Abstract
Mucosal vaccines are gaining much focus of interest to prevent virulence of harmful viruses which spreads through high viral load in surrounding environment of infected patient. These highly contagious pathogens can easily enter into nasal and oral mucosa of healthy patients and infect them. Nasal and oral vaccines can provide effective immunization at site of induction of disease and improves the safety of individuals. They can be used solely as preventive medications and in combination with conventional vaccines and medication to combat serious progression of disease. This review focuses on how nasal vaccines can effectively stimulate immune responses and their superiority to oral and other mucosal vaccines.
Keywords
Mucosal Vaccines, Immunization, Intra-nasal approach
Introduction
Nasal pathway is the primary inductive site for many airborne infections. It is an excellent pathway for the antigens to prevent harmful gastric juices and first pass metabolism in portal circulation. Conventional vaccines which are highly efficacious to stimulate immunogenic responses systemically cannot efficiently elicit mucosal immunity due to many limitations. The extent of mucosal immunization by needle based vaccines largely depends on antigen-adjuvant combination[1]. An overview of antibodies produced during nasal and intra muscular vaccination in illustrated below.

Figure 1: Antibody stimulating followed by intra nasal vaccination(left) and intra muscular vaccination(right) when same viral vector based vaccine is given
Ongoing research paves a way for development of efficient intra nasal vaccines. Unlike orally administered vaccines intra nasal vaccine is beneficious because of no exposure of formulation to gastric environment. Nasal mucosa is highly vascularized and contains specialized immune response system called Nasopharynx-associated lymphoid tissue (NALT). The immune response generated by nasal vaccine can prevent the viral pathogens right at their site of entry[2]. Nasal route for vaccination has a history dates back from 17th century to prevent mild smallpox. First documented evidence was found in china where it was mentioned as the emperor of Kangxi made his family and some important members of army to get inoculated a smallpox infected cotton swab with some fluid into nostrils of individuals [3]. Global pandemics of influenza and anthrax brought researchers interest in developing modern intra nasal vaccines. SARS CoV-19 pandemic led to rise in efforts of new nasal vaccines which are economical and facilitating mass vaccination in countries like India. Beside of its advantages nasal vaccines are limited in their use due to lack of evidences to support their efficacy and the pathways associated to theorize complicated induction of systemic humoral immunity through naso-mucosal immunization. The choice of drugs and adjuvant are limited based on information up-to-date due to sensitive nature of nasal mucosal cells. A detailed review on advancements in formulations, challenges associated will be discussed in the later sections [4].
NASO-MUCOSAL IMMUNE SYSTEM:
NASAL PHYSIOLOGY [4,5,6]:
Human nasal cavity is broadly classified into three regions namely vestibular, olfactory, and respiratory regions. The frontal part is named as vestibular region because it comprises of both vestibules and nasal hair. This region is less vascularized; thus, it is not considerably involved in drug absorption in intranasal delivery. The olfactory region is the upper part of the nasal cavity, with a surface area of ~10 cm2. This region has olfactory nerves connecting olfactory epithelia and olfactory bulb, which play a critical role in direct nose-to-brain drug and gene transport.
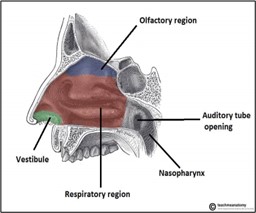
Figure 2:Various regions of nasal mucosa
The respiratory region is the largest area of the nasal cavity (~160 cm2) and is highly vascularized. Therefore, drug and gene absorption into the blood from the nasal cavity primarily occurs in this respiratory process. The respiratory epithelium in this region comprises of basal cells, mucus secreting goblet cells, and ciliated and non-ciliated columnar cells. Some respiratory cells are covered with cilia, which play a primary role in mucociliary clearance that protects the respiratory tract from pathogens or particles. In this region, the ophthalmic and maxillary branches of the trigeminal nerves are responsible for the transport of drugs and genes directly to the brain.

Figure 3:Detailed celluar structures in Olfactory and Respiratory regions
NASO MUCOSAL IMMUNOLOGY [7]:
Nasal cavity is multi functional in nature. Although its primary function is exchanging of gases and olfactory purpose, it serves as draining clearance for ocular region, to entrap and memorize pathogens in inhaled hair. The respiratory tract is covered with thick, viscoelastic gel like secretion called mucosa. Secreatory cells and goblet cells produce this nasal mucosa. Humidification, entrapment of dust and antigens through filtration are its primary functions.

