The orthopoxvirus, which causes monkeypox, is a pathogenic viral infection that has become a major global health concern. Monkeypox has lower death and transmission rates than smallpox, despite similarities between them. The 2022 global outbreak highlighted the virus's ability to impact an extensive spectrum of populations, owing to its new strain and its spreading. The epidemiological dynamics, clinical symptoms, diagnostic methods, and preventative measures for monkeypox are examined in this article. Significant results include the introduction of a novel strain of monkeypox, which mostly spreads from male to male, and the effectiveness of smallpox vaccinations in averting illness. The study further emphasizes the value of antiviral medications such as bicidofovir and tecovirimat in the treatment of monkeypox infections. A comprehensive approach including timely vaccination campaigns, better diagnostic methods, more surveillance, and the creation of revolutionary antiviral medications is required to effectively combat monkeypox. In order to contain the spread of this newly discovered infectious disease and avoid further outbreaks, coordination and awareness on a global scale are essential. Double-check Gemini's responses as it may disclose false information, including personal information. Gemini Apps and privacy functions as a new window.
Monkeypox, Orthopoxvirus, Epidemiology, Epidemiology, Vaccination, Antiviral medications, Global health.
The virus linked to monkeypox, which causes the zoonotic viral infection known as monkeypox, has become a major threat to global health. It is comparable to smallpox in that it causes a similar rash, but it is less successful in spreading from person to person and has lower case fatality rates. In the past, isolated epidemics mostly affected rural communities in West and Central Africa. However, a rebound of smallpox instances resulted from the vaccination's termination in the middle of the twentieth century [1, 2]. An important turning point came in 2022 when a global monkeypox outbreak surfaced, marked by population growth in nations outside of an endemic region. This unusual outbreak, which involved a new strain of the monkeypox virus, spread quickly, with over 78,000 cases documented globally by November of that year. The outbreak highlighted the virus's ability to spread quickly and its potential to affect a broad spectrum of populations. A rash that sometimes resembles pox is one of the most common clinical signs of monkeypox [3, 4]. Along with flu-like symptoms like a high body temperature, headache, muscle pain, and weariness. Although vaccinations have not been widely available, they can offer protection even though help is the mainstay of treatment. Containment tactics that work depend on public health interventions such as education, isolation, and contact tracing [5,6].
The global outbreak of monkeypox in 2022 highlighted the need for improved diagnostic tools, tracking systems, and treatment options to combat this newly emergent infectious disease. This global epidemic also made clear how crucial international cooperation and readiness are to halting the spread of viruses and bacteria over the world [7, 8].
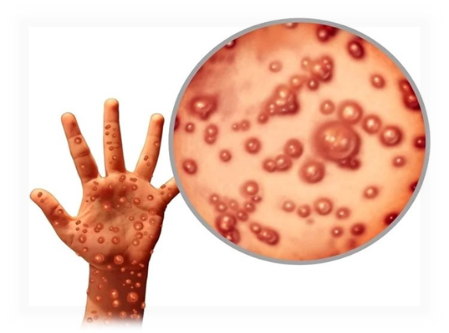
Fig. 1. Monkey-pox virus
Evolutionary Dynamics of Monkeypox
The orthopoxvirus that causes monkeypox is identified by its large genome (~200 kilobase pairs), double-stranded DNA, and synthesis of over 190 proteins. Its 200–250 nm brick-like virion structure is similar to that of the vaccine and smallpox viruses. In the past, two separate clades with differing toxicity profiles and geography distributions have been found in Africa [9, 10]. Clade 1, which is found in the Congo Basin and central Africa, has a higher case-fatality rate of 1–12%. Clade 2, on the other hand, is less virulent and is limited to West Africa. Its case-fatality rate is less than 0.1%. Within haplogroup 2b, a novel branch, B.1, has been found through recent research and linked to the global epidemic of 2022 [11, 12]. However, this lineage is showing a mutation rate that is far greater than earlier estimates. Most likely as a result of more human-to-human transfer. Research on how these variations in DNA effect the virus's virulence, tolerance to human hosts, and transmissibility remains essential [13, 14].
