Background:
It would appear that India makes substantial use of herbs, particularly in the form of medicinal plants, as a source of medical treatment. According to medicinal systems, plants are capable of accumulating more than 80 percent of the world's medications. Tulsi, also known as Holy Basil has been utilized in Ayurveda ever since its inception due to the curative properties it possesses.
Material/method:
Ocimum species are critically significant to the process of standardizing crude extracts due to the physical traits and medicinal potential of these species. Plants have a long history of use in medicine due to the fact that they are an excellent source of pharmaceuticals. Plants have been utilized in a broad number of sectors as a result of this feature. These fields include modern medicine, dietary supplements, folk medicines, health supplements, biopharmaceuticals, and biochemical moieties for the creation of synthetic medications.
Results:
The Ocimum plant, out of all the plants that were considered, was chosen to serve as a reference for the treatment of a wide variety of ailments, including cardiovascular impairment. The current study's findings established the quality profile requirements for the medicinal plant Ocimum sanctum Linn., Ocimum tenuiflorum, Ocimum gratissum and Ocimum basilicum. Finally, the plant has been detailed systematically to investigate and will be accepted throughout the world for use shortly.
Conclusion:
In both traditional and modern forms of herbal medicine, components of the Ocimum plant, including the leaves, stems, flowers, roots, and pollen are frequently employed. By means of a series of well-planned tests, the authors of this study intended to evaluate the antioxidant and antibacterial effects of Ocimum. In light of their potential therapeutic value, Ocimum species were selected for standardization in the present investigation
Tulsi, Morphological, Standardization, Pharmacognostical, TLC, Ocimum sanctum
Plants have a long history of being used extensively in medicine due to the high therapeutic source that they provide. Plants are utilized in a variety of fields, including modern medicines, dietary supplements, traditional medicines, health supplements, pharmaceuticals, and biochemical mobility for the production of pharmaceutical drugs. Physicians utilized plant species for the regular treatment of several illnesses in their ancient medical practice. Ocimum, which belongs to the Lamiaceae family, is an herbaceous perennial as well as a shrub of the genus Ocimum that has historically been used for medicinal purposes, including its leaves, stems, flowers, roots, seeds, and the entire plant. Plants from six inhabited continents were found at the site where tropical and warm temperatures were observed, with Africa having the highest number of species. The word okimon, which means basil in Greek, is the source of its name. Cooking basil (O. Basilicum) and tulsi (holy basil), O. Tenuiflorum, are the two species being used in cooking. In Ayurveda, tulsi is considered as ‘Mother of medicine in nature’, and ‘Queen of herbs’ (Singh et al, 2010).
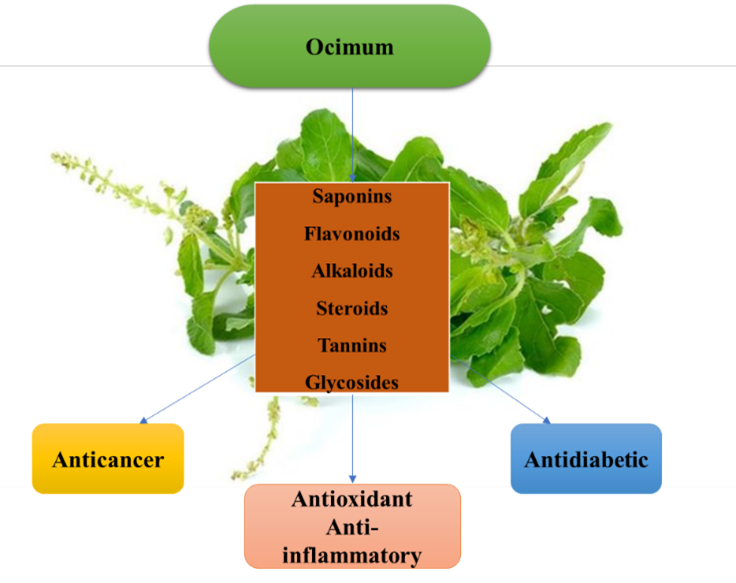
Fig no 1 Graphical abstract