Figure 4:Histology of nasal epithelium
The nasal mucosa consists of the epithelium, basement membrane, and lamina propria. There are four main cell types in nasal mucosa: basal cells, goblet cells, ciliated columnar cells, and non-ciliated columnar cells. Basal cells are found only on the basement membrane, and the other three cell types are found on the whole apical epithelial surface.Naso mucosal immunity mainly depends on specialized tissue called NALT (Naso-pharynx Associated Lymphoid Tissue).So this tissue is the target of interest for many developing intra nasal immune response activating formulations. Adenoids, lingual tonsils, palatal tonsils and NALT are collectively crucial components of the Waldeyer’s ring.


Figure 5:Anatomical location of Waldeyer's ring(left image) and its components(right image).
Nasal mucosal immunization [8]:
When an intra nasal vaccine is administered either one or both of the following mechanisms will be observed. First one is activation of NALT and its associated immune cells in upper respiratory tract which produces s-IGA. Second is by presenting antigen to BALT in bronchi region to elicit both IGA and IGG antibodies.
Mechanism of immunization in upper respiratory tract [2,8,9]:
Whenever intranasal vaccine is administered in solution form (nasal drops) or as a coarse mist, the attenuated antigen enters into nostrils and settles down onto mucosa in upper respiratory tract. Mucus secretions are hydrophilic in nature so the lipid cored vector is insoluble and remain in particulate form. The antigenic material is carried towards pharynx region due to ciliary movement. NALT is enclosed by group of epithelial cells called follicle-assoicated epithelial cells (FEC) .these celles are also known as villios M cells which are involved in processing and presenting antigen to NALT. Naso-pharynx asoociated lymphoid tissue is combination of Dentritic cells,T cells, B cells. Villious M cells present antigen to Dentritic cells and macrophages in NALT.CD4+ T cells will be activated and communicate with adjacent B cells. IGA committed B cells will be produced and migrates to effector site. These cells will deelop IGA producing plasma cells. The developed IGA dimerises and transports to effector site by binding to polymeric Ig receptor. S-IGA (antigen specific IGA) will be released in the mucus layer.

Figure 6:Cellular response cascade in upper respiratory tract immunization
Apart from this mechanism there is another theory that antigens which are hydrophilic are souble in mucus will directly reach systemic circulation through paracellular aqueous transport and elicit immune response which in turn leads to mild systemic immunity.
Mechanism of immunization in lower respiratory tract:
This event is observed only when the antigen manages to reach bronchioles region or the vaccine is designed in such intent. Similar to the above case the antigen is presented by M cells to Follicular dentritic cells (FDC) these cell clusters will activate B cells, native T cells. Activated B cells in turn develop IGA antibodies through maturation to IGA committed B cells(mucosal response).The t cell zone activates various kinds of CD4+ Th2 cells, CD4+ Th1 cells. These two types of cells activate IGg commited B cells (humoral response) and Natural killer cells (NK cells elicit cellular response) respectively. Additionally FDC cells activate Bronchus Associated Lymphoid Tissue (BALT).BALT is a specialized tissue which as line of defense as similar to NALT .Activation of BALT increases production of cytotoxic T lymphocytes. Cytotoxic T lymphocytes will lyses infected cells in bronchi region.

