected cases had been reported by the same day. In addition, 37 contacts from the confirmed patients were being monitored. As of August 20, there have been no recorded deaths.[25]

NETHERLANDS
Following worldwide alarms (EpiPulse, Early Warning and Response System), the first case of mpox in the Netherlands was discovered at a sexual health clinic in Amsterdam and verified on May 20, 2022. On August 8, 2022, the Netherlands announced the 1,000th case of mpox. With 31,112 confirmed cases of mpox reported globally, this equates to a cumulative incidence rate of 55 cases per million people, one of the highest rates in the world at the time, after Spain (104/million) and Portugal (69/million) [44]. Since the Netherlands discontinued its smallpox vaccination effort in 1974 and orthopoxvirus infections are uncommon, more than half (57%) of the population has never been exposed to orthopoxviruses and can be regarded as immunologically naïve [45,46]. In 1977, the World Health Organization suspended its global smallpox immunization program. The smallpox virus was declared extinct in 1980 [47]. Nonetheless, those who received the single dose of the first-generation smallpox vaccine may still benefit from cross-protection against (severe) mpox via MPXV-neutralizing antibodies [48, 49]. Here, we outline the features of the first 1,000 instances of mpox in the Netherlands, the public health reaction, and the estimated level of protection the first-generation smallpox vaccine offers against moderate-to-severe mpox symptoms.[43]

INDIA
Since the 2022 proclamation, 30 cases have been found in India; the most recent incidence was recorded in March 2024[50]
NIGERIA
In Nigeria, 39 cases of mpox have been confirmed in 2024; no deaths have been reported. In contrast, approximately 6000 suspected cases of cholera have been reported, with 176 deaths, the majority of which involve children under the age of five.[51]
BURUNDI
After the National Reference Laboratory of the National Institute of Public Health confirmed three cases, the Ministry of Health of Burundi announced an mpox outbreak on July 25, 2024. These three cases—one from Kamenge University Hospital, one from Kamenge Military Hospital, and one from Isare Health District—were discovered on July 22. They stated that fever, joint pain, and a generalized rash started on July 24. On July 25, a PCR test revealed that samples taken during a multidisciplinary inquiry tested positive with mpox. These cases of mpox are the first to be confirmed in Burundi. Since the outbreak proclamation, there have been 545 reports of mpox cases; as of August 17, 2024, 474 suspected cases (86.9%) had been looked into and confirmed. 142 (39.7%) of the 358 suspected cases that were tested were positive for MPXV. Clade Ib MPXV has been verified by genome sequencing study. As of August 17, there were no recorded deaths. Out of the 49 districts, 26 have reported confirmed cases (53.1%). With 54 of the 142 confirmed cases (38%), Bujumbura Nord, an urban region, is the most afflicted district. As at the time of reporting, no deaths had been reported.Of the cases, 44.4% are female and 55.6% are male. Children under five years old account for 28.9% of instances, with those between the ages of eleven and twenty (20.4%) and twenty-one and thirty (18.3%) making up the next three age groups.[52]
Year :- 2024
Case – 545
Positives:- 142
Death :- no death
SOUTH AMERICA
Year :- 2022- 23
Confirmed cases :- 22,293
Deaths :- 44
RWANDA
Two laboratory-confirmed mpox cases were reported to WHO by the IHR National Focal Point (NFP) for Rwanda on July 24, 2024. The Ministry of Health subsequently announced an outbreak of mpox in Rwanda on July 27. The two patients were a 34-year-old man (case 2) who had recently traveled to the Democratic Republic of the Congo and a 33-year-old woman (case 1) who visits the country regularly. Case 2 was discovered at the Kibagabaga hospital in the Gasabo district, and Case 1 was discovered at a point of entry (PoE) and isolated in the Rusizi district. According to reports, both instances are stable and receiving further medical care. These cases of mpox are the first to be confirmed in Rwanda. As of August 7, 2024, the nation had reported four confirmed cases of mpox and no deaths. One of the two new cases is a 34-year-old man who lives in Kigali’s Gasabo District. His symptoms, which included a fever, swollen lymph nodes, sore throat, and rashes on his face, arms, and genitalia, began on July 15, 2024. On July 12, 2024, he returned from Burundi and is presently being held in isolation. Five intimate contacts are being monitored. The other example is a 39-year-old male Rwandan living in Kicukiro District who has visited the Democratic Republic of the Congo in the past. He began experiencing similar symptoms on July 12, 2024, coupled with a headache.[52]
