Abstract
The eye is a highly important and delicate organ in the human body. Although there are now many various dose forms available, eye drops are the most often utilized since they are simple to use, safe, and convenient. Among the various eye conditions, dry eye syndrome is becoming more common. By concentrating on new developments in ocular formulation, symptomatic alleviation is seen. When giving the ocular formulation, a number of ocular obstacles are discovered; various solutions to these challenges are investigated. The synthesis and assessment of an eye drop including sodium chloride as a tonicity modifier, stabilized oxychloro complex as a preservative, and castor oil as lubricant is the main topic of my research paper. Castor oil is the active ingredient because it has been shown to demonstrate longer duration of time of eye retention along with anti inflammatory, lubricant and soothing activity.
Keywords
Ocular formulation , Dry eye eye syndrome, Eye retention time
Introduction
Eye drops are commonly used for a variety of eye conditions like dryness, redness, allergies, infections, and glaucoma. They are tailored to specific needs, with some offering lubrication for dryness, antihistamines for allergy relief, antibiotics for bacterial infections, and antivirals for viral infections. Glaucoma drops help reduce intraocular pressure to protect the optic nerve. Administering eye drops correctly is important. It involves following instructions from healthcare providers or manufacturers: wash hands, tilt the head back, create a pocket by pulling down the lower eyelid, and apply the prescribed drops. Closing the eyes briefly aids absorption. If discomfort or adverse reactions arise, seeking medical advice is crucial. It is also important to store eye drops properly and check expiry dates to ensure effectiveness and safety.
Parts of eye:
The eye is a complex organ with various essential components crucial for vision. Some of its key parts include:
Cornea:
This transparent outer layer covers the iris and pupil, essential for focusing light onto the retina.
Iris: Responsible for controlling the pupil's size, regulating the amount of light entering the eye.
Pupil:
The black circular opening in the center of the iris, enabling light to pass through to the inner eye structures.
Lens:
Located behind the iris, this transparent, flexible structure helps focus light onto the retina and adjusts its shape for clear vision.
Retina:
The innermost layer containing photoreceptor cells like rods and cones, converting light into electrical signals sent to the brain via the optic nerve.
Optic Nerve:
Comprising nerve fibers, it transmits visual information from the retina to the brain for image processing.
Sclera:
Known as the "white" of the eye, it's a tough, fibrous outer layer providing structural support and protection.
Choroid:
Situated between the retina and sclera, it supplies nutrients and oxygen to maintain retinal function.
Aqueous Humor:
A clear fluid between the cornea and lens, helping maintain the eye's shape and nourishing these structures.
Vitreous Humor:
This gel-like substance fills the space between the lens and retina, supporting the eye's structure. These components work together to enable vision, highlighting the eye's intricate design and functionality.[1][2]
Different dosage form into the eye:
Medications can reach the eye through various methods, each with its distinct advantages and considerations:
Topical Administration:
This common approach involves applying medications like eye drops, ointments, or gels directly onto the eye's surface (conjunctiva). While convenient and non-invasive, it may encounter challenges in terms of drug absorption and effectiveness.
Intraocular Injection:
Certain drugs are injected directly into the eye, ensuring swift and precise delivery to address conditions such as Macular degeneration or diabetic retinopathy. However, this method carries risks like infection and retinal detachment.
Subconjunctival Injection:
By administering drugs beneath the conjunctiva, sustained release and prolonged exposure to ocular tissues can be achieved. This proves beneficial for localized treatment, especially in cases of inflammation or infection.
Systemic Administration:
Drugs taken orally or intravenously can enter the eye through systemic circulation. Yet, their effectiveness may be hindered by metabolic processes and dilution, particularly for specific eye conditions.
Intravitreal Implants:
Implants placed within the eye release medication gradually over time. This offers sustained drug delivery and may lessen the need for frequent injections.
Transscleral Delivery:
Drugs applied onto the sclera (the eye's white part) penetrate intraocular tissues. Techniques such as patches, iontophoresis, or microneedle devices provide alternatives to invasive intraocular injections.
The choice of drug administration route hinges on factors like the eye condition, drug characteristics, patient preferences, and treatment goals.[3][4]
Ocular barriers while drug administering :
Numerous obstacles impede the effectiveness of ocular drug delivery systems, affecting their efficiency. These hindrances encompass:
Ocular Surface Barriers:
The eye's outer layer, composed of the cornea and conjunctiva, presents a formidable challenge to drug penetration due to factors like tear composition and tight junctions among epithelial cells.
Tear Film Dynamics:
Acting as a protective shield, the tear film swiftly eliminates foreign substances, including drugs, through drainage into the nasolacrimal duct.
Corneal Barrier:
While the cornea is the primary pathway for topical drug absorption, its structure and tight junctions can hinder drug penetration.
Blood-Aqueous Barrier:
Regulating the influx of substances from the bloodstream into the eye's anterior chamber, this barrier restricts systemic drugs' access to intraocular tissues.[2]
Blood-Retinal Barrier:
Tight junctions between retinal cells impede drug passage from the bloodstream into the retina and vitreous, complicating systemic drug delivery.
Metabolic Enzymes:
Ocular tissues house enzymes capable of metabolizing drugs, reducing their therapeutic availability.