Figure 7:cellular responses after antigen uptake in bronchi region
BENEFITS OF INTRA NASAL VACCINES[10]:
Nasal vaccines are mainly developed to avoid the traumatic procedure of conventional needle based vaccines. Apart from being painless administration the following reasons draw interest to developed vaccines using nasal route
- The antigen administered into nasal mucosa is not exposed to any gut enzymes and lower gastric pH conditions which highly improves its bioavailability.
- Since this rout bypasses first pass metabolism and other enzymatic degradations the dose of antigen incorporated will be reduced.
- Instilling nasal drops or as sprays are convenient to patient so they can be self administrable.
- Mucosal immunity along with systemic immunity can effectively prevent nasal infecting diseases like flu and HIV.
- The immune response generated is strong enough than conventional systemically delivered vaccines
- The production cost will be greatly reduced which makes the vaccine available for many people.
- Most preferred and practically feasible mucosal route to deliver vaccine (superior to oral route).
- Highly specific and long term immunity can be achieved because of specialized immune activations and high blood vasculature.
CHALLENGES ASSOCIATED[10,11]:
- Limited nostrils capacity to hold the formulation approx 25-200µl.
- Lack of human compatible adjuvants which are both compatible to antigen and efficient to elicit immune response.
- Rapid muco ciliary clearance makes difficult to estimate that required amount of antigen penetrated into cells.
- Difficult to administer vaccine when there is excess secretion of mucus or irritation in mucus layer.
- If proper device is not used the sterility of product may be disturbed in case of multi unit vaccine.
FORMULATION APPROACHES OF NASAL VACCINE DELIVERY:
Factors Effecting Nasal Absorption:
Designing of nasal vaccines needs little more effort, because we have to consider various factors like extent of mucus secretion, muco-ciliary clearance, age related factors[12]. These factors are broadly divided into physiological factors, formulation related and patient related factors.
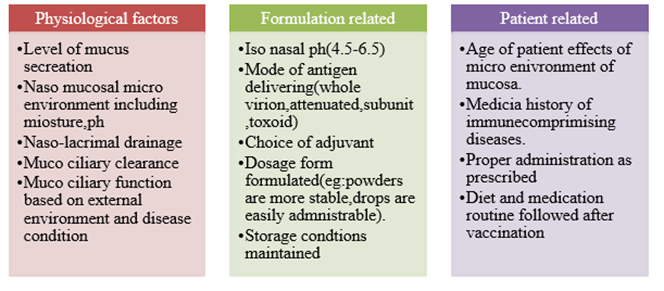
Approaches in Developing Delivery Systems:
Various strategies for ideal adjuvant combination are being explored and under development to successfully deliver antigen of interest via intra nasal route .According to M. Ramvikas et.al[2] and Junhu Tai et.al group studies[13] these strategies are broadly classified as replicating and non replicating delivery systems. Replicating delivery systems include utilization of suitable genetically modified viral and bacterial vectors. The selection of these vectors depends on the site of target in lower and upper respiratory tracts and also depends on antigen of interest. Adeno virus, rhino virus and rota virus are some of preferable vectors. Bacterial vectors like Shigella, Salmonella, Listeria are commonly employed. Development of these type of systems are first of choice but complicated to prevent virulence attenuated vaccine if change in storage conditions of vaccine. Non-replicating delivery systems comprises of Liposomes, Micro & Nano particulate systems, immune stimulating complexes (ISCOMs), Virus like particles, emulsions, Bio adhesive delivery systems.
Liposomes[14]:
Lipid containing micro vesicles protects hydrophilic antigens to remain as stable against enzymes in mucus layer. The immune response generated when antigen stimulate IGA mediated response provides long term immunity against pathogen. Chitosan based liposomes are getting widely developed because chitosan exhibits mucoadhesive property in nasal cavity.
Particulate systems[15]:
Nano particulate systems are gaining interest in targeted delivery of active ingredients and biological due to their fine size and improved bioavailability. Vehicles which are less than 1000nm (1µm) tend to have higher absorption rate due to high uptake by antigen presenting cells. Cationic nano particles have shown higher entrapment efficiency in recent studies performed by François Fasquelle et.al
ISCOMs[16]:
The Immune Stimulating Complex (ISCOM) is documented as a strong adjuvant and delivery system for parenteral immunization. Its effectiveness for mucosal immunization has also been proven with various incorporated antigens. Lövgren et al. were the first to demonstrate the capacity of influenza virus ISCOMs to induce mucosal immune response and protection after one comparatively low nasal dose. Further studies show that similar to Cholera toxin (CT) and Escherichia coli heat-labile toxin (LT), ISCOMs break immunological tolerance and exert strong mucosal adjuvant activity, resulting in secretory IgA and systemic immune responses.
Virus like particles[17]:
VLPs are formed by spontaneous interaction between one or more viral structural capsid proteins to form the final structure. VLPs are structurally and visually similar to live viruses but lack either a complete virus genome or lack the entire virus genome. The variety of structures adopted by different VLPs makes them structurally and functionally attractive. Spontaneous polymerization of different viral capsid proteins can produce VLPs with geometrical symmetry, usually in the form of icosahedral, spherical, or rod-like structures, depending on which virus from which they were derived. VLPs can generally be divided into different groups based on their structural complexity. Capsid proteins can be arranged in one, two or three layers. Other single layer VLPs can contain more than one structural protein.
Nano-emulsions:
nano dispersed systems are used to formulate when the drug is poorly water soluble. A novel optimized intranasal epitope-loaded NE delivery system for H. pylori vaccine developed by Yun Yang, Li Chen, Hong-wu Sun study group concluded that nano emusion of N-acetyl-neuroaminyllactose-binding hemagglutinin protein (epitope) loaded nano emulsion prepare with isopropyl myristate (IPM) as the oil phase, EL-35® (BASF, Germany) as the surfactant and propylene glycol as the co-surfactant, with water as the aqueous phase is effective as potent intra nasal vaccine[18].