Year : August 2024
Case:4
Death: No
WEST AFRICA
Côte d’Ivoire confirmed two mpox cases that did not result in death in July 2024. In the first occurrence, a 46-year-old patient in the Tabou area of San Pedro region, near the Liberian border, saw a doctor on July 1st, complaining of a fever, headache, and skin rash. On July 3 and July 14, respectively, the laboratory of the Institutes Pasteur of Côte d’Ivoire and the Institute Pasteur in Dakar verified the presence of mpox. The second instance is a 20-year-old patient who, on July 14, arrived with skin rash and oral mucosal lesions in the Koumassi health district of Abidjan. There is no known epidemiological connection between the first two incidents. Three health districts—Koumassi (one case), Tabou (one case), and Yopougon-Ouest-Songon (five cases)—had seven confirmed cases of mpox as of August 7, 2024. Of the seven confirmed cases, four (57%) are male and all older than 15 years. We have discovered forty contacts and are pursuing them. Although the nation has previously reported occurrences of mpox, no cases have been reported since the multi-country outbreak began in 2022. Clade II MPXV is the source of the newly discovered instances in 2024.
CONCLUSION
We were studying the monkeypox in detail. Firstly, we were studying the basic introduction of the monkeypox virus. Also, we study the aetiology, epidemiology, and pathophysiology of the monkeypox virus. And we were studying adverse drug reactions, causes, and risk factors of monkeypox virus. Also, in which country the 1st monkeypox virus is entered. Then how it is transmitted from person to person. Monkeypox is a communicable disease. Then we were studying stages of monkeypox disease, and we were also studying signs and symptoms of monkeypox disease. Monkeypox virus highly spread in Africa.We were studying to collect patients, how many cases were found in a specific country, and the death ratio of the monkeypox virus. In specific year or duration of spreading monkeypox virus in country.Also, we study how to diagnose the monkeypox virus and which drug is used in the treatment of the virus.
REFERENCES
- Mpox. Wikipedia. 2024 August 23.https://en.m.wikipedia.org/wiki/Mpox.
- 2.Monkeypox 2019 November 1.https://www.who.int/healthtopics/monkeypox#tab=tab_1.
- 3.Miranda MD, Caldas GC, Ferreira VN, Barth OM, da Silva APD, Silva MST, Grinsztejn B, Veloso VG, Souza TM, da Silva EE, Barreto-Vieira DF. Monkeypox (Mpox) virus isolation and ultrastructural characterisation from a Brazilian human sample case. Mem Inst Oswaldo Cruz. 2023 Aug 28;118:e230090. Doi: 10.1590/0074-02760230090. PMID: 37646742; PMCID: PMC10469757.
- Huang Y, Mu L, Wang W. Monkeypox: epidemiology, pathogenesis, treatment and prevention. Signal Transduction and Targeted Therapy. 2022 November 2;71. https://doi.org/10.1038/s41392-022-01215-4.
- Mpox. Cleveland Clinic. 2024 May 1. https://my.clevelandclinic.org/health/diseases/22371-monkeypox.
- Monkeypox.
- http://misc.medscape.com/pi/iphone/medscapeapp/html/A1134714-business.html Accessed: March 20, 2017.
- Karem, KL., Reynolds, M., Braden, Z., Lou, G., Bernard, N and Patton, J. 2005. Characterization of acute-phase humoral immunity to monkeypox: use of immunoglobulin M enzyme-linked immunosorbent assay for detection of monkeypox infection during the 2003 North American outbreak. Clin Diagn Lab immunol.12: 867-872.
- 8.Reynolds, MG., Yorita, KL., Kuehnert, MJ., Davidson, WB.,Huhn, GD and Holman, R. 2006. Clinical manifestations of human monkeypox influenced by route of infection. J Infect Dis.194: 773-780
- Li, Y.; Zhao, H.; Wilkins, K.; Hughes, C.; Damon, I.K. Real-time PCR assays for the specific detection of monkeypox virus West African and Congo Basin strain DNA. J. Virol. Methods 2010, 169, 223–227. [CrossRef] [PubMed]
- Laboratory Testing for the Monkeypox Virus: Interim Guidance. Available online: https://iris.who.int/handle/10665/354488 (accessed on 5 December 2022).