Efflux Transporters:
Proteins like P-glycoprotein actively eject drugs from ocular cells, lowering their intracellular concentration.
Physiological Factors:
Factors such as blinking, tear production, and ocular blood flow impact drug residence time and absorption in ocular tissues.
Overcoming these barriers is crucial to enhance ocular drug delivery effectiveness. Strategies like penetration enhancers, nanoparticles, prodrugs, mucoadhesive polymers, and sustained-release formulations hold promise in enhancing drug penetration and retention. Moreover, advancements in drug delivery technologies, including nanotechnology and microneedle arrays, offer innovative approaches to surmount ocular barriers and optimize drug delivery to the eye.[5][6]
Methods to overcome the ocular barrier:
To tackle the obstacles in ocular drug delivery, various targeted strategies are employed:
Nanoformulations:
Nanoparticles, liposome, or micelles are utilized to enhance drug solubility, stability, and bio availability. These vehicles facilitate drug penetration across ocular barriers, ensuring sustained release for better therapeutic outcomes.
Mucoadhesive Polymers:
The inclusion of mucoadhesive polymers in formulations extends drug contact time with the eye's surface. This enhances drug absorption by adhering to mucin glycoproteins in tears, thereby increasing residence time and bio availability.
Prodrugs:
Specifically designed prodrugs convert into active forms within ocular tissues, enhancing drug penetration while minimizing systemic side effects. They target specific ocular barriers, improving drug delivery efficiency.
In Situ Gelling Systems:
Formulations that undergo gelation upon contact with the ocular surface prolong drug residence time and ensure sustained release. This enhances bioavailability and patient compliance.
Ion-Activated Systems:
Hydrogels or polymers sensitive to pH or tear fluid ions release drugs at specific eye sites, enhancing targeting and efficacy.
Micro emulsions:
These formulations enhance drug solubility, stability, and penetration across ocular barriers, leading to increased bioavailability and reduced dosing frequency.
Nanoparticle-Mediated Penetration Enhancers:
Combining nanoparticles with enhancers improves drug delivery by enhancing solubility, stability, and penetration while minimizing systemic side effects.[7]
Intravitreal Implants:
Implanted devices provide sustained drug release directly to the retina and vitreous, offering prolonged therapeutic effects and reduced treatment frequency.
Surface Modification:
Modifying drug carriers or nanoparticles with ligands enhances their affinity for specific ocular tissues, improving delivery efficiency and targeting.
Physical Methods:
Techniques like iontophoresis, ultrasound, and electroporation temporarily disrupt ocular barriers, enhancing drug penetration and absorption without invasive procedures. These tailored approaches aim to overcome ocular drug delivery challenges, thereby enhancing efficacy and safety.[8]
Types of eye diseases :
Various eye diseases exist, each with unique causes, symptoms, and treatments. Some prevalent ones include:
Refractive Errors:
Myopia, hyperopia, and astigmatism result from the eye's shape hindering light focus on the retina, leading to blurred vision.
Cataracts:
The eye's lens becoming cloudy causes blurry or dim vision. While commonly associated with aging, cataracts may also develop due to injury or other medical factors.
Glaucoma:
This group of disorders damages the optic nerve, often due to increased eye pressure. Without treatment, it can result in vision loss and blindness.
Age-related Macular Degeneration (AMD): Gradual central vision loss, especially in older individuals, occurs due to AMD affecting the macula.
Diabetic Retinopathy:
Linked to diabetes, this condition damages the retina's blood vessels, leading to vision impairment and significant blindness risk among working-age adults.
Conjunctivitis (Pink Eye):
Inflammation of the thin tissue covering the eye's white part and inner eyelids can stem from infections, allergies, or irritants.
Dry Eye Syndrome:
Insufficient tear production or rapid tear evaporation causes discomfort, irritation, and blurry vision, Dry eye syndrome is an interpalpebral ocular surface problem also called as Keratoconjuctivitis sicca, which cause damage to interpalpebral eye surface due to deficiency of tear or uncontrolled evaporation. Resulting in the damage to the ocular surface which is getting worsen day by day.
Retinal Detachment:
The retina detaches from the eye's layers, potentially causing vision loss if not promptly treated.
Keratitis:
Inflammation of the cornea often occurs due to infection, injury, or extended contact lens wear.
Trachoma:
This bacterial eye infection may lead to eyelid and corneal scarring, posing blindness risks, particularly in regions with poor sanitation.[10][9]
Ocular formulation :
Ocular formulations are pharmaceutical products developed for application to the eye, aimed at treating various eye conditions while optimizing drug delivery, efficacy, and patient comfort. Some common ocular formulations include:
Eye Drops (Ophthalmic Solutions):
These are widely used ocular formulations containing drugs dissolved or suspended in a sterile aqueous solution. Eye drops are used for conditions such as dry eyes, glaucoma, and eye infections.
Ointments:
Ophthalmic ointments are semisolid formulations with drugs suspended or dissolved in a base like mineral oil or petrolatum. They provide prolonged drug contact with the ocular surface and are used for conditions requiring sustained drug release, such as severe dry eye syndrome.
Gels:
Ophthalmic gels are viscous formulations that provide extended drug contact and increased residence time on the ocular surface compared to eye drops. They are beneficial for moderate to severe dry eye syndrome, requiring prolonged lubrication and hydration.