Epidemiological Characteristics and Public Health Implications"
A significant global comeback of monkeypox, an infectious viral disease native to Central and West Africa, was observed in 2022. The current situation was different from earlier periods in that it had been defined by a sharp rise in cases globally. In 2003, the first events of monkeypox outside of Africa were related to contact with tired prairie dogs kept as animals in the United States. Later, isolated cases surfaced in a number of nations, primarily among Nigerian travelers returning home [15, 16]. But the 2022
growing saw a significant rise in cases, with over 16,000 cases reported in 75 countries in a short amount of time. The greatest number of deaths happened on Africa, despite the region reporting fewer cases [17]. A multicountry study that studied 528 cases of monkeypox infections found that a majority of those impacted were men, particularly gay or bisexual men who were HIV positive. With the virus DNA found in the seminal fluid from multiple individuals, sexual transmission proved to be the main mechanism of transmission. Even though HIV is highly prevalent among those who are afflicted, this particular study failed to report any deaths [18,19]. In 2022, there were also a few occasions of monkeypox in India, mainly affecting men who had previously traveled abroad. Concerns about domestic infection were brought up, meanwhile, by the appearance of a case without a travel history. Many factors played a role in the worldwide spread of monkeypox. A favorable environment for the virus to spread was produced by the virus's changing biological makeup, climate change, and decreased immunity as a result of smallpox vaccine elimination. High-risk sexual activities and a surge in foreign travel also contributed to the virus's spread. [20] The global importance of monkeypox and the urgent need for coordinated action led to the World Health Organization (WHO) labeling it as a public health emergency of international significance. Ongoing tracking, inquiry, and public health activities are required to lessen the outbreak's effects and stop it from growing.[21]
Mechanism of transmission and pathogenesis
The virus develops in a number of cell types after infection, including skin cells, fibroblasts The viral orthopoxvirus that causes monkeypox may circulate via the skin and the respiratory among and cells called endothelial cells (the skin route) and respiratory route epithelial cells in the airways [22]. The virus is further delivered to the lymph system by cells that present antigen, where it grows even more and causes a rise in The virus then spreads to the skin, liver, and spleen, among other organs, giving rise to the typical clinical physical appearance [23].
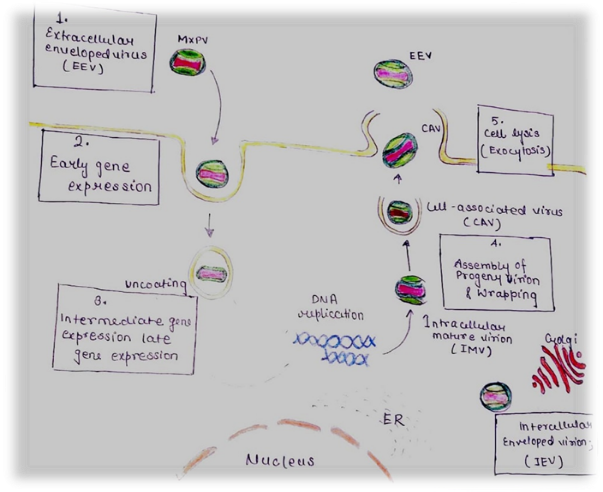
Fig. 2. Mechanism of transmission of Monkey-pox virus
Monkeypox is transmitted from animal to human via the skin or the respiratory system. Given the paucity of data, skin inoculation is typically linked to regional illness, as the vaccinia virus vaccine demonstrates [24]. While the monkeypox virus primarily affects the skin, it can also involve other systems, including the digestive, genitourinary, and respiratory tracts. However, historical outbreaks have indicated that respiratory transmission often leads to more widespread symptoms. The skin lesions associated with monkeypox exhibit specific clinical characteristics [25]. In Both humoral in nature and cellular factors are involved in the immune response to monkeypox. IgG and IgM antibodies are produced by the humoral response in response to various viral objectives resulting in the development of long-term memory B cells that offer defense against additional exposure [26]. The growth of activated CD4+ and CD8+ lymphocytes called T lymphocytes, which are necessary for destroying infected cells, is a component of the cellular response. As demonstrated by the vaccinia virus, memory T cells can survive for decades [28]. While CD8+ T cells eliminate infected phages, CD4+ T cells help B cells produce antibodies. If their CD4+ count is higher than 350, people with HIV can develop a T-cell response related to the viral infection [29]. It's yet unknown how those with low CD4+ levels will react, though. Studies on primates other than humans have shown that B-cell responses are essential for protecting against monkeypox, whereas CD4+ or CD8+ T cell inhibition has just a small impact [30].