- Scarpa, F.; Sanna, D.; Azzena, I.; Cossu, P.; Locci, C.; Angeletti, S.; Maruotti, A.; Ceccarelli, G.; Casu, M.; Fiori, P.L.; et al. Genetic Variability of the Monkeypox Virus Clade IIb B.1. J. Clin. Med. 2022, 11, 6388. [CrossRef]
- Gentile, M.; Gelderblom, H.R. Rapid viral diagnosis: Role of electron microscopy. New Microbiol. 2005, 28, 1–12. [PubMed]
- Müller, M.; Ingold-Heppner, B.; Stocker, H.; Heppner, F.L.; Dittmayer, C.; Laue, M. Electron microscopy images of monkeypox Virus infection in 24-year-old man. Lancet 2022, 400, 1618. [CrossRef]
- Reed, K.D.; Melski, J.W.; Graham, M.B.; Regnery, R.L.; Sotir, M.J.; Wegner, M.V.; Kazmierczak, J.J.; Stratman, E.J.; Li, Y.; Fairley,J.A.; et al. The detection of monkeypox in humans in the Western Hemisphere. N. Engl. J. Med. 2004, 350, 342–350. [CrossRef]
- Erez, N.; Achdout, H.; Milrot, E.; Schwartz, Y.; Wiener-Well, Y.; Paran, N.; Politi, B.; Tamir, H.; Israely, T.; Weiss, S.; et al. Diagnosis of imported monkeypox, Israel, 2018. Emerg. Infect. Dis. 2019, 25, 980. [CrossRef]
- Hutson, C.L.; Kondas, A.V.; Mauldin, M.R.; Doty, J.B.; Grossi, I.M.; Morgan, C.N.; Ostergaard, S.D.; Hughes, C.M.; Nakazawa, Y.; Kling, C.; et al. Pharmacokinetics and efficacy of a potential smallpox therapeutic, brincidofovir, in a lethal monkeypox virus animal model. MSphere 2021, 6, 10–1128. [CrossRef]
- Weaver, J.R.; Isaacs, S.N. Monkeypox virus and insights into its immunomodulatory proteins. Immunol. Rev. 2008, 225, 96–113.[CrossRef] [PubMed]
- Gilchuk, I.; Gilchuk, P.; Sapparapu, G.; Lampley, R.; Singh, V.; Kose, N.; Blum, D.L.; Hughes, L.J.; Satheshkumar, P.S.; Townsend,M.B.; et al. Cross-neutralizing and protective human antibody specificities to poxvirus infections. Cell 2016, 167, 684–694.[CrossRef] [PubMed]
- Dubois, M.E.; Hammarlund, E.; Slifka, M.K. Optimization of peptide-based ELISA for serological diagnostics: A retrospective Study of human monkeypox infection. Vector-Borne Zoonotic Dis. 2012, 12, 400–409. [CrossRef] [PubMed]
- Duong MT, Tebas P, Ancha B, et al. Combination of Extended Antivirals with Antiretrovirals for Severe Mpox in Advanced HIV Infection: Case Series of 4 Patients. Open Forum Infectious Diseases. 2024 February 27. https://doi.org/10.1093/ofid/ofae110.
- Stafford A, Rimmer S, Gilchrist M, et al. Use of cidofovir in a patient with severe mpox and uncontrolled HIV infection. Lancet Infect Dis 2023; 23:e218–26.Google ScholarCrossrefPubMedWorldCat
- Lanier R, Trost L, Tippin T, et al. Development of CMX001 for the treatment of poxvirus infections. Viruses 2010; 2:2740–62.Google ScholarCrossrefPubMedWorldCat
- Facp MBGM Fidsa,. Monkeypox (Mpox) Treatment & Management: Medical Care, Prevention, Long-Term Monitoring n.d. https://emedicine.medscape.com/article/1134714-treatment?form=fpf#d9.
- Patauner F, Gallo R, Durante-Mangoni E, Bertolino L. Monkeypox infection, 18 months later: A vanishing epidemic? European Journal of Internal Medicine. 2024 April 1;122:35–7. https://doi.org/10.1016/j.ejim.2024.02.017.