Inserts/Implants:
Solid or semisolid drug delivery systems placed in the conjunctival sac, releasing drugs slowly over time. They are useful for chronic conditions like glaucoma or postoperative inflammation.
Suspensions:
Ophthalmic suspensions contain finely dispersed drug particles suspended in a liquid vehicle. They are used when a drug's solubility is limited or when extended contact with the ocular surface is necessary, such as in allergic conjunctivitis.[11]
Solutions for Irrigation: Sterile saline solutions used for irrigation and cleansing during procedures or for removing foreign bodies from the eye.
Eye Washes:
These are used for rinsing the eyes to alleviate discomfort or remove irritants, typically comprising isotonic saline or buffered solutions specifically designed for ocular use.
Ocular formulations undergo thorough testing to ensure accurate dosing, minimal systemic absorption, and compatibility with ocular structures. They are evaluated for safety, efficacy, and tolerability before clinical use. Advances in drug delivery technology continually improve ocular formulations, leading to better therapeutic outcomes and patient care.[12]
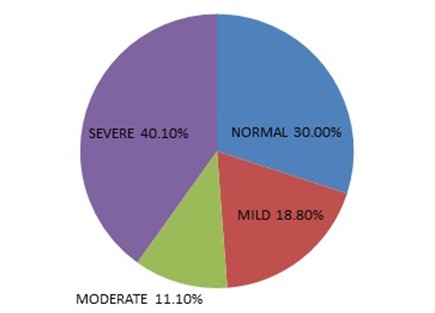
Fig. 1 Pie chart showing severity of Dry Eye Syndrome
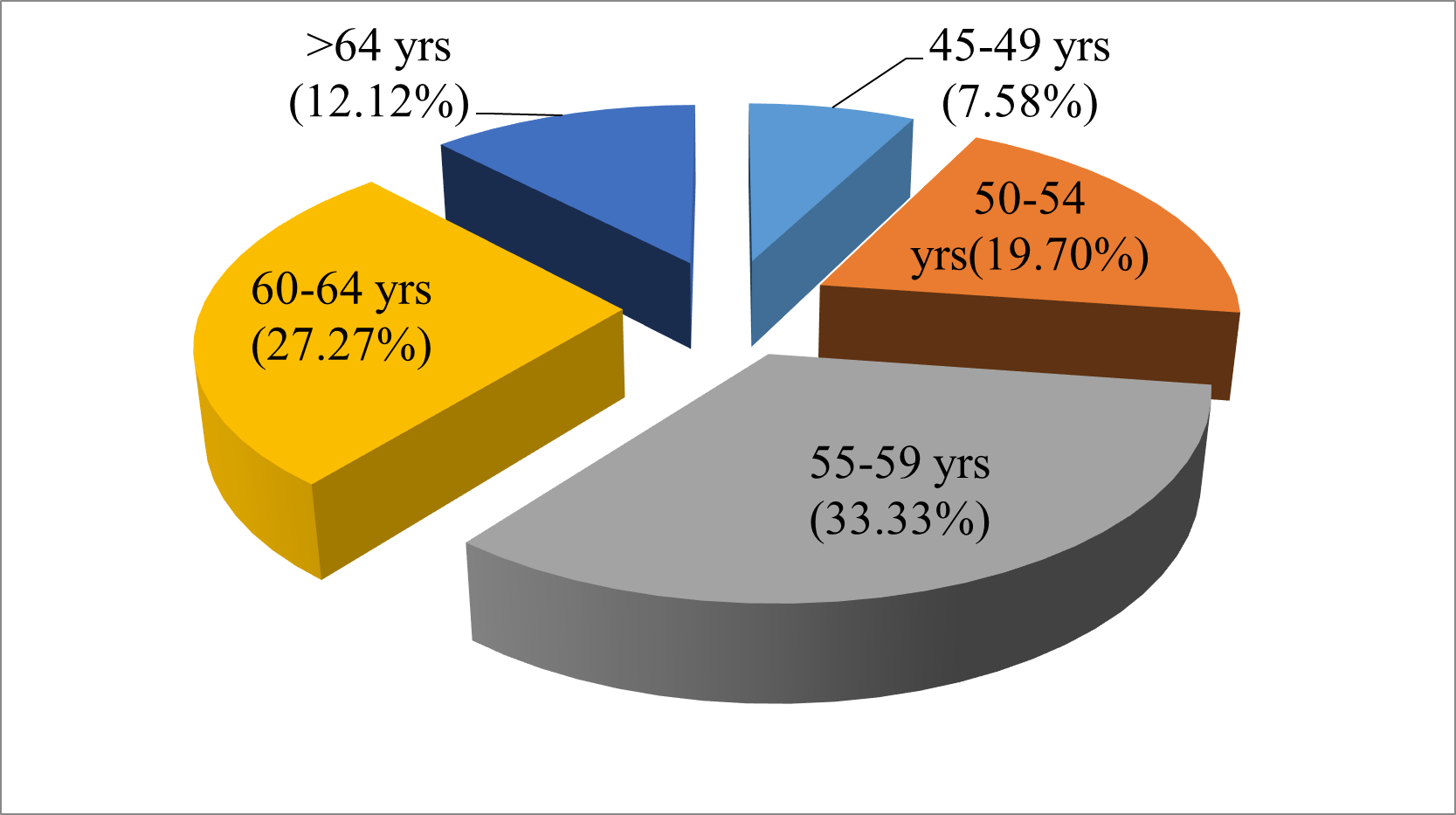