Clinical Manifestations, Risk Factors, and Complications
A pathogenic orthopoxvirus infection known as monkeypox is currently an international health problem. Though usually self-limiting and in some people it might cause serious problems. The main clinical features, risk factors, and possible effects of a monkeypox infection are provided in this article [31]. Monkeypox takes five to twenty-one days to develop, with a median of six to thirteen days. All age groups are dependent, however with time, the median age of those impacted has increased. In comparison with females, males are more frequently impacted. Similar to smallpox, monkeypox is characterized by a precursor phase followed by a rash in its clinical physical appearance [32, 33]. Patients may have fever, headache, weakness, discomfort, and lymphadenopathy as during the phase known as prodromal. The rash is frequently variegated and severe, and it usually emerges a few days after the first onset of symptoms [34]. It develops in multiple stages, including pustules as vesicles, papules, and macules. The cheeks, palms, soles, oral mucous membranes, genitalia, and ocular are among the common sites of exposure. A person may be more susceptible to a severe case of monkeypox if they are younger, have underlying immune system problems, or have never received a smallpox vaccination [35, 36]. Significant complications include corneal involvement, sepsis, dementia, pneumonia and resulting infections. Several factors, including the monkeypox clade, host characteristics, vaccination status, and availability to care, increase the case fatality rate [37]. Embryonic infection brought on by vertical transmission of monkeypox can result in malformations and even fetal death. Compared to clades 2 and 3, clade 1 of the virus is linked to a higher risk of severe consequences. In summary, monkeypox is a potentially harmful disease that needs to be identified and treated quickly. Effective prevention and control addresses require an understanding of the clinical features, risk factors, and possible implications [38, 39].
Diagnostic Challenges and Techniques
Because monkeypox shares clinical features with other viruses such as pox including chickenpox and smallpox, diagnosing it offers special difficulties. Though the clinical picture may offer first signals, test confirmation is necessary for a definitive diagnosis and successful public health program [40, 41]. Electron microscopy is able to distinguish between ortho pox viruses and monkey pox viruses, but not between orf and bovine stomatitis. PCR, ELISA, and viral isolation are more accurate techniques. Although histopathology cannot identify a virus by species, it can aid in separating between herpes and poxviruses [42, 43]. For the diagnosis of monkeypox, PCR methods—especially real-time PCR assays that target panortho pox virus or MPXV-specific sequences—are regarded as the gold standard. Recent years have seen a development in the availability of quick diagnostic techniques, which helps with early disease detection and management [44, 45].
Efficacy and Safety Profiles of Smallpox Vaccines for Monkeypox Prevention
Vaccines versus smallpox have shown promise in providing immunity to infections from monkeypox. According to previous research, receiving smallpox vaccinations prior to getting monkeypox can either significantly decrease the chance of developing the disease or lessen the severity of its clinical symptoms [46, 47]. There are now three smallpox vaccines available in the US: APSV, ACAM2000®, and JYNNEOSTM (MVA-BN). The FDA has approved JYNNEOSTM and ACAM2000® for the prevention of smallpox and monkeypox [48, 49].
Although both vaccines promote protection, their qualities are different. ACAM2000® is a replication-competent vaccinia virus, whereas JYNNEOSTM is a modified, non-replicating Ankara virus. Various safety profiles are the result of this difference [50, 51]. An increased chance of accidental vaccination, erythema vaccinatum, progressive vaccinia, myopericarditis, and post-vaccine encephalitis is linked to ACAM2000®. On the other hand, because JYNNEOSTM is non-replicating, it does not present these hazards [52, 53]. Another smallpox vaccine, APSV, may be administered with an Emergency Use Authorization, or EUA, or an investigational new drug (IND). Its usefulness against monkeypox, however, is still unknown [54, 55]. Pre-exposure prophylactics is suggested for people who may be infected with orthopoxviruses at work. In order to offer protection prior to potential exposure, vaccination is required. Post-exposure prophylaxis (PEP) is advised for those who have been exposed to monkeypox, especially for high-degree exposure [56, 57]. Still, the choice to provide PEP for exposures of a middle level should be taken separately, taking into account the unique set of circumstances. It important to remember that proactive measures for smallpox and monkeypox may change in the future. Getting the most recent advice on these issues requires speaking with public health officials or medical experts.