Innovative Nasal Vaccine Delivery Devices:
Nasal vaccine formulations are generally delivered in form of drops and fine mist. Dry powder inhalers are also developed in order to increase shelf life of product. Since mucus clearance is an important factor which influencing penetration of antigen loaded vector hydrogels are coming into focus in this area of research. Hydrogels containing Malto dextrin, Chitosan[19] and also insitu polymers are developed and their efficacy is being studied by various works. Bio materials like mRNA as adjuvants in effective delivery of viral vaccines has been under study[20]. APTAR Pharma developed patented nasal syringe which can able to delivery almost any type of nasal vaccine formulation ranging from liquids ,gels to Dry powder with high accuracy and maintaining sterility of product[21]. Various types of model are being introduced in order to meet effective and safe delivery of single dose and multi dosage intra nasal vaccines.

Figure 8: Novel nasal syringe developed by APTAR Pharma( from left to right ) nasal dropper outer compartment,inner delivering syringe,punching cylinder,piston.
Nasal mist and spray type devices are also under clinical trials of which Laval nozzle is well known device. These types of devices provide liquid formulations as fine mist same ass observed from nebulizer. Through optimizing of the Laval nozzle structure and testing experiments on spray particle size, spray velocity, spray angle and spray rate, a set of parameters which is applicable to optimal nasal spray vaccination is obtained[22].

Figure 9:Components of laval nozzle spray device
NASAL VACCINES UNDER CLINICAL TRAILS:
INFLUENZA VACCINES:
The first nasal vaccine approved is under this category is Flumist by USFDA in 2003.This vaccine is commonly known as universal flu vaccine in US. But this vaccine is not effective in immunizing all age groups .Hence alternatives are being searched since 2014[23]. FluMos-v1 and FluMos-v2 are modified versions of Flumist which are currently under phase-1 clinical trials according to National Institute Of Health US. The approach of intra nasal vaccine facilitating mass vaccination for the most infectious disease in that country.
HIV VACCINES:
The vaccines which are intended to prevent HIV transmission must be capable to stimulate both systemic and mucosal immunity sine the virus can easily cross mucosal barriers. Viral vetor and liposomal based formulations are succeeding in many trails. One of such is Intranasal Vaccination against HIV-1 with Adenoviral Vector-Based Nanocomplex Using Synthetic TLR-4 Agonist Peptide as Adjuvant performed by Man Li, Yuhong Jiang, Tao Gong , Zhirong Zhang, and Xun Sun[24]. Intranasal vaccination not only stimulates systemic immunity but also elicits mucosal immunity that provides first defense for mucosally transmitted diseases like HIV-1. Adjuvants such as TLR agonists are usually codelivered with antigens to enhance the immunogenicity of vaccines. They prepared nano complex of adeno vector with novel TLR agonist to improve the efficacy of viral vector.TLR agonists have been attributed with the ability to suppress the invasive behaviour of tumour cells as well as being able to suppress angiogenesis. Therefore they may be projected as ideal candidates to target the process of metastatic spread.
SARSCoV-19 VACCINES[25,26]:
Various countries are trying to develop potent formulations which can preventSARSCoV-19 viral shredding in respiratory tract. But very few of them managed to go through phase-2&phase-3 human trails. One of them is iNCOVACC Adeno vector based vaccine developed by Bharat Biotech. This multi unit based liquid vaccine is the world first intra nasal vaccine which released into consumer market in India. Other nasal vaccines like nasal spray vaccine developed by Can Sino Biologics are claimed to be succeed phase-2 clinical trials in 2022. Detailed lists of vaccines are mentioned in the following figure.