Fig. 2 Pie chart showing age wise distribution of dry eye diseases
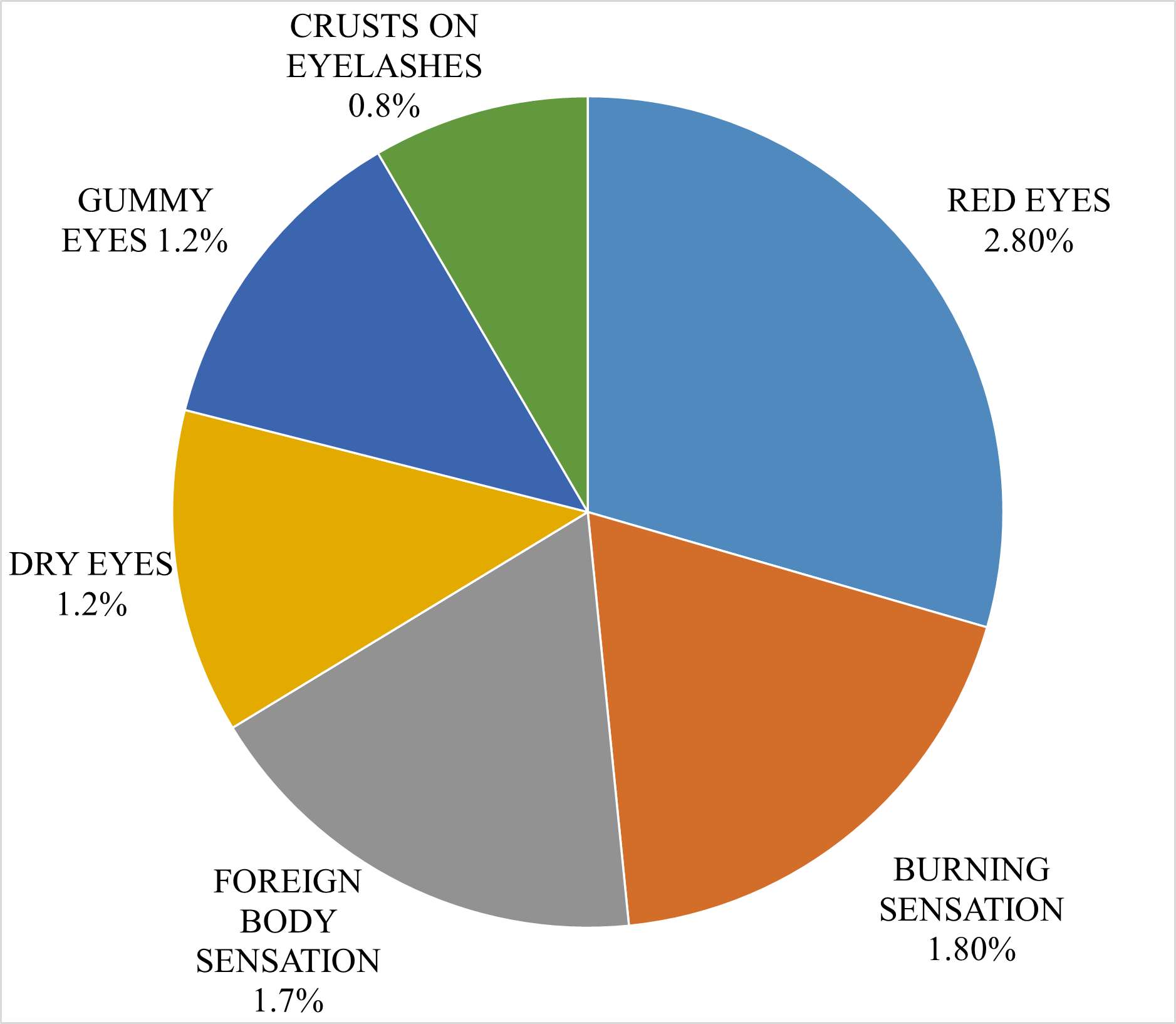
Fig .3 Pie chart showing common symptoms of dry eye
Advantage of eye drop:
Convenience:
They are easy to administer and do not require specialized skills or medical expertise. Patients can self-administer eye drops at home without assistance from healthcare professionals.
Non-Invasive:
Eye drops are non-invasive, causing minimal discomfort and side effects compared to injections or implants. They are generally well-tolerated by most patients.
Rapid Onset of Action:
Eye drops provide quick delivery of drugs to the target tissues of the eye. Upon instillation, they rapidly come into contact with the ocular surface, initiating therapeutic effects, which makes them suitable for acute conditions or symptomatic relief.
Localized Delivery:
They deliver drugs directly to ocular tissues, enabling targeted treatment while minimizing systemic exposure. This localized delivery reduces the risk of systemic side effects associated with other forms of drug delivery.
Flexible Dosage:
Eye drops allow for dosage adjustment based on the severity of the condition and individual patient response. Healthcare providers can easily adjust the dosage frequency or concentration to optimize therapeutic outcomes.