- Item 2024 August 22. https://www.who.int/emergencies/disease-outbreak-news/item/2024-DON528#:~:text=As of 7 August 2024,in Gasabo District in Kigali.
- Nolen LD, Osadebe L, Katomba J, et al. Extended Human-to-Human Transmission during a Monkeypox Outbreak in the Democratic Republic of the Congo. Emerging Infectious Diseases. 2016 June 1;226:1014–21. https://doi.org/10.3201/eid2206.150579.
- Mpox in the Democratic Republic of the Congo. Wikipedia. 2024 August 21. https://en.wikipedia.org/wiki/Mpox_in_the_Democratic_Republic_of_the_Congo.
- Ryan ET, Hill DR, Solomon T, Endy TP, Aronson N. Hunter’s Tropical Medicine and Emerging Infectious Diseases E-Book. Elsevier Health Sciences; 2019 March 25.
- Arita I, Henderson DA. Monkeypox and whitepox viruses in West and Central Africa. Bull World Health Organ. 1976;53(4):347-53. PMID: 186209; PMCID: PMC2366520.
- Cho CT, Wenner HA. Monkeypox virus. Bacteriol Rev. 1973 Mar;37(1):1-18. Doi: 10.1128/br.37.1.1-18.1973. PMID: 4349404; PMCID: PMC413801.
- Poxviruses.GoogleBooks.N.d.https://books.google.com/books/about/Poxviruses.html?id=F18FC71UH-sC#v=onepage&q=Outbreak of monkeypox at Rotterdam Zoo&f=fals.
- Rimoin AW, Kisalu N, Kebela-Ilunga B, Mukaba T, Wright LL, Formenty P, Wolfe ND, Shongo RL, Tshioko F, Okitolonda E, Muyembe JJ, Ryder R, Meyer H. Endemic human monkeypox, Democratic Republic of Congo, 2001-2004. Emerg Infect Dis. 2007 Jun;13(6):934-7. Doi: 10.3201/eid1306.061540. PMID: 17553242; PMCID: PMC2792850.
- Item 2020 October 1. https://web.archive.org/web/20220605103903/https://www.who.int/emergencies/disease-outbreak-news/item/monkeypox-democratic-republic-of-the-congo.
- Hobson G, Adamson J, Adler H, Firth R, Gould S, Houlihan C, Johnson C, Porter D, Rampling T, Ratcliffe L, Russell K, Shankar AG, Wingfield T. Family cluster of three cases of monkeypox imported from Nigeria to the United Kingdom, May 2021. Euro Surveill. 2021 Aug;26(32):2100745. Doi: 10.2807/1560-7917.ES.2021.26.32.2100745. PMID: 34387184; PMCID: PMC8365177.
- Malik YS, Singh RK, Dhama K. Animal-Origin Viral Zoonoses. Springer Nature; 2020 September 23.
- Mi36.Rimoin AW, Mulembakani PM, Johnston SC, Lloyd Smith JO, Kisalu NK, Kinkela TL, Blumberg S, Thomassen HA, Pike BL, Fair JN, Wolfe ND, Shongo RL, Graham BS, Formenty P, Okitolonda E, Hensley LE, Meyer H, Wright LL, Muyembe JJ. Major increase in human monkeypox incidence 30 years after smallpox vaccination campaigns cease in the Democratic Republic of Congo. Proc Natl Acad Sci U S A. 2010 Sep 14;107(37):16262-7. Doi: 10.1073/pnas.1005769107. Epub 2010 Aug 30. PMID: 20805472; PMCID: PMC2941342.
- Brown K, Leggat PA. Human Monkeypox: Current State of Knowledge and Implications for the Future. Trop Med Infect Dis. 2016 Dec 20;1(1):8. Doi: 10.3390/tropicalmed1010008. PMID: 30270859; PMCID: PMC6082047.