With reference to the holistic lifestyle approach to health, tulsi is recognized as one of the best herbs in Ayurveda. It is recommended that tulsi consumption on a daily basis will promote health, well-being, and longevity of life by protecting against diseases. It is also beneficial for enhancing the complexion luster, the sweetness of voice, beauty, intelligence, stamina, and calmness of mind (Singh et al, 2010; Mahajan et al, 2013; Mohan et al, 2011; Pattanayak et al, 2010). In addition to this, tulsi has shown tremendous effect against several disorders like anxiety, cough, asthma, diarrhea, fever, dysentery, arthritis, eye diseases, skin diseases, etc. (Mondal et al, 2009). Cancer treatment has become a herculean task since it was discovered mostly in living beings. The major emphasis of all medicinal chemists in response to that challenge would be to stop the disease from progressing and the effects of the condition. (Bos, 1989) As of now, numerous medicines for cancer therapy and progression have been licensed by a variety of regulatory bodies. However, side effects and resistance are significant obstacles(Valeriote and van Putten,1975). Modification of the medication is done in a variety of ways to improve its safety profile and activity, and its compounds also receive clearance from several authorities to identify the ideal drug for treatment. The effort was excessive in comparison to the success, leading one to consider the finding (Qian et al., 2012 and Manir et.al 2012) Presently, modifications to the current medication have been made to improve pharmacokinetic characteristics. (Stathopoulos et al., 2005) Drugs with superior pharmacokinetic parameters are less toxic than parent drugs, and some of them are effective in clinical study investigations (NIH, 2019), as well as combination or single drugs(Koukourakiset al., 2010).It has been estimated that tulsi is also beneficial for cancer prevention caused due to toxicity and helps to reduce DNA damage (Siddiqueet al, 2007). In precancerous and cancerous cells, tulsi will induce apoptosis thereby inhibiting the growth of tumors and increasing the survival rate. (Jhaet al, 2012; Manikandanet al, 2008) The journey of plants with their by-products over the last few decades has proven that they are essential and necessities from which pharmaceuticals and food may be derived for humans. It is constantly assisting humanity by delivering new and unique treatments. (Kamboj et al., 2000; Ojewole et al., 2007; WHO et al., 1993; Sharma et al., 2012) Herbs are indeed a rich source of drugs that exist naturally in the atmosphere, making our lives healthy and disease-free, as well as helping to treat to save us against a range of illnesses. World Health Organization reports state that around 80% of people of nations throughout utilize traditional methodologies such as plants with medicinal properties regularly for treating disease. (Porter, 1998) Owing to its excellent cultural acceptance in developed countries, the rising compatibility also with human use of natural medicine has shown a significantly lower number of adverse effects since last year. (Prakash Kala and colleagues, 2006) About 17,000 plant species are present across India, with a large number of them, i.e.,7500, being employed for their excellent medicinal properties. Thus, when compared to any other nation on the globe, India has the largest ratio of traditional medicines with therapeutic effectiveness, making our country a trademark in existing flora. (Agrawal and Paridhavi, 2012; Grover et al., 2002; Kirtikar and Basu, 1935; Nayar S.L. Chopra R.N. Chopra I.C., 1956).