Improved Patient Compliance:
Due to their ease of use and non-invasive nature, eye drops may improve patient compliance with medication regimens. Patients are more likely to adhere to prescribed treatment schedules when using eye drops compared to other delivery methods.[13]
Stability:
Many drugs formulated as eye drops have good stability and shelf life when stored properly. This ensures that the medication remains effective throughout its intended duration of use, minimizing the risk of drug degradation.
Cost-Effectiveness:
Eye drops are often more cost-effective than other delivery methods, such as injections or implants. They require fewer specialized resources for administration, making them accessible to a broader patient population.[14]
Disadvantage of eye drop:
Limited Residence Time:
Eye drops may not remain on the eye's surface for extended periods, reducing their effectiveness and necessitating frequent dosing.
Variable Absorption:
Drug absorption from eye drops can fluctuate due to factors such as tear production and corneal permeability, impacting treatment consistency.
Potential Wastage:
Precise administration of eye drops can be difficult, leading to medication wastage because of incorrect instillation techniques or excessive bottle squeezing.
Difficulty in Administration:
Certain patients, particularly the elderly or those with impaired vision, may struggle to administer eye drops accurately, compromising treatment adherence and efficacy.
Ocular Irritation:
Eye drops containing preservatives or additives may induce irritation or allergic reactions. Prolonged use can result in ocular surface toxicity or discomfort.[15]
Systemic Absorption:
Despite being intended for ocular use, some drugs from eye drops may be absorbed systemically, potentially causing side effects or interactions with other medications.
Incompatibility with Contact Lenses:
Some eye drop formulations may not be suitable for use with contact lenses, necessitating their removal before administration, which can be inconvenient for users.
Limited Efficacy for Posterior Segment Diseases:
While effective for anterior segment conditions, eye drops may not adequately treat diseases affecting the posterior segment of the eye, necessitating alternative delivery methods such as systemic or intraocular administration.[16]
Pharmacognosy of castor oil:
Botanical Source:
Castor oil is sourced from the seeds of the castor plant (Ricinus communis), a perennial flowering plant native to Africa and India. Belonging to the Euphorbiaceae family, the castor plant is cultivated worldwide for its seeds, which are the primary raw material for castor oil production.
Chemical Composition:
Castor oil primarily comprises triglycerides, with ricinoleic acid being the predominant fatty acid component, constituting approximately 85-95% of the oil. Ricinoleic acid, a unique fatty acid, imparts castor oil with its characteristic viscosity, stability, and therapeutic attributes. Other fatty acids present in smaller amounts include oleic acid, linoleic acid, stearic acid, and palmitic acid.
Pharmacological Properties:
Castor oil exhibits diverse pharmacological properties primarily attributed to ricinoleic acid. These properties encompass:
Laxative:
Orally ingested, castor oil functions as a stimulant laxative by stimulating intestinal contractions, thereby facilitating bowel movements.[17]
Anti-inflammatory:
Ricinoleic acid demonstrates anti-inflammatory effects in preclinical studies, rendering castor oil beneficial for mitigating inflammation and minor skin irritations.
Moisturizing and Emollient:
Castor oil is extensively employed in skincare and hair care products for its moisturizing and emollient qualities, assisting in hydrating and conditioning both the skin and hair.
Therapeutic Applications:
Castor oil has a historical tradition of use for various therapeutic purposes, including:
Constipation Relief:
Due to its laxative properties, castor oil is commonly used as a natural remedy for constipation. However, its oral consumption for this purpose should be approached cautiously and under professional guidance.
Skincare:
Topically applied in skincare formulations, castor oil moisturizes, nourishes, and soothes the skin, promoting overall skin health and addressing minor irritations.
Hair Care:
Castor oil is widely employed to stimulate hair growth, moisturize the scalp and hair, minimize frizz, and enhance hair.[18]
Phytochemical screening of castor oil:
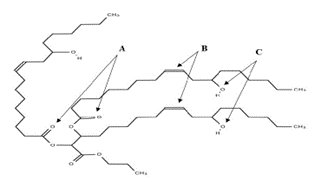
Fig.4 :Structure of Castor Oil
Phytochemical screening of castor oil involves the identification and analysis of various chemical compounds found in the plant. Ricin and ricinine, toxic proteins present in castor seeds, are detected using immunoassays, chromatography, and spectroscopic methods. Gas chromatography is utilized to quantify fatty acids, predominantly ricinoleic acid, in castor oil. Phenolic compounds, known for their antioxidant properties, are screened using techniques such as spectrophotometry, chromatography, and colorimetric assays. Triterpenoids, sterols, alkaloids, flavonoids, saponins, tannins, proteins, enzymes, and carbohydrates are among other compounds present in castor seeds, each requiring specific screening methods such as TLC, GC, HPLC, colorimetric assays, or biochemical assays for detection and analysis. These screenings provide valuable insights into the medicinal and industrial uses of castor.[19]
- Ricin and Ricinine, toxic proteins present in castor seeds, undergo screening utilizing immunoassays, chromatography , and spectroscopic methods.
- For Fatty Acids, particularly ricinoleic acid which is the main component in castor oil, gas chromatography is employed to identify and measure them accurately.
- Phenolic Compounds in castor oil, recognized for their antioxidant properties, are assessed using methods like spectrophotometry, chromatography, and colorimetric assays.