Smallpox Vaccines as a Defence against Monkeypox
Vaccines have been used for a long time to fight off infectious diseases, as proven by their effectiveness in eradicating smallpox. Today, they continue to be an essential instrument in combating new viral dangers, like the recent monkeypox surge. A number of vaccines, such as ACAM2000, JYNNEOSTM, and one created by Aventis, have demonstrated successful defense against monkeypox. These vaccines provide an important defense against the transmission of monkeypox and reducing its effects on public health [59, 60]. The non-replicating vaccine JYNNEOSTM is especially notable because it has been approved for both smallpox and monkeypox. Research has indicated that providing smallpox vaccines containing the vaccinia virus may successfully avoid monkeypox by 85%. Although IMVANEX has been approved in Europe for smallpox, the UK has used it safely off-label to treat cases of monkeypox [61, 62]. During the current global epidemic, ACAM2000, a well-known smallpox vaccine, has been frequently used as a vaccination against smallpox and, especially, has been advised for non-variola orthopoxviral diseases, such as monkeypox [63, 64]. The neutralizing antibodies are the basic part of cross-protection against monkeypox and are essential in preventing the virus from replicating. A decrease in population immunity to orthopoxviruses as a result of the regular smallpox vaccination program stopping has made conditions conducive for the emergence of monkeypox [65, 66]. In addition to vaccine-based methods, antiviral drugs have been created to address monkeypox. a virus called has a broad antiviral spectrum that includes infections caused by herpes, the virus known as and orthopoxvirus. It was first regulated for treating chlamydia eye disorders [67,68]. Since it was first licensed to treat smallpox, bicidofovir has also been used to treat adenovirus, orthopoxvirus, and viral. Tecovirimat is an additional antiviral medication that has been used to treat cowpox and monkeypox infections. It has demonstrated effectiveness against smallpox. Furthermore, problems related to vaccinia infection have been treated with vaccinia-vaccinations and antiviral therapies [69, 70].
Future perspective
Our approach to preparation for future viral outbreaks has been strongly influenced by the lessons learned from prior outbreaks, specifically the COVID-19 pandemic. Three key elements of these efforts have emerged: greater financial allocations, faster biologics development, and improved surveillance networks [71]. To improve preparedness as well as reaction roles, new agencies have been formed, like the US Department of Health and Human Services and the Department of Pandemic and Epidemic Diseases at the World Health Organization. The current MPX outbreak provides a focus on boosting control approaches, carrying out extensive research, and assessing available treatment possibilities [72, 73] A solid foundation in biosecurity, along with strict compliance to biosafety guidelines and a thorough understanding of pathogen-host interactions, is essential to effectively minimizing the effects of future viral outbreaks. When combined with concerted global efforts, technological developments could lead to the development of successful intervention measures that could lessen the catastrophic effects of future pandemic occurrences [74, 75].
CONCLUSION
The global monkeypox outbreak of 2022 highlighted the virus's alarming capacity for rapid transmission and its potential to cause widespread societal disruption. To effectively contain the outbreak and prevent future occurrences, a comprehensive and multifaceted approach is essential. This includes the swift implementation of vaccination campaigns to bolster immunity, the development of more accurate and efficient diagnostic methods, enhanced surveillance efforts to track the virus's spread, and the creation of novel antiviral drugs to combat the infection. Furthermore, international cooperation and awareness are paramount to halting outbreaks and mitigating the spread of this newly emerged infectious disease. By fostering collaboration among nations, sharing information and resources, and raising public awareness about the risks and prevention measures, we can strengthen our collective response to monkeypox. Additionally, it is crucial to address the underlying factors that contribute to the emergence and spread of infectious diseases, such as deforestation, wildlife trade, and climate change. By addressing these root causes, we can reduce the likelihood of future outbreaks and protect public health on a global scale. In conclusion, the eradication of monkeypox requires a coordinated effort that combines vaccination, diagnostics, surveillance, antiviral development, international cooperation, and addressing the underlying causes of infectious disease emergence. By implementing these strategies, we can successfully eliminate monkeypox and safeguard global health for generations to come.
REFERENCES
- Mitjà O, Ogoina D, Titanji BK, Galvan C, Muyembe JJ, Marks M, Orkin CM. Monkeypox. The Lancet 2023 Jan 7;401(10370):60-74.
- Parker S, Nuara A, Buller RM, Schultz DA. Human monkeypox: an emerging zoonotic disease.Future microbiology. 2007 Feb 1;2(1):17-34.
- Rosenberg R. Detecting the emergence of novel, zoonotic viruses pathogenic to human. Cellular and molecular life sciences. 2015 Mar; 72:1115-25.
- Mudhasani RR, Golden JW, Adam GC, Hartingh TJ, Kota KP, Ordonez D, Quackenbush CR, Tran JP, Cline C, Williams JA, Zeng X. Orally available nucleoside analog UMM-766 provides protection in a murine model of orthopox disease. Microbiology Spectrum. 2024 Apr 2;12(4):e03586-23.
- Roychoudhury S, Das A, Sengupta P, Dutta S, Roychoudhury S, Choudhury AP, Ahmed AF, Bhattacharjee S, Slama P. Viral pandemics of the last four decades: pathophysiology, health impacts and perspectives. International journal of environmental research and public health. 2020 Dec;17(24):9411.