Figure 10:Progress of intra nasal vaccines developed aganist SARSCoV-19 according to literature review study ppublished in name of Intranasal vaccines for SARS-CoV-2: From challenges to potential in COVID-19 management by Vivek P. Chavda et.al
FUTURE DEVELOPMENTS:
The research of intra nasal vaccine is in higher interest due to feasibility of self administration and fast immunization in mass populated paved a way of interest in many researchers. Although many research works are supporting are proving the efficacy of naso mucosal immunization still the under lying mechanisms are yet to be clarified only by in depth study. The complexity and lack of potent mucosal adjuvants are delaying newer nasal vaccines. Inhalation vaccines are extensively being researched because of their superiority in generating strong immune responses.
REFERENCES
- Hartwell BL, Melo MB, Xiao P, Lemnios AA, Li N, Chang JYH, Yu J, Gebre MS, Chang A, Maiorino L, Carter C, Moyer TJ, Dalvie NC, Rodriguez-Aponte SA, Rodrigues KA, Silva M, Suh H, Adams J, Fontenot J, Love JC, Barouch DH, Villinger F, Ruprecht RM, Irvine DJ. Intranasal vaccination with lipid-conjugated immunogens promotes antigen transmucosal uptake to drive mucosal and systemic immunity. Sci Transl Med. 2022 Jul 20;14(654):eabn1413. doi: 10.1126/scitranslmed.abn1413. Epub 2022 Jul 20. PMID: 35857825; PMCID: PMC9835395.
- M. Ramvikas, M. Arumugam, S.R. Chakrabarti, K.S. Jaganathan, Chapter Fifteen - Nasal Vaccine Delivery, Editor(s): Mariusz Skwarczynski, Istvan Toth, In Micro and Nano Technologies, Micro and Nanotechnology in Vaccine Development, William Andrew Publishing, 2017, Pages 279-301, ISBN 9780323399814,
- Boylston, Arthur (July 2012). "The origins of inoculation". Journal of the Royal Society of Medicine.
- Nian X, Zhang J, Huang S, Duan K, Li X, Yang X. Development of Nasal Vaccines and the Associated Challenges. Pharmaceutics. 2022 Sep 20;14(10):1983. doi: 10.3390/pharmaceutics14101983. PMID: 36297419; PMCID: PMC9609876.
- Tai, J.; Han, M.; Lee, D.; Park, I.-H.; Lee, S.H.; Kim, T.H. Different Methods and Formulations of Drugs and Vaccines for Nasal Administration. Pharmaceutics 2022, 14, 1073. https://doi.org/10.3390/ pharmaceutics14051073.
- Kehagia E, Papakyriakopoulou P, Valsami G. Advances in intranasal vaccine delivery: A promising non-invasive route of immunization. Vaccine. 2023 Jun 1;41(24):3589-3603. doi: 10.1016/j.vaccine.2023.05.011. Epub 2023 May 11. PMID: 37179163; PMCID: PMC10173027.
- Su F, Patel GB, Hu S, Chen W. Induction of mucosal immunity through systemic immunization: Phantom or reality? Hum Vaccin Immunother. 2016 Apr 2;12(4):1070-9. doi: 10.1080/21645515.2015.1114195. Epub 2016 Jan 11. PMID: 26752023; PMCID: PMC4962944.
- Kehagia E, Papakyriakopoulou P, Valsami G. Advances in intranasal vaccine delivery: A promising non-invasive route of immunization. Vaccine. 2023 Jun 1;41(24):3589-3603. doi: 10.1016/j.vaccine.2023.05.011. Epub 2023 May 11. PMID: 37179163; PMCID: PMC10173027.
- Chavda VP, Vora LK, Apostolopoulos V. Inhalable Vaccines: Can They Help Control Pandemics Vaccines (Basel). 2022 Aug 13;10(8):1309. doi: 10.3390/vaccines10081309. PMID: 36016197; PMCID: PMC9413847.
- Meenakshi S, Kumar VU, Dhingra S, Murti K. Nasal vaccine as a booster shot: a viable solution to restrict pandemic? Clin Exp Vaccine Res. 2022 May;11(2):184-192. doi: 10.7774/cevr.2022.11.2.184. Epub 2022 May 31. PMID: 35799869; PMCID: PMC9200647.
- Yusuf, H., & Kett, V. (2017). Current prospects and future challenges for nasal vaccine delivery. Human Vaccines & Immunotherapeutics, 13(1), 34–45. https://doi.org/10.1080/21645515.2016.1239668