- Triterpenoids and Sterols found in castor seeds are examined through techniques such as Thin-layer chromatography, Gas Chromatography, and High-performance liquid chromatography.
- Alkaloids, albeit in trace amounts, are detectable in castor seeds using colorimetric assays, Thin Layer Chromatography, or High Performance Liquid Chromatography.
- Flavonoids, known for their antioxidant properties, are screened using methods including UV-visible spectrophotometry and chromatography.
- The presence of Saponins, glycosides found in castor and other plants, is determined through foam or hemolysis tests.
- Tannins, if present in castor seeds, are identified using precipitation methods or colorimetric assays.
- Proteins and Enzymes, apart from ricin, are identified using various biochemical assays and immunoassays in castor seeds.
- Carbohydrates such as polysaccharides in castor seeds are analyzed using techniques like thin-layer chromatography and colorimetric assays.[20]
Sofzia (Stabilized Oxychloro Complex) Preservative:
When it comes to preserving sterility and prolonging the A replacement to Benzalkonium chloride is Sofzia®. It is a component of some eye medicines.Sofzia® comprises an ion-buffered solution of borate, zinc, and sorbitol in place of Benzalkonium chloride. The intent of this preservation system is to retain its antibacterial qualities while minimizing the toxicity to the surface of the eyes.In the same way as travoprost maintained with Sofzia® efficiently reduces Intra ocular pressure in individuals with normal tension glaucoma[21]. As an alternative to eye drops kept in Benzalkonium chloride, it shows promise.Sofzia is an ionic-buffered preservative with zinc, propylene glycol, sorbitol, and borate. It has antibacterial and antifungal characteristics and acts as an oxidizing preservative[22]. When the components come into contact with cations on the ocular surface, they break down quickly, causing less cytotoxicity than Benzalkonium chloride. Preservatives are used to amplify the shelf life of drug by decreasing the oxidation of active ingredient and excipient by reducing the microbial production. An ideal preservatives are effective at low concentration against all microorganisms,be non toxic and compatible with other constituent of the preparation and be stable for the shelf life of the preparation.[24]
Sodium Chloride:
It has been found from studies that hyper osmolarity and tear instability, affects tear instability, hyperosmolar levels in the tear film may momentarily surge, causing ocular irritation and stimulating sensory neurons resulting in increase symptoms of dry eye condition.[23]This increase the importance of creating either hypotonic or isotonic solution.Dry eye symptoms were effectively improved by administering 0.1%, 0.15%, and 0.3% Sodium chloride, as well as improving the objective signs. Together with the well-tolerated profiles of 0.1%, 0.6%, and 0.3% Sodium chloride, these results demonstrate that it is a successful therapeutic approach for treating dry eye.[25]
LITERATURE REVIEW
AIM :
Formulation and In Vitro Evaluation of Eye Drop for Dry Eye Syndrome.
OBJECTIVES:
- Identify the component of eye and the ocular barrier.
- To study about dry eye syndrome and different eye diseases.
- Briefly discuss ingredient use for preparation of eye drop, such as the use of preservatives, tonicity modifier and use of lubricant.
- To evaluate the different parameters for eye drop preparation.
PLAN OF WORK:
- Introduction
- Literature review
- Selection of ingredient
- Preparation of eye drop
- Evaluation of eye drop
MATERIALS AND METHOD
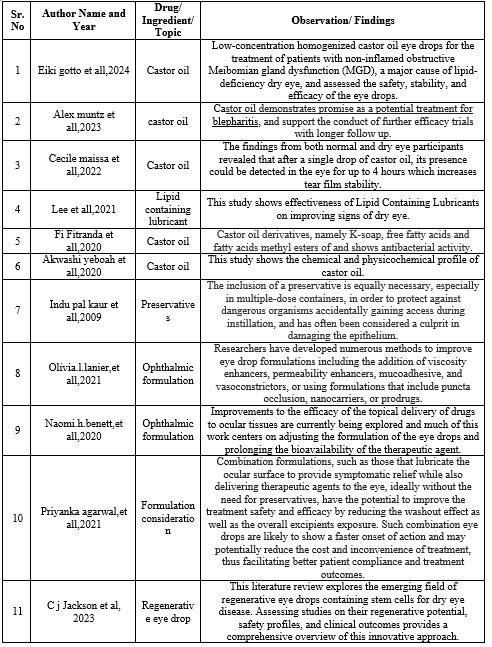
Formulation:
- All the ingredients are weight in weighing balance and mixed starting with 0.6 mg of sodium chloride in 100ml of beaker contains distilled water, followed by addition of 0.25mg of stabilized oxychloro complex in 100ml of distilled water and 1mg of glycerin in 100ml of distilled water, 0.25?stor oil is dissolved in 100ml of propylene glycol.
- After this from sodium chloride solution 4ml is taken, 3ml is taken from stabilized oxychlorocomplex, 1ml of glycerin and 2ml is taken from castor oil for sample 1.
- For sample 2, 2ml of castor oil is taken from prepared solution,5ml of sodium chloride solution,2ml of stabilized complex is taken and 1ml of glycerin.
- For sample 3, 2 ml of castor oil is taken, 2ml of sodium chloride is taken, 2ml of stabilized oxychloro complex is taken, 2ml of glycerin is taken.