- Davis M. The monster enters: COVID-19, avian flu, and the plagues of capitalism. Verso Books; 2022 Feb 1
- Tambo E, Al-Nazawi AM. Combating the global spread of poverty-related Monkeypox outbreaks and beyond. Infectious Diseases of Poverty. 2022 Jul 7;11(1):80.
- Mohapatra RK, Tuli HS, Sarangi AK, Chakraborty S, Chandran D, Chakraborty C, Dhama K. Unexpected sudden rise of human monkeypox cases in multiple non-endemic countries amid COVID-19 pandemic and salient counteracting strategies: Another potential global threat?. International journal of surgery. 2022 Jul 1;103:106705.
- Wittek R. Organization and expression of the poxvirus genome. Experientia. 1982Mar;38(3):285-97.
- Prichard MN, Kern ER. Orthopoxvirus targets for the development of new antiviral agents. Antiviral research. 2012 May 1;94(2):111-25.
- Rifkin RF, Vikram S, Ramond JB, Cowan DA, Jakobsson M, Schlebusch CM, Lombard M. Ancient DNA of Rickettsia felis and Toxoplasma gondii implicated in the death of a hunter-gatherer boy from South Africa, 2,000 years ago. BioRxiv. 2020 Jul 23:2020-07
- de Filippo C, Barbieri C, Whitten M, Mpoloka SW, Gunnarsdóttir ED, Bostoen K, Nyambe T, Beyer K, Schreiber H, de Knijff P, Luiselli D. Y-chromosomal variation in sub-Saharan Africa: insights into the history of Niger-Congo groups. Molecular biology and evolution. 2011 Mar 1;28(3):1255-69
- Steel J, Lowen AC, Mubareka S, Palese P. Transmission of influenza virus in a mammalian host is increased by PB2 amino acids 627K or 627E/701N. PLoS pathogens. 2009 Jan 2;5(1):e1000252.
- Brites D, Gagneux S. Co?evolution of M ycobacterium tuberculosis and Homo sapiens. Immunological reviews. 2015 Mar;264(1):
- Alakunle E, Moens U, Nchinda G, Okeke MI. Monkeypox virus in Nigeria: infection biology, epidemiology, and evolution. Viruses. 2020 Nov 5;12(11):1257.
- Kumar N, Acharya A, Gendelman HE, Byrareddy SN. The 2022 outbreak and the pathobiology of the monkeypox virus. Journal of autoimmunity. 2022 Jul 1;131:102855.
- Parkin DM. The global health burden of infection?associated cancers in the year 2002. International journal of cancer. 2006 Jun 15;118(12):3030-44.
- Thornhill JP, Barkati S, Walmsley S, Rockstroh J, Antinori A, Harrison LB, Palich R, Nori A, Reeves I, Habibi MS, Apea V. Monkeypox virus infection in humans across 16 countries—April–June 2022. New England Journal of Medicine. 2022 Aug 25;387(8):679-91.
- Singhal T, Kabra SK, Lodha R. Monkeypox: a review. Indian journal of pediatrics. 2022 Oct;89(10):955-60.
- Petersen E, Kantele A, Koopmans M, Asogun D, Yinka-Ogunleye A, Ihekweazu C, Zumla A. Human monkeypox: epidemiologic and clinical characteristics, diagnosis, and prevention. Infectiou Disease Clinics. 2019 Dec 1;33(4):1027-43.
- Sklenovská N, Van Ranst M. Emergence of monkeypox as the most important orthopoxvirus infection in humans. Frontiers in public health. 2018 Sep 4;6:383729.
- Fleming SB, Wise LM, Mercer AA. Molecular genetic analysis of orf virus: a poxvirus that has adapet to skin. Viruses. 2015 Mar 23;7(3):1505-39
- Nossal GJ, Abbot A, Mitchell J, Lummus Z. Antigens in immunity: XV. Ultrastructural features antigen capture in primary and secondary lymphoid follicles. The Journal of experimental medicine. 1968 Feb 1;127(2):277-90
- McCollum AM, Damon IK. Human monkeypox. Clinical infectious diseases. 2014 Jan15;58(2):260-7.
- Patel A, Bilinska J, Tam JC, Fontoura DD, Mason CY, Daunt A, Snell LB, Murphy J, Potter J, Tuudah C, Sundramoorthi R. Clinical features and novel presentations of human monkeypox in a central London centre during the 2022 outbreak: descriptive case series. bmj. 2022 Jul 28;378.