Plant: A rich medicinal source:
The plants but also their semi-synthetic derivatives are known for being a rich source of crude medicines in earlier medicinal systems, making them an important contributor to the healthcare industry. (Grynkiewicz and Gadzikowska, 2008) For example, “Ayurveda” is an ancient Indian medical system that comprises about 2000 traditional therapeutic plants utilized according to the Unani and Siddha systems. The Charak Samhita (Srivastava et al., 1996; Ahmed et al., 2023) is just an ancient herbal therapy that documented the development of 340 folk medicine derived from plants and their use. Currently, around 25 percent of the total medicines are plant derivatives acquired directly, and a significant number of pharmaceuticals are created by modifying, i.e., synthetic equivalents or models found in plant species listed in the contemporary pharmacopeia. (Gupta et al., 2015; Mishra et al., 2001; Mukherjee, 2003; Karki H., 2014; Vidya et al., 2017) The environmental source of active components is indeed considered an effective source of contemporary medicines, nutraceuticals, foods, folk remedies, medicinal products, and chemical motives for the creation of synthetic medications. (Veeresham, 2012, Chaudhary, 2012,Lahlou, 2013) Plants are photochemically used as a result of the human being's productive physiologic activity. Photochemical plant products produced from metabolic reactions are physiologically active plant products. These chemical compounds are considered nutrition and medication to offer biological advantages contrary to different situations of sickness and therefore protect human health. (Bayliss, 1920; Hackney et al. 1983; Kriplani P et al 2019; Mulabagal and Tsay 2004; Stoskopf 1983; Chung IM et al 2005); Some possible herbal medicines are frequently utilized as a therapeutic option in the method of treatment. These medicines, for instance, include morphine, quinine, atropine, digitoxin, reserpine, vincristine, pilocarpine, vinblastine, etc. (Sampedro and Valdivia, 2014, Farnsworth and Soejarto, 1991; Khaled et al., 2013) Traditional remedies produced from plants normally cost US$14 billion a year for commercial reasons, in India around US$1 billion a year and in the rest of the globe about US$40 billion. Okigbo and Ogbonnaya, 2002. Orafidiya et al., 2006, WHO et al., 1993, 1995. (Nakamura et al. 1999. Ojewole, 2007. Kamboj, 2000. In the current situation, WHO estimates that demand would rise steadily to 5 trillion USD in 2050 after medical herbs and raw materials have been used by around 15-25%. (Porter, 1998). The many functions of tulsi are frequently attributed to their high phenolic content and anti-oxidant characteristics; however, the Krishna tulsi (black/purple type) demonstrates the same in larger proportion as opposed to the white Vana (wild) tulsi. (Wangcharoen et al, 2007). According to research conducted in laboratories, tulsi increases the antioxidant capacity of the body, such as glutathione, and also boosts the activity of antioxidant enzymes, such as superoxide dismutase and catalase, which helps to manage the damage caused by free radicals when oxygen is in short supply (Panda et al, 2009; Dhankhar et al, 2023; Mittal et al, 2023) in addition to other harmful compounds (Shivananjappa et al, 2012;Manikandan et al, 2007).
Table no 1 Plant classification.
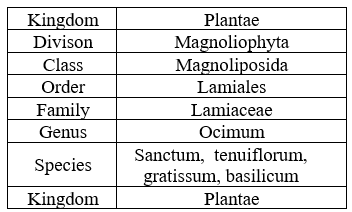
Tulsi plants are a very important source of medicines and drugs. It belongs to the sanctum species with kingdom Plantae, genus Ocimum, and Lamiaceae family, which are known for their several therapeutic actions and medicinal properties [Table 1.1.]. Tulsi is a Sanskrit word that means ‘matchless one’ or ‘no herb equivalent to this’. It has many Sanskrit synonyms [Table 1.2.] like:
Surasa – Having Good Taste
Gramya – Found Commonly In Every Household
Bahumanjari- Has A Beautiful Inflorescence
Sulabha - Auspicious
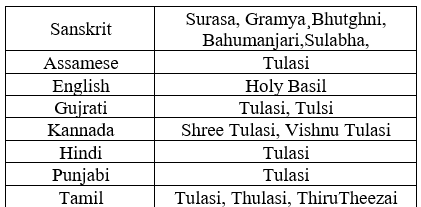
Tulsi is also known to have several vernacular names according to different regions of India. [Table 1.2.]. Ocimum sanctum is classified as two varieties namely: Krishna tulsi (black one) and Rama tulsi (green one).
Table 2. Tulsi synonyms:
PHYTOCONSTITUENTS:
Tulsi contains essential oils that are good to one's health and are known to contain, among other things, eugenol, apigenin, apigenin-7-O-glucuronide, vicenin, gallic acid, and ursolic acid. The makeup of OS is as follows: it accounts for 0.7 percent of total volatile oils, contains approximately 71% eugenol, and contains approximately 20% methyl eugenol. Carvacrol and the sesquiterpene hydrocarbon caryophyllene are other components. Isothymusin, rotameric acid circimaritin, cirsilineol, apigenin, rotameric acid, and a considerable quantity of eugenol are phenolic components recovered from stems and leaves (fresh) in OS extract. The product is obtained by isolating components such as ursolic acid, apigenin, luteolin, apigenin-7-O-glucuronide, luteolin, and molludistine, which are produced from either aqueous OS extracts or leaf extracts. Ursolic acid is one of the components that is used in the production of the product. Orientine and Vicenin are two examples of flavonoids. Bornyl acetate, ?-element, neral, ?- and ?-pinenes, camphene, pastoral, cholesterol, stigmasterol, and ?-sitosterol are all loaded with ODs of sesquiterpenes and monoterpenes, respectively.