- All this different sample is mixed individually in beaker.
- And the resultant sample is filled in different eye drop container
Table 1: Formulation Table
PROCEDURE:
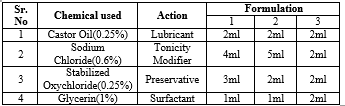
1.pH:
Normal ph of eye drop falls under 7.4, and the ph of eye drop is performed by ph paper, the samples of eye drop prepared are tested by pouring 1-2 drops on the ph paper, the change in colour shows the acidic or basic nature of the eye drop, the different prepared solution of eye drop fall under the ph scale of 7.0.

Fig 5 : pH Paper
2.STERILITY:
Sterility test of eye drop are performed by two different method that is:
-
- Direct inoculation method
- Membrane filtration method.
As per the direct inoculation method the chemicals used are fluid thioglycollate medium, which is incubated at 30 to 35 degree Celsius for 7 days, for detection of aerobic and anaerobic bacteria, if the medium shows no growth sample pass the test. The medium is prepared as followed :
- Suspend 29g of the powder in 1 litre of distilled water.
- Mix well and heat to boil, shaking frequently until completely dissolved.
- Dispense into appropriate containers.
- Sterilize by autoclave at 121°C for 15 minutes.
- Prepare freshly or boil and cool the medium just before use.
- Inoculate the medium following aseptic technique.
- Incubate the broth at 35-37°C as soon as possible.

Fig 6 : Fluid Thioglycollate Medium
3.CLARITY TEST:
In this test the eye drop is passed against the light background, to check discoloration, particulate matter,cloudiness, the solution prepared of 3 different samples passes the test, no particulate matter is observed.

Fig 7 : Clarity Test
4.LEAKAGE TEST:
In this test the eye drop is kept up down position in the stand for few hours, and the leakage is observed, the three different prepared solution of eye drop pass the test.
5.STERILLIZATION TEST:
In this test all the glass apparatus are firstly sterilized through hot air oven at a temperature at 160 degree Celsius for 45 min,after filling with 5ml syringe twice the container it is sterilized in autoclave at 115 degree Celsius for 30 min.

Fig 8 : Hot air oven
7.VISCOSITY:
In this test the viscosity is measured by Ostwald viscometer, the sample with the help of dropper is inserted into the bulb 1 and than followed by bulb 2 till the mark point after this time is noted which is required by the different sample, the time required by the water is 21sec whereas the time required by the eye drop sample is 50 sec, 1 min, 1 min 20 sec by sample 1,2,3 respectively. After this the density of the sample is calculated by weighing through empty specific gravity bottle, with water,with sample individually and the weight is noted, by putting given mentioned formula.
Density = mass of liquid/ mass of equal volume of water
= W3 - W1/ W2 - W1
= 23.89 - 15.60/42.6 - 15.60
= 7.92 g/ml
Below is the formula for calculating viscosity of the sample
Where pw (0.75) is density of water at given temperature, tw(91) is the time required by the water to travel from distance A to B, nw(0.01) is viscosity of water at given temperature, ps(7.92) is the density of given sample,ts(99) is the time required by the sample to travel from point A to B.

Fig 9: Ostwald viscometer
7.SURFACE TENSION:
In this test Stalagmometer is used to measure surface tension of the different eye drop, prior to this weight of the specific gravity bottle with and without water, with unknown sample is measured, followed by counting the drop from point A to point B of the Stalagmometer of different sample and the time required is noted, by using above formula calculation is done.
Calculation of specific gravity of unknown liquid = W3 -W1/ W2 - W1 = 0.15
W3 = Weight of specific gravity bottle and unknown liquid (23.89)
W2 = Weight of specific gravity bottle and water(42.62)
W1 = Weight of empty specific gravity bottle (16.34)
Surface tension of unknown sample = (nw /nL) Yw SL = 30nM/m
Nw = number of drops of water(45) , Yw = surface tension of water(44nM/m)
NL = number of drops of sample(10), SL = specific gravity of the unknown liquid(0.15)
Nw1 = 42 , Nw2 = 45, Nw3 = 40
NL1 = 9 , NL2 = 10 , NL3 = 8

Fig 10: Stalagmometer for surface tension determination

Fig 11 : Specific gravity bottle
Table 2: Evaluation Table
RESULTS & DISCUSSIONS
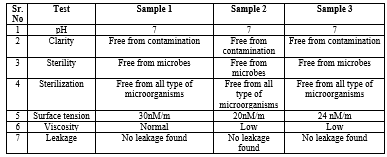
From this result it is found that the following mentioned list of ingredient for preparation and evaluation of eye drop of three different sample eye drop namely 1, 2 and 3 with same ingredient shows different pharmaceutical activity, sample 1 posses the best pharmaceutical property than other two this is concluded from different evaluation parameter which best fits the test.
Table 3: Result Table
CONCLUSION
The eye's vital function in our daily lives enables us to perceive and engage with the world visually. However, modern lifestyle habits such as excessive screen time, prolonged close work, and exposure to environmental pollutants can harm eye health. Conditions like digital eye strain, dry eye syndrome, and myopia are increasingly prevalent due to these lifestyle factors. This study explores the creation of an eye drop formula containing castor oil for its antioxidant and lubricating properties, sodium chloride to adjust tonicity, and sofzia as a preservative. These components are believed to enhance eye health by supporting critical eye structures like the retina and lens. Out of the three sample prepared the best result is shown by sample 1 for eye drop in which different evaluation parameter are best suited. To sustain and enhance overall eye health, integrating natural ingredients into one's diet or incorporating them into eye care products may yield advantages.