- Seifert M, Küppers R. Human memory B cells. Leukemia. 2016 Dec;30(12):2283-92.
- Xu R, Johnson AJ, Liggitt D, Bevan MJ. Cellular and humoral immunity against vaccinia virus infection of mice. The Journal of Immunology. 2004 May 15;172(10):6265-71.
- Giorgi JV, Liu Z, Hultin LE, Cumberland WG, Hennessey K, Detels R. Elevated levels of CD38+ CD8+ T cells in HIV infection add to the prognostic value of low CD4+ T cell levels: results of 6 years of follow-up. JAIDS Journal of Acquired Immune Deficiency Syndromes. 1993 Aug 1;6(8):904-12.
- Fang M, Sigal LJ. Antibodies and CD8+ T cells are complementary and essential for natural resistance to a highly lethal cytopathic virus. The Journal of Immunology. 2005 Nov 15;175(10):6829-36.
- Silva NI, de Oliveira JS, Kroon EG, Trindade GD, Drumond BP. Here, there, and everywhere: the wide host range and geographic distribution of zoonotic orthopoxviruses. Viruses. 2020 Dec 30;13(1):43.
- Hopkins DR. The greatest killer: smallpox in history. University of Chicago press; 2002 Sep 15.
- Vidal J. Fevered Planet: How Diseases Emerge when We Harm Nature. Bloomsbury Publishing; 2023 Jun 22.
- Luo Q, Han J. Preparedness for a monkeypox outbreak. Infectious Medicine. 2022 Jun 1;1(2):124-
- Damon IK. Poxviruses. Manual of clinical microbiology. 2011 May 16:1647-58.
- Pourriyahi H, Aryanian Z, Afshar ZM, Goodarzi A. A systematic review and clinical atlas on mucocutaneous presentations of the current monkeypox outbreak: with a comprehensive approach to all dermatologic and nondermatologic aspects of the new and previous monkeypox outbreaks. Journal of medical virology. 2023 Feb;95(2):e28230.
- Skvortsov T, Maillard JY. Viruses and Other Acellular Infectious Agents: Characteristics and Control. Hugo and Russell's Pharmaceutical Microbiology. 2023 Jan 1.
- Huang Y, Mu L, Wang W. Monkeypox: epidemiology, pathogenesis, treatment and prevention. Signal Transduction and Targeted Therapy. 2022 Nov 2;7(1):1-22.
- Adler H, Gould S, Hine P, Snell LB, Wong W, Houlihan CF, Osborne JC, Rampling T, Beadsworth MB, Duncan CJ, Dunning J. Clinical features and management of human monkeypox: a retrospective observational study in the UK. The Lancet Infectious Diseases. 2022 Aug 1;22(8):1153-62.
- Cho CT, Wenner HA. Monkeypox virus. Bacteriological reviews. 1973 Mar;37(1):1-8.
- Huhn GD, Bauer AM, Yorita K, Graham MB, Sejvar J, Likos A, Damon IK, Reynolds MG, Kuehnert MJ. Clinical characteristics of human monkeypox, and risk factors for severe disease. Clinical infectious diseases. 2005 Dec 15;41(12):1742-51.
- Damon IK. Poxviruses. Manual of clinical microbiology. 2011 May 16:1647-58.
- Pauli G, Blümel J, Burger R, Drosten C, Gröner A, Gürtler L, Heiden M, Hildebrandt M, Jansen B, Montag-Lessing T, Offergeld R. Orthopox viruses: infections in humans. Transfusion Medicine and Hemotherapy. 2010 Dec;37(6):351.
- Huggett JF, French D, O’Sullivan DM, Moran-Gilad J, Zumla A. Monkeypox: another test for PCR. Eurosurveillance. 2022 Aug 11;27(32):2200497.
- Dumont C, Irenge LM, Magazani EK, Garin D, Muyembe JJ, Bentahir M, Gala JL. Simple technique for in field samples collection in the cases of skin rash illness and subsequent PCR detection of orthopoxviruses and varicella zoster virus. PloS one. 2014 May 19;9(5):e96930.
- Edghill-Smith Y, Golding H, Manischewitz J, King LR, Scott D, Bray M, Nalca A, Hooper JW, Whitehouse CA, Schmitz JE, Reimann KA. Smallpox vaccine–induced antibodies are necessary and sufficient for protection against monkeypox virus. Nature medicine. 2005 Jul 1;11(7):740-7
- Hammarlund E, Lewis MW, Carter SV, Amanna I, Hansen SG, Strelow LI, Wong SW, Yoshihara P, Hanifin JM, Slifka MK. Multiple diagnostic techniques identify previously vaccinated individuals withprotective immunity against monkeypox. Nature medicine. 2005 Sep 1;11(9):1005-11.