TRADITIONAL USES:
Natural products are extensively utilized and are a significant source of physiologically active chemicals from human civilization. OS is regarded as the elixir of life because of its longevity enhancement. Ever since, it has been widely employed in the treatment of healthcare and illnesses including cold, flu, earaches, fever, colic, asthma, malaria, and hepatic disorders, in systems like Ayurveda and Siddha. Tulsi has beneficial oils that comprise a variety of active ingredients, some of which include eugenol, apigenin, apigenin-7-O-glucuronide, vicenin, gallic acid, and ursolic acid, amongst others.
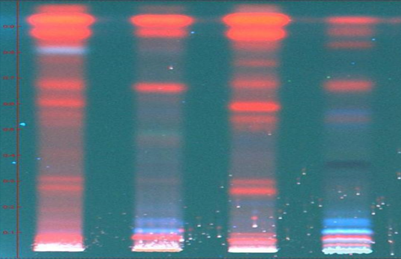
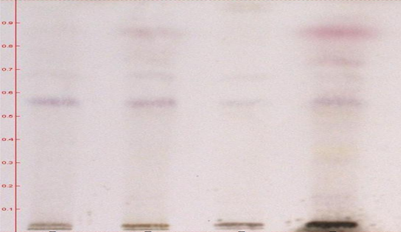
T.L.C. Methodology:
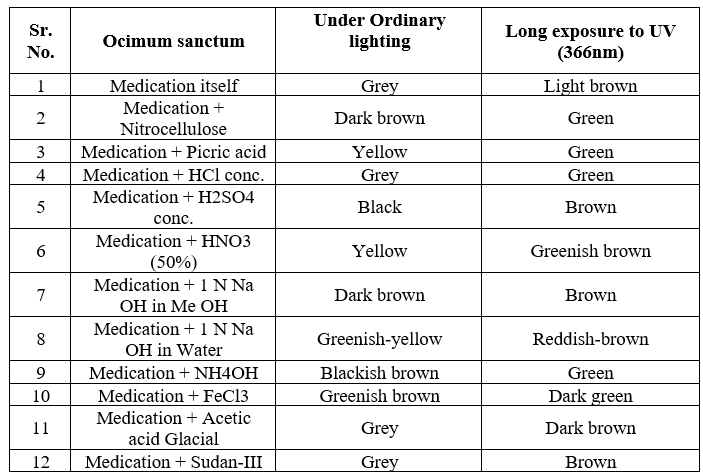
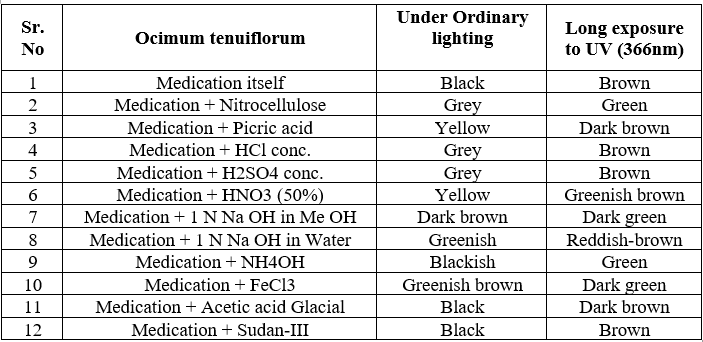
For HPTLC, 2 gm of each sample were extracted with 25 ml of methanol on boiling water bath for 20 min. Filtered through Whatman filter paper no. 1 and concentrated. TLC of methanolic extracts of all the samples were carried out on silica gel 60 F254 percolated plates (0.2 mm thickness; from Merck India Limited Mumbai). An applicator from Camag Linomat-5 Camag Switzerland: 140443) was used for band application and photo documentation unit (Camag Reprostar-3: 140604) was used for documentation of chromatographic fingerprints. The mobile phase used Toluene: Ethyl acetate (7:3). All the plates were developed over a distance of 8 cm in a saturated development chamber (Twin through chamber 20X10 cm with SS lid and visualized under 254nm, 366nm and visible light. After spraying with 5% methanolic sulphuric acid followed by heating at 1100C for 10 min
RESULTS AND DISCUSSION:
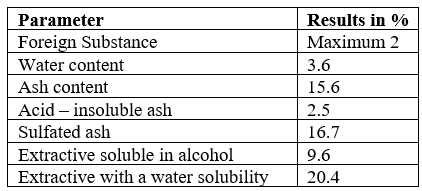
The O. sanctum leaf extracts were analysed for their strength and purity on various parameters like foreign substance, water content, ash content and its solubility in acid, solubility of extract in water and alcohol (Table 2.1). Majority of the extract solubility found in water (20.4%), followed by alcohol (9.6%). The estimation on foreign substance (maximum 2), water content (3.6%), and ash content (15.6%) reveals the purity and strength of the sample. Further, sulphated ash and acid–insoluble ash were 16.7% and 2.5% respectively.