- Adler H, Gould S, Hine P, Snell LB, Wong W, Houlihan CF, Osborne JC, Rampling T, Beadsworth MB, Duncan CJ, Dunning J, Fletcher TE, Hunter ER, Jacobs M, Khoo SH, Newsholme W, Porter D, Porter RJ, Ratcliffe L, Schmid ML, Semple MG, Tunbridge AJ, Wingfield T, Price NM; NHS England High Consequence Infectious Diseases (Airborne) Network. Clinical features and management of human monkeypox: a retrospective observational study in the UK. Lancet Infect Dis. 2022 Aug;22(8):1153-1162. Doi: 10.1016/S1473-3099(22)00228-6. Epub 2022 May 24. Erratum in: Lancet Infect Dis. 2022 Jul;22(7):e177. Doi: 10.1016/S1473-3099(22)00353-X. Erratum in: Lancet Infect Dis. 2022 Jul;22(7):e177. Doi: 10.1016/S1473-3099(22)00372-3. PMID: 35623380; PMCID: PMC9300470.
- More than 600 dead in spreading DR Congo mpox outbreak as Republic of Congo reports its first cases. CIDRAP. 2024 March 15.
- Stawiska Z. Deadly Mpox Transmission In DR Congo Happening Under Radar; Most Victims Are Children – Health Policy Watch. Health Policy Watch. 2024 March 13. https://healthpolicy-watch.news/deadlly-mpox-transmission-in-dr-congo-happening-under-radar-children-main-victims/.
- Cohen J. Deadlier strain of mpox spreads to multiple African countries. Science. 2024 August 9;3856709:591. https://doi.org/10.1126/science.ads2948.
- WHO considers public health emergency as mpox cases mount in Africa. CIDRAP. 2024 August 5.
- Van Ewijk CE, Miura F, Van Rijckevorsel G, et al. Mpox outbreak in the Netherlands, 2022: public health response, characteristics of the first 1,000 cases and protection of the first-generation smallpox vaccine. Eurosurveillance. 2023 March 23;2812. https://doi.org/10.2807/1560-7917.es.2023.28.12.2200772.
- Mathieu E, Spooner F, Dattani S, Ritchie H, Roser M. Mpox (monkeypox). OurWorldInData.org. [Accessed: 24 Aug 2022]. Available from: https://ourworldindata.org/monkeypox
- Statistics Netherlands (CBS). Leeftijdsverdeling. Hoe ziet de leeftijdsopbouw van de Nederlandse bevolking eruit? [Age distribution – What is the age structure of the Dutch population?]. The Hague: CBS. [Accessed: 9 Jan 2023]. Dutch. Available from: https://www.cbs.nl/nl-nl/visualisaties/dashboard-bevolking/leeftijd/bevolking
- Dutch National Institute of Public Health and the Evironment (RIVM). Pokken. [Smallpox]. Bilthoven: RIVM; 2020. Dutch. Available from: https://www.rivm.nl/pokken
- World Health Organisation (WHO). History of smallpox vaccination. Geneva: WHO. [Accessed: 9 Jan 2023]. Available from: https://www.who.int/news-room/spotlight/history-of-vaccination/history-of-smallpox-vaccination
- Fenner F, Henderson DA, Arita I, Jezek Z, Ladnyi ID. Smallpox and its eradication. Geneva: World Health Organisation; 1988. 1460 p. Available from: https://apps.who.int/iris/handle/10665/39485
- Zaeck LM, Lamers MM, Verstrepen BE, Bestebroer TM, van Royen ME, Götz H, et al. Low levels of monkeypox virus-neutralizing antibodies after MVA-BN vaccination in healthy individuals. Nat Med. 2023;29(1):270-8. https://doi.org/10.1038/s41591-022-02090-w PMID: 36257333
- Singh SG. Mpox outbreak: What travellers from US, India, & other countries must know. WwwBusiness-StandardCom. 2024 August 22.
- Adepoju P. Mpox declared a public health emergency. The Lancet. 2024 August 1;40410454:e1–2. https://doi.org/10.1016/s0140-6736(24)01751-3.
- Item 2024 August 22. https://www.who.int/emergencies/disease-outbreak-news/item/2024-DON528#:~:text=These are the first confirmed,%) tested positive for MPXV.


 Borphale Pallavi Rajendra *
Borphale Pallavi Rajendra *
 Patel Muskan Rajjak
Patel Muskan Rajjak
 Dharashive Pradnya Shivsamb
Dharashive Pradnya Shivsamb
 Giram Tushar Bankat
Giram Tushar Bankat












 10.5281/zenodo.13787539
10.5281/zenodo.13787539