- Rizk JG, Lippi G, Henry BM, Forthal DN, Rizk Y. Prevention and treatment of monkeypox. Drugs. 2022 Jun;82(9):957-63.
- Ullah A, Shahid FA, Haq MU, Tahir ul Qamar M, Irfan M, Shaker B, Ahmad S, Alrumaihi F, Allemailem KS, Almatroudi A. An integrative reverse vaccinology, immunoinformatic, docking and simulation approaches towards designing of multi-epitopes based vaccine against monkeypox virus. Journal of Biomolecular Structure and Dynamics. 2023 Nov 2;41(16):7821-34.
- Abdelaal A, Reda A, Lashin BI, Katamesh BE, Brakat AM, Al-Manaseer BM, Kaur S, Asija A, Patel NK, Basnyat S, Rabaan AA. Preventing the next pandemic: is live vaccine efficacious against monkeypox, or is there a need for killed virus and mRNA vaccines? Vaccines. 2022 Aug29;10(9):1419.
- Letafati A, Sakhavarz T. Monkeypox virus: A review. Microbial Pathogenesis. 2023 Mar 1;176:106027.
- Saadh MJ, Ghadimkhani T, Soltani N, Abbassioun A, Pecho RD, Kazem TJ, Yasamineh S, Gholizadeh O. Progress and prospects on vaccine development against monkeypox infection. Microbial Pathogenesis. 2023 Jul 1;180:106156.
- Karagoz A, Tombuloglu H, Alsaeed M, Tombuloglu G, AlRubaish AA, Mahmoud A, Smajlovi? S, ?ordi? S, Rabaan AA, Alsuhaimi E. Monkeypox (mpox) virus: Classification, origin, transmission, genome organization, antiviral drugs, and molecular diagnosis. Journal of infection and public health. 2023 Apr 1;16(4):531-41.
- Soheili M, Nasseri S, Afraie M, Khateri S, Moradi Y, Mortazavi SM, Gilzad-Kohan H. Monkeypox: virology, pathophysiology, clinical characteristics, epidemiology, vaccines, diagnosis, and treatments. Journal of Pharmacy & Pharmaceutical Sciences. 2022 Sep 21;25:297-322.
- Chakraborty S, Mohapatra RK, Chandran D, Alagawany M, Sv P, Islam MA, Chakraborty C, Dhama K. Monkeypox vaccines and vaccination strategies: Current knowledge and advances. An update–Correspondence. International Journal of Surgery. 2022 Sep 1;105:106869.
- Minhaj FS, Ogale YP, Whitehill F, Schultz J, Foote M, Davidson W, Hughes CM, Wilkins K, Bachmann L, Chatelain R, Donnelly MA. Monkeypox outbreak—Nine states, May 2022: Weekly/June 10, 2022/71 (23); 764–769.
- Long B, Koyfman A, Gottlieb M, Liang SY, Carius BM, Chavez S, Brady WJ. Monkeypox: A focused narrative review for emergency medicine clinicians. The American Journal of Emergency Medicine. 2022 Nov 1;61:34-43.
- Ajmera KM, Goyal L, Pandit T, Pandit R. Monkeypox–An emerging pandemic. IDCases. 2022 Jan 1;29:e01587.
- Saied AA, Dhawan M, Metwally AA, Fahrni ML, Choudhary P, Choudhary OP. Disease history, pathogenesis, diagnostics, and therapeutics for human monkeypox disease: a comprehensive review. Vaccines. 2022 Dec 7;10(12):2091.
- Anwar F, Haider F, Khan S, Ahmad I, Ahmed N, Imran M, Rashid S, Ren ZG, Khattak S, Ji XY. Clinical manifestation, transmission, pathogenesis, and diagnosis of monkeypox virus: a comprehensive review. Life. 2023 Feb 14;13(2):522.
- Kumar S, Subramaniam G, Karuppanan K. Human monkeypox outbreak in 2022. Journal of medical virology. 2023 Jan 1;95(1).
- Li H, Huang QZ, Zhang H, Liu ZX, Chen XH, Ye LL, Luo Y. The land-scape of immune response to monkeypox virus. EBioMedicine. 2023 Jan 1;87.