Table no 3 Strength, identity & Purity
The O. gratissum leaf extracts were analysed for their strength and purity on various parameters like foreign substance, water content, ash content and its solubility in acid, solubility of extract in water and alcohol (Table 2.3). Majority of the extract solubility found in water (22.10%), followed by alcohol (10.6%). The estimation on foreign substance (maximum 1.5), water content (7.8%), and ash content (14.01%) reveals the purity and strength of the sample. Further, sulphated ash and acid–insoluble ash were 11.2% and 3.01% respectively.
Table no 4 Strength, identity & Purity
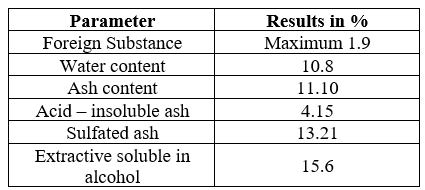
The O. Basilicum leaf extracts were analysed for their strength and purity on various parameters like foreign substance, water content, ash content and its solubility in acid, solubility of extract in water and alcohol (Table 2.4). Majority of the extract solubility found in water (17.07%), followed by alcohol (15.06%). The estimation on foreign substance (maximum 1.9), water content (10.8%), and ash content (11.10%) reveals the purity and strength of the sample. Further, sulphated ash and acid–insoluble ash were 13.21% and 4.15% respectively.
Table no 5 Strength, identity & Purity

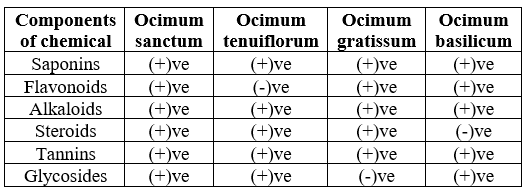
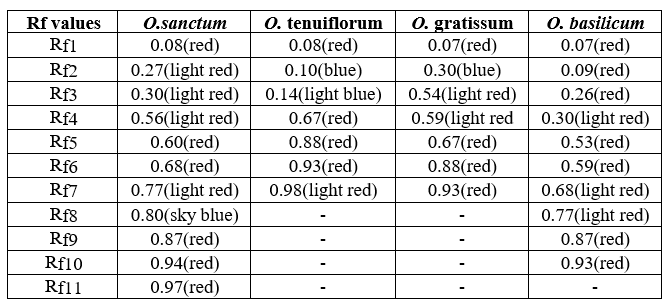
To evaluate the presence of specific bioactive compounds in O. sanctum, Ocimum tenuiflorum, Ocimum gratissum, Ocimum basilicum leaf extracts, phytochemical screening was done for saponins, flavonoids, alkaloids, steroids, tannins, and glycosides by using different methods(Ayoola et al. 2008;Joshi et al2013)(Table 2.2).


 Prerna Sharma*
Prerna Sharma*
 Satish Kumar Sharma
Satish Kumar Sharma







 10.5281/zenodo.10927242
10.5281/zenodo.10927242