- Hu X, Yue X, Wu C, Zhang X. Factors affecting nasal drug delivery and design strategies for intranasal drug delivery. Zhejiang Da Xue Xue Bao Yi Xue Ban. 2023 Jun 25;52(3):328-337. English, Chinese. doi: 10.3724/zdxbyxb-2023-0069. PMID: 37476944; PMCID: PMC10412955.
- 13, Tai J, Han M, Lee D, Park IH, Lee SH, Kim TH. Different Methods and Formulations of Drugs and Vaccines for Nasal Administration. Pharmaceutics. 2022 May 17;14(5):1073. doi: 10.3390/pharmaceutics14051073. PMID: 35631663; PMCID: PMC9144811.
- Duong, V.-A.; Nguyen, T.-T.-L.; Maeng, H.-J. Recent Advances in Intranasal Liposomes for Drug, Gene, and Vaccine Delivery. Pharmaceutics 2023, 15, 207. https://doi.org/10.3390/ pharmaceutics15010207
- Lê MQ, Carpentier R, Lantier I, Ducournau C, Fasquelle F, Dimier-Poisson I, Betbeder D. Protein delivery by porous cationic maltodextrin-based nanoparticles into nasal mucosal cells: Comparison with cationic or anionic nanoparticles. Int J Pharm X. 2018 Dec 10;1:100001. doi: 10.1016/j.ijpx.2018.100001. PMID: 31545856; PMCID: PMC6733295.
- Ke-Fei Hu, Karin Lövgren-Bengtsson, Bror Morein, Immunostimulating complexes (ISCOMs) for nasal vaccination, Advanced Drug Delivery Reviews, Volume 51, Issues 1–3, 2001, Pages 149-159, ISSN 0169-409X, https://doi.org/10.1016/S0169-409X(01)00165-X.
- Nooraei, S., Bahrulolum, H., Hoseini, Z.S. et al. Virus-like particles: preparation, immunogenicity and their roles as nanovaccines and drug nanocarriers. J Nanobiotechnol 19, 59 (2021). https://doi.org/10.1186/s12951-021-00806-7.
- Yang, Y., Chen, L., Sun, Hw. et al. Epitope-loaded nanoemulsion delivery system with ability of extending antigen release elicits potent Th1 response for intranasal vaccine against Helicobacter pylori. J Nanobiotechnol 17, 6 (2019). https://doi.org/10.1186/s12951-019-0441-y.
- Popescu R, Ghica MV, Dinu-Pîrvu CE, Anu?a V, Lupuliasa D, Popa L. New Opportunity to Formulate Intranasal Vaccines and Drug Delivery Systems Based on Chitosan. Int J Mol Sci. 2020 Jul 16;21(14):5016. doi: 10.3390/ijms21145016. PMID: 32708704; PMCID: PMC7404068.
- 20.Cahn, D., Amosu, M., Maisel, K. et al. Biomaterials for intranasal and inhaled vaccine delivery. Nat Rev Bioeng 1, 83–84 (2023). https://doi.org/10.1038/s44222-022-00012-6
- https://aptar.com/products/pharmaceutical/intranasal-vaccines/
- Wang, Z., Zhang, Z., Wang, Q. et al. A nasal spray vaccination device based on Laval nozzle and its experimental test. Sci Rep 13, 6267 (2023). https://doi.org/10.1038/s41598-023-33452-0.
- https://www.astrazeneca-us.com/media/press-releases/2023/us-food-and-drug-administration-accepts-for-review-astrazenecas-supplemental-biologics-license-application-for-self-administration-of-flumist-quadrivalent-influenza-vaccine-live-intranasal.html
- Li M, Jiang Y, Gong T, Zhang Z, Sun X. Intranasal Vaccination against HIV-1 with Adenoviral Vector-Based Nanocomplex Using Synthetic TLR-4 Agonist Peptide as Adjuvant. Mol Pharm. 2016 Mar 7;13(3):885-94. doi: 10.1021/acs.molpharmaceut.5b00802. Epub 2016 Feb 15. PMID: 26824411.
- Chavda VP, Vora LK, Pandya AK, Patravale VB. Intranasal vaccines for SARS-CoV-2: From challenges to potential in COVID-19 management. Drug Discov Today. 2021 Nov;26(11):2619-2636. doi: 10.1016/j.drudis.2021.07.021. Epub 2021 Jul 29. PMID: 34332100; PMCID: PMC8319039.
- https://healthlibrary.askapollo.com/incovacc-worlds-new-nasal-covid-19-vaccine-from-india/


 Rama Vaishnavi Vedantam*
Rama Vaishnavi Vedantam*
 M.Tulasi Maheswari
M.Tulasi Maheswari













 10.5281/zenodo.13356950
10.5281/zenodo.13356950