- Hatch GJ, Graham VA, Bewley KR, Tree JA, Dennis M, Taylor I, Funnell SG, Bate SR, Steeds K, Tipton T, Bean T. Assessment of the protective effect of Imvamune and Acam2000 vaccines against aerosolized monkeypox virus in cynomolgus macaques. Journal of virology. 2013 Jul 15;87(14):7805-15.
- Petersen BW. Use of vaccinia virus smallpox vaccine in laboratory and health care personnel at risk for occupational exposure to orthopoxviruses—recommendations of the Advisory Committee on Immunization Practices (ACIP), 2015. MMWR. Morbidity and mortality weekly report. 2016;65.
- Gilchuk I, Gilchuk P, Sapparapu G, Lampley R, Singh V, Kose N, Blum DL, Hughes LJ, Satheshkumar PS, Townsend MB, Kondas AV. Cross-neutralizing and protective human antibody specificities to poxvirus infections. Cell. 2016 Oct 20;167(3):684-94.
- Zaeck LM, Lamers MM, Verstrepen BE, Bestebroer TM, Van Royen ME, Götz H, Shamier MC,Van Leeuwen LP, Schmitz KS, Alblas K, Van Efferen S. Low levels of monkeypox virus-neutralizing antibodies after MVA-BN vaccination in healthy individuals. Nature medicine. 2023 Jan;29(1):270-8.
- Hatmal MM, Al-Hatamleh MA, Olaimat AN, Ahmad S, Hasan H, Ahmad Suhaimi NA, Albakri KA, Abedalbaset Alzyoud A, Kadir R, Mohamud R. Comprehensive literature review of monkeypox. Emerging Microbes & Infections. 2022 Dec 31;11(1):2600-31.
- Parker S, Handley L, Buller RM. Therapeutic and prophylactic drugs to treat orthopoxvirus infections. Future Virology. 2008 Nov 1;3(6):595-612.
- Siegrist EA, Sassine J. Antivirals with activity against mpox: a clinically oriented review. Clinical infectious diseases. 2023 Jan 1;76(1):155-64.
- McCarthy MW. Therapeutic strategies to address monkeypox. Expert Review of Anti-infective Therapy. 2022 Oct 3;20(10):1249-52.
- Ibrahim NK. Epidemiologic surveillance for controlling Covid-19 pandemic: types, challenges and implications. Journal of infection and public health. 2020 Nov 1;13(11):1630-8.
- Farahat RA, Sah R, El-Sakka AA, Benmelouka AY, Kundu M, Labieb F, Shaheen RS, Abdelaal A, Abdelazeem B, Bonilla-Aldana DK, Franco-Paredes C. Human monkeypox disease (MPX). Le infezioni in Medicina. 2022;30(3):372.
- Laurenson-Schafer H, Sklenovská N, Hoxha A, Kerr SM, Ndumbi P, Fitzner J, Almiron M, de Sousa LA, Briand S, Cenciarelli O, Colombe S. Description of the first global outbreak of mpox: an analysis of global surveillance data. The Lancet Global Health. 2023 Jul 1;11(7):e1012-23.
- Singh BK, Delgado-Baquerizo M, Egidi E, Guirado E, Leach JE, Liu H, Trivedi P. Climate change impacts on plant pathogens, food security and paths forward. Nature Reviews Microbiology. 2023 Oct;21(10):640-56.
- Waage JK, Mumford JD. Agricultural biosecurity. Philosophical Transactions of the Royal Society B: Biological Sciences. 2008 Feb 27;363(1492):863-76.


 Arnab Roy* 1
Arnab Roy* 1
 Ankita Singh 2
Ankita Singh 2
 Suraj Kumar 3
Suraj Kumar 3
 Jiten Goray 4
Jiten Goray 4
 Shivam Kashyap 5
Shivam Kashyap 5
 Gangadhar Singh 6
Gangadhar Singh 6
 Sudarshan Rawani 7
Sudarshan Rawani 7
 Priyanshu Kumar Singh 8
Priyanshu Kumar Singh 8
 Ayush Kumar Verma 9
Ayush Kumar Verma 9
 Rashmi Kumari 10
Rashmi Kumari 10
 Purnima Kumari 11
Purnima Kumari 11
 Nisha Kumari 12
Nisha Kumari 12
 Anisha Kumari 13
Anisha Kumari 13
 Sweta Verma 14
Sweta Verma 14
 Megha Chattaraj 15
Megha Chattaraj 15
 Prince Verma 16
Prince Verma 16
 Dr. K. Rajeswar Dutt 17
Dr. K. Rajeswar Dutt 17


 10.5281/zenodo.14533746
10.5281/zenodo.14533746