REFERENCES
-
-
- Collin E Willoughby MD, “Anatomy and physiology of the human eye: effects of mucopolysaccharidoses disease on structure and function – a review”, Clinical and Experimental Ophthalmology, 2010, vol38, pp1442- 9071.
- Kishore Cholkar et all, “Eye: anatomy, physiology and barriers to drug delivery”, Wood head Publishing Journal in Bio medicine, 2013, pp1-36.
- Furquan Maulvi et all, “Recent advances in ophthalmic preparations: Ocular barriers, dosage forms and routes of administration”, International Journal of Pharmaceutics, 2021, vol638, pp121-105.
- Sriram Gunda et all, “Barriers in Ocular Drug Delivery”, Opthalmology Research Journal, 2008, pp 399 413.
- S. Nikam et all, “Advancement in Ocular Drug Delivery System to Overcome Ocular Barrier”, International Journal of Pharmaceutical Sciences Review and Research, 2021, vol71(2), pp 90-97.
- Karin Attebo, et all “Knowledge and beliefs about common eye diseases”. Australian and New Zealand Journal of Ophthalmology, 2009, vol25(3).
- A Patel et all, “Ocular drug delivery systems: An overview”, world journal pharmacology, 2013, vol2 (2), pp 47- 64.
- Ja Smith et all, “The epidemiology of dry eye disease”, Acta Opthalmological Journal, 2007, vol85 240.
- Horse rewitt Md, “Dry Eye Disease: The Scale of the Problem”, Survey of opthalmology, 200, vol45 (2), pp199-202.
- Smith, et all, “Traditional Lubricants in Dry Eye Management: A Review”, Journal of Ocular Health, 15(3), 123-135.
- Johnson, C. D. (2022), “Innovative Nano formulations for Dry Eye Therapy: A Comprehensive Assessment”, Journal of Nanomedicine, 7(2), 56-68.
- J C lang etall, “Ocular drug delivery”, conventional ocular formulations Journal, 12(4), 210-223.
- Arjun Watane et all, “The Effect of Market Competition on the Price of Topical Eye Drops”, Seminars in Opthalmology, 2022, vol37 pp42-48.
- Ying Li, “Comparison of 0.3% Hypotonic and Isotonic Sodium chloride Eye Drops in the Treatment of Experimental Dry Eye”, Current Eye Research Journal, 2017, vol 42(8), pp1108-1114.
- Andjelka Racic,et all. “Formulation of olopatadine hydrochloride viscous eye drops – physicochemical, biopharmaceutical and efficacy assessment using in vitro and in vivo approaches”, European Journal of Pharmaceutical Sciences, 2021, vol 166.
- William G Gensheimer, et all, “Novel Formulation of Glycerin 1% Artificial Tears Extends Tear Film Break-Up Time Compared with Systane Lubricant Eye Drops”, Journal Of Ocular Pharmacology And Therapeutic, 2012, vol28(8).
- Sukhbir S Chirai, et all, “Drop size and initial dosing frequency problems of topically applied ophthalmic drugs”, Journal of Pharmaceutical sciences, 1974, vol63(3), pp333- 338.
- Manoj kumar,et all, “A Review on Phytochemical Constituents and Pharmacological Activities of Ricinus communis L. Plant”, International Journal of Pharmacognosy and Phytochemical Research, 2017, vol9(4), pp 466-472.
- Dr.Chandana A. Virkar, et all. “Study Of Efficacy Of Erandtail (Castor Oil) Ashchotana (Eye Drops) In Dry Eye Syndrome”, World Journal of Pharmaceutical Research , vol4(4), pp660-669.
- Wasi Yeboah,et all, “Castor oil (Ricinus communis): a review on the chemical composition and physicochemical properties”, Food science and technology, campinas, 2020, pp1001-2061,
- M I Fitranda,et all, “Physicochemical Properties and Antibacterial Activity of Castor Oil and Its Derivatives”, IOP Conference Journal :Materials science and Engineering, 2019, 1757-8999, vol83(3).
- Stephen C, et all, “Ophthalmic preservatives the past present and future”, Continuing medical education journal, 208, vol3(21).
- Haxia Liu, et all, “A link between hyper osmolarity and tear instability in dry eye syndrome”, Investigative opthalmology and visual science and, 2014, vol50(8), pp3671-3679.
- Indu Pal Kaur, et all, “Ocular Preservatives: associated Risk and Newer option”, Cutaneous and ocular toxicology, 2008, vol28(3).
- Yulli Park, et all “A Randomized Multi center Study Comparing 0.1%, 0.15%, and 0.3% Sodium Chloride with 0.05% Cyclosporine in the Treatment of Dry Eye” Journal of Ocular Pharmacology and Therapeutics, 2017, vol33(2).


 smriti kumari*
smriti kumari*
 Kanchan Singh
Kanchan Singh















 10.5281/zenodo.12559083
10.5281/zenodo.12559083